Abstract
Despite the availability of a protective vaccine for over 3 decades, the number of persons with chronic HBV infection remains high. These persons are at risk for complications of cirrhosis and hepatocellular carcinoma (HCC). Current treatment is effective at inhibiting viral replication and reducing complications of chronic HBV infection, but is not curative, and in the case of nucleos(t)ide analogues must be administered long-term, if not indefinitely, due to persistence of the covalently closed circular (cccDNA) in the hepatocyte. Thus, there is a need for novel, finite therapy that can cure chronic HBV infection. A number of agents are in early phase development and can be broadly viewed as direct acting antiviral agents that either target key viral or host proteins important for the viral life cycle or immunemodulators that boost or restore an ineffective host immune response. This review will highlight key developments in antiviral/immunomodulatory therapy, the rationale for choosing these approaches and possible therapeutic regimens.
Keywords: Vaccines, Infection, Cirrhosis, Hepatocellular Carcinoma, Hepatocyte, Antiviral/immunomodulatory therapy
Introduction
Chronic hepatitis B virus (HBV) infection is a substantial global public health problem. Despite the availability of a protective vaccine for over 3 decades, the prevalence of infection remains high. Worldwide it is estimated there are 257 million persons with chronic HBV infection, that results in 887,000 deaths annually primarily from complications of cirrhosis and hepatocellular carcinoma (HCC).1 Although currently approved treatments, peginterferon and nucleos(t)ide analogues, are effective at inhibiting viral replication and reducing complications of chronic HBV infection, they are not curative and in the case of nucleos(t)ide analogues must be administered long-term, if not indefinitely, due to persistence of the covalently closed circular (cccDNA) in the hepatocyte nucleus.2 Therefore, there is a need for new treatments that could lead to sustained, off-treatment inhibition of viral replication and loss of hepatitis B surface antigen (HBsAg). The search for new antiviral agents with activity against HBV has been hampered by the small and compact nature of the HBV genome with relatively few druggable viral targets and a lack of experimental systems or animal models that faithfully recapitulate the viral life cycle in the presence of an intact immune system. Nevertheless, recent improvements in model systems have facilitated the identification of a number of novel therapeutic approaches against HBV, as illustrated in Figure 1. There are now over 30 agents under investigation that either directly or indirectly target the HBV.3 This review will highlight key developments in antiviral/immunomodulatory therapy, the rationale for choosing these approaches and possible therapeutic regimens.
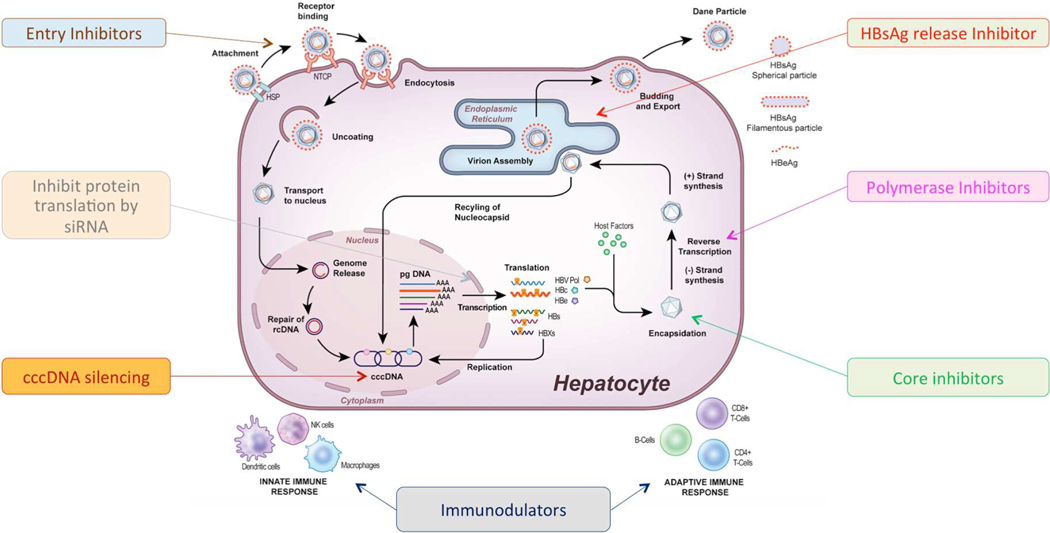
Figure 1: Hepatitis B cell life cycle and main classes of medications under development.
rcDNA-relaxed circular DNA, cccDNA-covalently closed circular DNA, pgRNA-pregenomic RNA, HBsAg-hepatitis B surface antigen, HBeAg- hepatitis B e antigen.
Goals of novel therapy and definitions of cure
The goals of novel therapies for chronic hepatitis B are to cure the chronic infection and thereby prevent complications of chronic liver disease, cirrhosis, HCC and liver-related death. It is important to appreciate that any definition of a cure should encompass eradication of the chronic viral infection as well as resolution of the underlying liver disease. It is expected that complete viral eradication should result in resolution of liver disease, but this may not be true for patients with cirrhosis. The optimal treatment endpoint would be eradication of intrahepatic cccDNA and integrated HBV DNA, loss of HBsAg and undetectable HBV DNA referred to as a complete sterilizing cure. This endpoint may not be a feasible one, as currently, it is not possible to eradicate cccDNA and integrated HBV DNA. Additionally, complete sterilizing cure may not be necessary to reduce complications of chronic HBV infection. Rather, current efforts are focused on achieving loss of HBsAg and undetectable HBV DNA in serum with or without seroconversion to hepatitis B surface antibody (anti-HBs) after completion of a finite course of treatment, resolution of residual liver injury, and a reduced risk of cirrhosis and HCC, referred to as functional cure. This may still be a challenging endpoint given the recent observation that HBsAg may originate from integrated HBV DNA.4 An alternate and perhaps more attainable goal may be complete viral suppression but persistence of HBsAg in serum after completion of a finite course of treatment, referred to as a partial cure. This endpoint is observed in a proportion of patients, but in the absence of HBsAg loss, is unlikely to be durable as there is a 15–40% lifetime risk of disease reactivation and risk of HCC may be higher compared to patients who achieve HBsAg loss.5
New therapeutic approaches for chronic hepatitis B
A greater understanding of the HBV lifecycle and the host immune response has led to the development of many new therapeutic approaches to treat chronic HBV infection. Broadly they can be viewed as agents that directly target the virus (direct acting antiviral) or indirectly through modulation of a host factor (indirect acting antiviral) or the host immune response (immunotherapy). These various strategies will be discussed in more detail.
HBV life cycle
To better understand the mechanism of action of direct acting antiviral agents under development, review of the viral life cycle will be informative, Figure 1. HBV initiates infection by first loosely attaching to heparan sulfate proteoglycans on the hepatocyte membrane, then binds to its entry receptor, the hepatocyte-specific, bile acid transporter, sodium taurocholate co-transporting polypeptide (NTCP) through an interaction with the pre-S1 lipopeptide of the large (L) envelope protein. This is followed by fusion of the HBV envelope with the endosomal membrane and endocytosis of the virus.6–8 Next, there is uncoating with release of the double-stranded relaxed circular DNA genome (rcDNA) and its transport into the hepatocyte nucleus. In the nucleus, host cellular enzymes repair the rcDNA to form cccDNA.2,9 The cccDNA serves as the transcriptional template for all mRNAs as well as the pregenomic RNA, which is the template for viral replication.
Four viral transcripts, polymerase, core, surface and X are exported to the cytoplasm where they translated into 7 viral proteins. In the cytoplasm, core proteins self-assemble into a viral nucleocapsid and the pgRNA and viral polymerase are packaged into the newly formed nucleocapsid through an encapsidation reaction. Viral replication occurs through an RNA intermediate step within the nucleocapsid. The mature viral capsids containing rcDNA are then enveloped with the small, medium and large (S, M, L) surface proteins in the endoplasmic reticulum and secreted from the infected cell as intact virions or transported back to the nucleus to replenish the cccDNA pool.
Entry inhibitors
HBV entry into hepatocytes requires the coordinated binding of the virus to heparin sulfate proteoglycans, a low affinity receptor, that mediates hepatocyte attachment, followed by a high affinity interaction with the NTCP receptor for viral internalization.8,10 The NTCP receptor confers the species-specificity to HBV.11,12 Knowledge of this process has led to the development of several classes of specific and non-specific inhibitors of viral entry, see Table 1.13–16 Entry inhibitors may lead to clearance of cccDNA by preventing de-novo infection of uninfected hepatocytes.
Table 1:
List of Agents in the Development Pipeline for Chronic Hepatitis B
| Target | Mechanism of Action | Class | Compounds in Development |
|---|---|---|---|
| Viral entry | Antibodies targeting pre-S1 or small surface protein | Monoclonal antibodies | GC1102 |
| Attachment inhibitors that block viral interaction with entry receptors | Heparin | ||
| Poly-L-lysine | |||
| Reversibly/irreversibly block the NTCP receptor | Conjugated bile salts | ||
| Synthetic N-acylated pre-S1 | Myrcludex B (Bulevirtide) | ||
| Cyclosporine | |||
| cccDNA | Inactivate cccDNA | Zinc finger nucleases | |
| Transcription activator-like effector nuclease | |||
| CRISPR/cas9 system | EBT106 HBV CRISPR-CAS-9 Lipid Nanoparticle | ||
| Degrade cccDNA | Interferon α, γ, | ||
| TNF-α | |||
| Lymphotoxin-β receptor agonists | |||
| Functionally silence cccDNA | Epigenomic modifiers | ||
| Viral transcripts | Degrade mRNA | siRNA | AB-729 ARB-1467 ARB-1740 DCR-HBVS Hepbarna (BB-HB-331) JNJ-3989 (ARO-HBV) Lunar-HBV Vir-2218 (ALN-HBV) |
| Bind viral mRNA to prevent viral protein production | Antisense oligonucleotides | IONIS-HBVRx (GSK3228836) IONIS-HBVLRx (GSK33389404) RG6004 RO7062931 |
|
| Cause degradation of HBV RNA in the nucleus | DHQ | AB452 RG7834 |
|
| Downregulate viral mRNA | FXRα agonist | EYP001 | |
| Core protein assembly modulators | Inhibit encapsidation of pregenomic RNA or nucleocapsid assembly | Heteroaryldihydropyrimidines (HAPS) | Morphothiadin (GLS4) |
| Phenylpropenamide | AT-61; AT-130 | ||
| Pyridazinone derivatives | 3711 | ||
| Sulfamoylbenzamide | AB-423, AB 506 JNJ-6379, JNJ-0440, NVR 3-778 |
||
| Isothiafludine | NZ-4 | ||
| 2-amino-n-(2,6-dichloropyridin-3-yl) acetamide derivatives | BCM-599 | ||
| 5,5’-bis[8-(phenylamino)-1-naphthalenesulfonate] | Bis-ANS | ||
| ABI-H2158, ABI-H0731, ABI-H3733 | |||
| RG7907 | |||
| QL-007 | |||
| EDP-514 | |||
| CB-HBV-001 | |||
| HBsAg Release Inhibitors | Synthetic oligonucleotides that bind HBsAg | Nucleic acid polymers | Rep 2139 Rep 2165 |
| Targeting Host Pathways | Apoptosis | Apoptosis inducer | APG-1387 |
| Cyclophilin | Cyclophilin Inhibitor | CRV 431 (CPI 431-32) | |
| Boost Innate immunity | Agonists of sensing arm of innate immune system | Toll-like receptor-7 agonist | AL-034 ANA773 RO6864018 (RG7795) RG7854 |
| Toll-like receptor-8 agonist | GS-9688 | ||
| RIG-I and NOD2 agonist | Inarigivir (SB9200) | ||
| STING agonists | |||
| TCR-like antibodies | |||
| Boost humoral immunity | Anti-HBs | HBIg | |
| Anti-HBs monoclonal antibody | GC1102 | ||
| Boost adaptive immunity | Checkpoint inhibitors | Anti-CTLA-4 | |
| Anti-PD-1 | Nivolumab | ||
| Engineering new HBV-specific T cells | TCR gene transfer | LTCR-H2-1 | |
| CAR-T cells | |||
| Therapeutic vaccines | Induction of HBV-specific B and T cells | T-cell vaccines | HepTcell |
| DNA vaccines | HB-110 INO-1800 JNJ 64300535 |
||
| Viral vectors expressing HBV proteins | TG1050 TomegaVax HBV |
||
| Virus-like particle vaccine | VBI-2601 | ||
| Inactivated parapoxvirus (iPPVO) Non-specific vaccine | AIC 649 | ||
| Antigen-antibody fusion vaccine | Chimigen HBV |
Agents such as heparin, suramine, synthetic anti-lipopolysaccharide peptides can bind to the virus or cellular heparan sulfate proteoglycans (poly-l-lysine) and non-specifically interrupt viral attachment to proteoglycans but none of them have been evaluated in the clinic 10,17–19.
More specific agents are ones that either target the antigenic loop of the HBV S-domain or N-terminal epitopes in the pre-S1 domain such as neutralizing antibodies (Ma18/7, KR127, 17.1.41/19.79.5, hepatitis B immunoglobulin [HBIg]), or ones that reversibly (taurocholate and ezetimibe) 6,15 or irreversibly bind to NTCP (myrcludex B, cyclosporin A and its derivatives such as SCYX1454139).10,20,21 HBIg, a mixture of antibodies that target HBsAg purified from the plasma of vaccinated individuals, is the only approved medication but requires parenteral administration and large doses to be effective. It has not been tested as a therapeutic agent in clinical studies.22–25 Similarly, taurocholate and ezetimibe require high concentrations to be effective but their short half-life at the receptor may limit their clinical application.
In contrast, Myrcludex B (Bulevirtide), a HBV large surface protein-derived synthetic lipopeptide, has a long half time at the receptor and irreversibly blocks the NTCP receptor in non-saturating concentrations.26 Myrcludex was evaluated in a phase IIa proof of concept study in 40 patients with HBeAg-negative CHB without cirrhosis. Patients were randomized to receive myrcludex-B at one of 5 doses, 0.5 mg, 1 mg, 2 mg, and 5 mg for 12 weeks and 10 mg once daily for 24 weeks. HBV DNA declined >1 log in 6/8 patients in the 10 mg dosing group and in 7/32 patients in the lower dosing groups however, no patient experienced HBsAg loss.27 Studies of mycludex either alone or in combination with pegylated interferon in patients with chronic delta hepatitis yielded more promising results. Among 60 patients with HBV/HDV co-infection who were randomized to receive pegylated interferon alone, pegylated interferon plus myrcludex-B 2 or 5 mg, or myrcludex-B 2 mg for 48 weeks, 3/15 (20%) patients in the pegylated interferon plus 2 mg myrcludex-B arm achieved HBsAg loss 24 weeks off therapy and this number increased to 4/15 (27%) 48 weeks off therapy. No HBsAg loss was observed in any of the other treatment arms.28 Thus, myrcludex-B in combination with other antiviral agents may be a promising treatment for chronic delta hepatitis.
Targeting cccDNA
cccDNA plays a key role in the viral life cycle where it serves as the template for viral transcription and the pgRNA, the template for viral replication. Elimination of cccDNA is considered the holy grail of HBV treatment because its persistence in the nucleus of infected hepatocytes is the major reason why cure of HBV is currently not possible. Strategies to block cccDNA formation, enhance its destruction, silence its transcription and to stimulate cell division to promote its elimination are currently under investigation.
DNA cleavage enzymes such as homing endonucleases or meganucleases, zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats associated system 9 (CRISP/Cas-9) proteins act by inducing targeted breaks in double stranded DNA. The breaks in double stranded DNA are repaired by homologous repair in the case of homologous template such as double strand DNA and single strand DNA or non-homologous end repair in the absence of template and the mutated cccDNA is eventually lost. In-vitro studies demonstrating inhibition of HBV replication has provided evidence that such an approach can work, however, specificity for viral targets and efficient and safe delivery of the gene editing agent to eliminate cccDNA from all infected hepatocytes are major challenges that need to be overcome.29–36
Another approach being pursued is to use agents that upregulate apolipoprotein B mRNA editing enzyme catalytic subunit 3A and 3B (APOBEC3A/B) deaminases. Upregulation of APOBEC3A/B by interferon-α and lymphotoxin-b has been shown to lead to non-cytolytic degradation of cccDNA in-vitro, but the degradation of the cccDNA pool is incomplete.37 Other cytokines including IFN-γ, TNF-α and IL-1β can also lead to cccDNA degradation via a similar mechanism.38,39
In the nucleus, the viral cccDNA is organized into a chromatin-like structure which makes it amenable to epigenetic manipulation.40 Several compounds have been shown in-vitro to silence cccDNA transcription. Interferon-α inhibits transcription of genomic and subgenomic RNAs derived from cccDNA, both in HBV-replicating cells in culture and in HBV-infected chimeric uPA/SCID mice repopulated with primary human hepatocytes.41 Interestingly, the HBV X protein (HBX) which is essential for viral transcription has been shown to act through degradation of the host structural maintenance of chromosomes (Smc) complex Smc5/6 which was shown to selectively block extrachromosomal DNA transcription. HBX destroys the Smc5/6 complex, thereby removing the inhibition on transcription and permits hepatitis B virus gene expression.42,43 Thus, targeting the HBX might be a viable approach to silencing cccDNA. Indeed, pevonedistat, a NEDD8-activating enzyme inhibitor, restored Smc5/6 protein levels and suppressed viral transcription in cultured hepatocytes.44 However, the observation that there is reactivation of cccDNA as soon as HBX becomes available again may be a major limitation to this approach 45,46. At present, no cccDNA targeting strategies have been evaluated in clinical trials.
Targeting viral transcripts
It has been proposed that high levels of viral antigens may inhibit the host immune response and contribute to persistence of HBV, based mostly on evidence from host evasion strategies of other viruses. Thus, targeting the viral mRNA, through the use of molecular approaches such as RNA interference, antisense oligonucleotides and ribozymes, may be an effective way to control HBV infection.9
Four viral genes are transcribed from the cccDNA template by host RNA polymerase II in the hepatocyte nucleus. All mRNA transcripts share a common 3’ terminus and encode the seven viral proteins, core, e, polymerase, small, middle and large surface and X. Therefore, targeting this region with a small interfering RNA, that can induce the cell’s RNA-induced silencing complex/argonaute 2, could lead to degradation of all viral transcripts and would allow simultaneous suppression of all viral proteins. Several siRNAs have been designed for this purpose including ARB-1467, ARB-1740, ALN-HBV, Hepbarna (BB-HB-331), Lunar-HBV, ARC-520 and ARC-521.47–50 In clinical trials, ARC-520 and ARC-521 in combination with entecavir, showed good tolerance and a decrease of HBsAg, total HBV DNA and cccDNA in both HBeAg-negative and HBeAg-positive patients.51 An interesting observation from these studies was that the decline in HBsAg levels was lower among HBeAg-negative compared to HBeAg positive patients. This reduced efficacy among HBeAg-negative patients was subsequently shown in a chimpanzee model to be due to production of HBsAg from integrated HBV DNA and absence of binding sites for the siRNA on the 3’ end of the transcripts.4 Use of a second siRNA targeting another region of preS/S was able to result in reduction of HBsAg levels in HBeAg-negative chimpanzees that was similar to that achieved in HBeAg-positive animals. However, studies with this particular compound were discontinued due to the possible fatal toxicity of the delivery vehicle in non-human primates.
An alternate approach to block viral protein expression is to use liver-directed antisense oligonucleotides that act through steric hindrance and/or RNA degradation by ribonuclease H cleavage. Two antisense molecules, IONIS-HBVRx (GSK3228836) and IONIS-HBVLRx (GSK33389404), linked to a trimer of N-acetylgalactosamine (GalNAc) moieties, allows delivery of the antisense molecule to the liver via the hepatocyte-expressed asialoglycoprotein. Such an approach may reduce off target toxicities associated with antisense oligonucleotides.52 These agents are currently in phase I trials. It is important to note that both siRNA and antisense oligonucleotides do not eliminate cccDNA and rebound of HBsAg to pre-treatment levels after treatment is stopped have been observed, leading to concerns about the durability of response. In addition, viral mutations and quasispecies may contribute to rebound and limit the applicability of this approach. Therefore, it is likely that repeated courses of therapy or use in combination with other approaches would most likely be required.53
RG7834 is a novel, small molecule compound belonging to the dihydroquinolizinones chemical class that leads to selective degradation of HBV transcripts in infected hepatocytes and in the human liver chimeric urokinase-type plasminogen activator/severe combined immunodeficiency (uPA/SCID) mouse model that acts similar to siRNA but through a different mechanism.54
Targeting viral nucleocapsid assembly
The HBV core protein is not only a structural component of the viral nucleocapsid, but is involved in nearly every stage of the HBV life cycle, including subcellular trafficking and release of the HBV genome, RNA metabolism, capsid assembly and transport, reverse transcription 55 and may modulate the host innate immune response, making the HBV core protein a promising target for HBV treatment.
Several compounds referred to as core protein assembly modulators (CpAMs) are under investigation. All the compounds bind to a hydrophobic pocket on the capsid formed at the interface between core protein dimers. These molecules either inhibit nucleocapsid assembly, encapsidation of pregenomic RNA, or both, leading to inhibition of rcDNA synthesis from pgRNA, as reverse transcription takes place only in the viral nucleocapsid.56,57 Two classes of CpAMs have been identified based on their mechanism of action. Class I typified by heteroaryldihyropyridines, increase the kinetics of capsid formation and lead to the formation of misassembled capsids. Class 2 typified by phenylpropenamides accelerate capsid assembly and form morphologically normal capsids that are empty and lack viral pgRNA and HBV polymerase. Some CpAMs may have additional effects such as affecting the conversion of rcDNA to cccDNA. Importantly, CpAMs appear to be active against most HBV genotypes and some have demonstrated activity against nucleos(t)ide analogue resistant strains.58–61 There are now a number of CpAMs in differing stages of clinical development, Table 1.
NVR 3–778, a sulfamoyl benzamide derivative was the first in class CpAM evaluated either alone or with peginterferon in a proof of concept phase 1b trial among HBeAg positive patients without cirrhosis. Reductions in both HBV DNA and HBV RNA were observed and were greatest in the group that received NVR 3–778 plus peginterferon alfa (~2 log10 copies/mL for both respectively), compared with the groups given NVR 3–778 or peginterferon alfa alone (reduction in HBV DNA of 1.43 log10 copies /mL and 1.06 log10 copies /mL respectively and (reduction in HBV RNA of 1.42 log copies/mL and 0.89 log10 copies/mL, respectively). NVR 3–778 dosed at 400 mg or lower was ineffective at suppressing HBV DNA and HBV RNA. Viral rebound was observed after treatment was stopped. Overall treatment was well tolerated.62
A novel CpAM, JNJ 56136379 (JNJ-6379), showed promise as an antiviral in treatment naive patients with CHB without cirrhosis. Efficacy and safety of JNJ-6379 at three doses of 25mg, 75mg and 150 mg administered for 28 days was evaluated in a randomized, double-blind, placebo-controlled phase 1b study among 36 HBeAg positive and negative patients. Substantial dose-dependent, reductions in HBV DNA and HBV RNA from baseline were observed with up to 2.9 log IU/ml reduction in HBV DNA and 1.7 log reduction in HBV RNA observed with the 150 mg and 75 mg dose, respectively. The drug showed no dose-limiting toxicities, with exposure increasing in a dose-dependent manner.63
RO7049389 is a small-molecule, Class I HBV CpAM that induces formation of abnormal HBV core aggregates, resulting in defective capsid assembly and thereby suppressing HBV replication. RO7049389 administered in single and multiple ascending dosing among healthy volunteers was shown to be safe and well tolerated across all tested doses. RO7049389 was administered at 200 mg bid for 28 days to six patients with chronic HBV infection, and demonstrated a median decline in HBV DNA of 2.7 log10 IU/ml from baseline and below the limit of detection in 3/6 patients. These preliminary but promising results suggest that RO7049389 has excellent anti-HBV activity.64
Preliminary results from a phase 1b trial of AB-506, a potent, oral class II capsid inhibitor, evaluating multiple doses of AB-506, with or without a nucleos(t)ide analogue, once daily for 28 days among HBeAg-positive or -negative CHB subjects reported a mean decline in HBV DNA of −2.0 log (160 mg dose) and −2.8 log (400 mg dose) from baseline at day 28 and a mean decline in HBV RNA of −2.4 log from baseline at day 28 for both doses.65 Unfortunately, further development of AB-506 was discontinued due to the observation of two cases of acute hepatitis in the phase 1a 28-day clinical trial in healthy volunteers.
ABI-H0731 is a potent and selective oral HBV core protein inhibitor. Interim results are available from two randomized, double-blind, placebo-controlled phase 2a studies. In the first study (ABI-H0731–201), ABI-H0731, 300 mg once-daily or placebo was administered to HBeAg-positive or negative individuals with chronic HBV infection already suppressed on standard-of-care nucleos(t)ide analogues (n=73). In the second study, (ABI-H0731–202), ABI-H0731 300 mg plus entecavir or entecavir plus placebo daily was given to treatment-naïve HBeAg-positive individuals (n=25). In the nucleos(t)ide suppressed HBeAg+ cohort (ABI-H0731–201), significant reductions in HBV RNA levels were observed in the group receiving nucleos(t)ide analogues plus ABI-H0731compared to the those receiving nucleos(t)ide analogues plus placebo at week 12 (2.34 log10 IU/ml vs 0.05 log10 IU/ml, p<0.001). In the treatment-naïve HBeAg+ cohort (ABI-H0731–202), significantly greater declines in HBV DNA levels were noted with the combination of ABI-H0731 and entecavir compared to entecavir plus placebo at week 12 (4.54 vs 3.29 log10 IU/mL, p<0.011) and HBV RNA levels at week 12 (2.27 vs 0.44 U/mL, p<0.005). No patient lost HBeAg or HBsAg, although decreases in HBeAg and HBsAg levels were noted in some individuals in both studies.66 These encouraging early data suggest that capsid inhibitors can result in substantial reduction in HBV DNA and HBV RNA levels. Longer term studies alone and in combination with other antiviral agents will be needed to determine if CpAMs will result in loss of serum HBsAg, HBeAg and cccDNA.
HBV polymerase inhibitors
All currently approved, nucleos(t)ide analogues target the DNA polymerase activity of the viral polymerase. The purpose of developing new HBV polymerase inhibitors is to augment their antiviral efficacy, improve oral bioavailability and safety compared to the current nucleos(t)ide analogues.9 Besifovir, CMX-157, AGX-1009 and lagociclovir are newer HBV polymerase inhibitors in development. 53,67,68
Besifovir has entered phase III trials and in doses of 90 mg or 150 mg has shown the same antiviral efficacy as 96 weeks of treatment with 0.5 mg entecavir.69 Besifovir had good antiviral activity against wild type and drug resistant mutant strains.70 The drug was well tolerated but dose dependent carnitine depletion was observed in some patients and as a result these patients had to receive carnitine supplementation.71
Additional approaches are focused on targeting the ribonuclease H (RNase H) activity of the HBV polymerase, which is required for production of new virions. Abolishing RNAse H activity results in the formation of defective HBV DNA. Currently there are no compounds that inhibit RNAse H activity in clinical trials.
Targeting HBsAg
Clearance of HBsAg is the primary focus of drug development. Therefore, compounds that can directly target HBsAg resulting in its clearance would be highly desirable. Nucleic acid polymers (NAPs) are synthetic oligonucleotides that bind HBsAg and block its release through a poorly understood mechanism.72 It has been proposed that NAPs possibly interfere with the assembly/release of HBV subviral particles.73,74 REP-2139 and REP-2165 are candidate NAPs in clinical development and in combination with pegylated interferon 2a or thymosin alpha-1, showed significant decline of HBV DNA and HBsAg levels with anti-HBs seroconversion in some HBeAg-positive Asian patients.75 REP 2139 and REP 2165 have been studied in combination with tenofovir and pegylated interferon 2a. All patients received lead in tenofovir for 24 weeks after which they were continued on tenofovir and randomized to receive pegylated interferon 2a plus REP 2139 or pegylated interferon 2a plus placebo for 24 weeks. Significant declines in HBsAg levels were noted in patients receiving REP 2139 with 9/10 patients achieving >4 log decline in HBsAg levels and 8/10 experiencing HBsAg loss compared to only 1/10 patients not receiving REP 2139 24–48 weeks off therapy.76 Further follow-up is required to determine if these interesting results are sustained.
Modulating the host immune response
The immunological response in patients with chronic HBV infection is characterized by a weak innate and HBV-specific cellular immune response. The focus of current immunological approaches are to restore the host immune response to HBV that may lead to viral clearance.77 Therapeutic strategies being pursued to induce innate immunity include the use of cytokines and pattern-recognition receptor agonists to stimulate production of interferon. Strategies to reconstitute functional HBV-specific immunity include non-specific approaches such as checkpoint inhibitors to re-awaken the T-cell response and more specific approaches using genetically engineered/modified T-cells and therapeutic vaccines.
Boosting Innate Immunity
HBV is considered a stealth virus and is not associated with induction of a strong innate immune response.78 However, several lines of evidence suggest that an adequate innate immune response can suppress HBV replication.79 As an example, interferon-α, interferon-γ, tumor necrosis factor-α (TNF-α), interleukin-1a (IL1a), produced by non-parenchymal liver cells, can suppress or even eradicate HBV from infected hepatocytes through a non-cytolytic mechanism.41,80 In addition, lymphotoxin-b-mediated activation of APOBEC or activation of (RIG-I) have been shown to suppress HBV replication.37,81 These data suggests that strategies to boost the innate immunity are a rational therapeutic approach.
Vesatolimod (GS-9620), a TLR-7 agonist, activates intrahepatic dendritic cells triggering the production of type I and II interferons and activating intrahepatic natural killer (NK) and mucosal associated invariant T (MAIT) cells. In proof-of-principle studies, vesatolimod was shown to reduce HBV DNA and HBsAg in chimpanzees and woodchucks but not in humans.82–84 Vesatolimod was tested in a phase II, double-blind, randomized, placebo-controlled study, in 162 patients stratified by HBsAg levels and serum HBeAg status. Patients received once-weekly oral vesatolimod (1-, 2-, or 4-mg doses) or placebo for 4, 8 or 12 weeks. No significant decline in HBsAg was observed at the primary (week 24) or secondary endpoints (weeks 4, 8,12, and 48).85 Differences in response observed in animal and human studies may relate to activity of TLR-7 and doses used in animal and human studies.
GS-9688, a TLR-8 agonist activates intrahepatic dendritic cells and other myeloid cells leading to production of IL-12/IL-18, activation of NK and MAIT cells and eventually decline in serum woodchuck hepatitis virus (WHV) DNA and clearance of woodchuck hepatitis surface antigen. GS-9688 is currently under investigation.86
Inarigivir (SB9200), an oral dinucleotide RIG-I agonist, has demonstrated antiviral activity against HBV via a combination of activation of innate immunity and as a direct acting antiviral effect as a non-nucleotide reverse transcriptase inhibitor (NNRTI).87 The ACHIEVE trial randomized 80 HBeAg-positive and negative patients to receive one of four doses of inarigivir (25, 50, 100 and 200 mg) daily or placebo in a 4:1 ratio for 12 weeks after which all patients were switched to tenofovir for 12 weeks. HBV DNA reduction was achieved in a dose dependent fashion for both HBeAg-positive and negative patients with a maximal reduction of 3.26 log10 in the 200 mg dose. HBV RNA levels paralleled reductions in HBV DNA levels but HBsAg decline was not dose dependent indicating the potential importance of the host response to inarigivir. The medication was well-tolerated. ALT flares were observed in 6 treated patients (10%) and 4 placebo patients (25%) and 1 patient required dose discontinuation for ALT > 400 IU. Further studies at doses of up to 400 mg daily in combination with TDF or added to tenofovir-suppressed patients are in progress.88
An agonist of the mouse stimulator of IFN genes (STING), 5,6-dimethylxanthenone-4-acetic acid (DMXAA), was found to induce a robust cytokine response in macrophages that efficiently suppressed HBV replication in mouse hepatocytes by reducing the amount of cytoplasmic viral nucleocapsids.89
An alternate strategy to augment the intrahepatic innate immune response is to selectively deliver cytokines to HBV infected hepatocytes using T-cell receptor (TCR)-like antibodies conjugated with cytokines like IFNα.90 These TCR-like antibodies bind to the human leukocyte antigens (HLA) that present HBV peptides at the surface of the infected hepatocytes and they deliver the cytokines into the microenvironment of the infected cells. Studies in animal models or humans with TCR-like antibodies have not yet been conducted.
Finally, IFN-γ and TNF-α are known to control HBV in a non-cytolytic fashion. In additional lymphotoxin-b-mediated activation of APOBEC or activation of retinoic acid-inducible gene-I (RIG-I) have been shown to suppress HBV replication. Lymphotoxin-a (LTa), lymphotoxin-b (LTb), and CD258 are the natural ligands of lymphotoxin-b receptor (LTbR). However, the potential for severe side effects with these cytokines limits their therapeutic use. Superagonistic tetravalent bi-specific antibody (BS1) and a bivalent anti-LTbR monoclonal antibody (CBE11) are LTbR agonists. BS1 and CBE11 have been tested in-vitro experiments using HBV-infected differentiated HepaRG (dHepaRG) cells and have shown to decrease levels of cccDNA, intracellular HBV DNA, pgRNA and secreted HBeAg by ~90% without toxicity.37
Adaptive immunity central to control of HBV
Control and resolution of acute HBV infection is dependent on the generation of a complex repertoire of viral-specific B- and T-cells. HBV-specific CD8 T-cells secrete cytokines such as IFN-γ that induce non-cytolytic HBV clearance as well as recruitment of other inflammatory immune cells that are critical for clearance of HBV. This process is regulated by HBV-specific CD4 T-cells.91 In chronic HBV infection, there are quantitative and functional defects of the HBV-specific T-cell response that contribute to viral persistence. T-cell exhaustion is a relatively well-characterized phenomenon in chronic HBV infection and the underlying mechanisms are thought to be the persistent exposure of T-cells to HBV antigens 92 and the increased expression of multiple co-inhibitory molecules on HBV specific T-cells, programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte–associated antigen 4 (CLTA4), lymphocyte activation gene-3 (LAG-3), CD160, T-cell immunoglobulin domain and mucin 3 (TIM-3), and 2B4.93,94 The TRAIL-death receptor, TRAIL-2, is upregulated by the exhausted T-cells making them susceptible to TRAIL-dependent NK cell lysis.95 Several other mechanisms within the liver contribute to diminished T-cell function. The release of enzymes from damaged hepatocytes deplete essential amino acids for T-cell function such as arginine and tryptophan.96 In addition, myeloid suppressor cells can also produce arginase, which also depletes arginine.97 Finally, T regulatory cells, B cells and stellate cells can secrete cytokines, such as IL-10 and TGF-β that suppress T-cell function.98,99 Restoration of an adaptive immune responses is crucial for the successful control of HBV.
Non-specific approaches to stimulate adaptive immunity
Non-specific inhibition of PD-1, programmed death-ligand 1 (PD-L1), T-cell inhibitory receptor Tim-3, and CTLA-4 could restore vigorous immune responses and this approach has been used in treatment of malignancies. However, a concern with this approach is that non-specific activation of the immune system may lead to autoimmunity and/or flares of hepatitis.
Given the upregulation of PD1 in patients with chronic hepatitis B, anti-PD-1 was evaluated to restore exhausted HBV specific T-cell function.100,101 The administration of a combination of entecavir with an anti-PD-1 ligand monoclonal antibody together with a WHV DNA vaccine to woodchucks with chronic WHV infection was associated with restoration of WHV-specific T-cell responses and clearance of WHsAg. No significant increase in serum markers of hepatic injury was observed in treated compared to untreated, control animals.102 Data on safety of nivolumab, a PD-1 inhibitor, in patients with chronic hepatitis B is available from patients with HCC. In one study, among 51 patients with HBV-related HCC treated with nivolumab, all of whom were receiving nucleos(t)ide analogues with HBV DNA <100 IU/ml, none had reactivation of HBV and no patient experienced anti-HBs seroconversion.103 A single dose of nivolumab with or without GS-4774 (a therapeutic T-cell vaccine) was evaluated in HBeAg-negative, non-cirrhotic patients without HCC in a phase 1 study. The regimen demonstrated a good safety profile and modest decreases of HBsAg levels were observed.104 The safety of check point inhibitors with prolonged use and whether these medications can be safely used in patients with cirrhosis, those with underlying autoimmune disease or who are listed for liver transplantation are important issues that will need to be addressed.
Specific approaches to stimulate adaptive immunity
Restoring an adequate HBV-specific T-cell response in chronic HBV patients is likely to be challenging due to their low frequency and exhausted phenotype. A possible solution may be the adoptive transfer of newly engineered HBV-specific T-cells.77 Evidence from patients with leukemia and chronic HBV infection who received bone marrow transplantation from donors who developed HBV-specific T-cell responses, either from vaccination or prior exposure to HBV with spontaneous recovery, demonstrating clearance of HBsAg and development of anti-HBs suggests that the approach of adoptive transfer of HBV-specific T-cells may be feasible.105,106 Genetic reprogramming to create functional T-cells to eliminate HBV infected hepatocytes could be achieved through T cell receptor (TCR) gene transfer or use of chimeric antigen receptor (CAR) T cells. The main difference between the two approaches are the type of antigen receptors used for re-programming. In the case of TCR gene transfer, it is the native alpha beta chains of the TCR whereas in the case of CAR, it is the extracellular domain of virus specific antibody. HBV-specific T-cells with CAR or classical TCRs tested in-vitro and in HBV transgenic mice demonstrated selective elimination of HBV-infected cell lines and control of HBV replication with only transient liver damage, respectively.107,108
Proof of principle of using adoptive transfer of re-programmed HBV-specific T-cells was demonstrated in a patient with metastatic HCC targeting the malignant cells expressing HBV antigens. Tumor cells were recognized in-vivo by lymphocytes engineered to express an HBV specific T-cell receptor. The genetically modified T-cells survived, expanded and mediated a reduction in HBsAg levels without exacerbation of liver inflammation or other toxicity but clinical efficacy was not observed in this patient.109
Therapeutic vaccination
Although HBsAg-based vaccines have demonstrated good efficacy at inducing anti-HBs production and protective immunity in vaccinees, the use of vaccines as a therapeutic modality has been generally disappointing. Vaccination has been used therapeutically to break T-cell tolerance to HBV proteins and stimulate HBV specific T-cell responses with the goal of achieving sustained suppression of HBV replication and ultimately HBsAg loss in patients with chronic HBV. Unfortunately, studies of current protein, DNA and T-cell vaccines in patients with chronic hepatitis B have been unsuccessful, perhaps because they only targeted HBsAg.110
The development of newer DNA vaccines, heterologous prime-boost approaches, vaccines against multiple HBV proteins and novel adjuvants, has renewed interest in vaccines as a therapeutic modality for CHB.111 DNA vaccines, such as INO-1800 or JNJ-64300535 are in phase 1 trials. INO-1800 is a mixture of recombinant DNA vaccines that encode the HBsAg and the consensus sequence of the HBcAg. However, DNA vaccination alone is not very immunogenic. INO-1800, alone or in combination with IL-12 as an immune activator is currently being tested in adults with chronic hepatitis B. Preliminary results have shown it is safe, well-tolerated and generated virus-specific T-cells, including CD8+ killer T-cells.112 JNJ-64300535 is being studied in combination with nucleos(t)ide analogues and results are awaited.111,113
A DNA prime-adenovirus boost approach was tested in woodchuck hepatitis virus-transgenic mice and was shown to elicit a potent and functional WHcAg-specific CD8+ T-cell response that resulted in the reduction of the woodchuck hepatitis virus load below the detection limit in more than 70% of animals. In addition, the combination of entecavir and DNA prime-adenovirus boost immunization in chronic woodchuck hepatitis virus carriers resulted in woodchuck hepatitis surface antigen- (WHsAg) and woodchuck hepatitis core antigen-specific CD4+ and CD8+ T-cell responses, which were not detectable in controls animals that received entecavir alone.114 Woodchucks receiving the combination therapy showed a prolonged suppression of woodchuck hepatitis virus replication and lower WHsAg levels compared to entecavir-treated controls.114 Moreover, two of four immunized carriers remained WHV negative after the end of entecavir treatment and developed anti-WHs antibodies. A clinical trial using a DNA prime-adenovirus boost approach is planned.
Vaccines using multiple HBV proteins produced by different vectors in combination with adjuvants have shown some efficacy in-vitro and in animal models.77,114,115 GS-4774 (a yeast based, heat inactivated, T-cell vaccine containing HBV core, surface and X protein) and TG1050 (a non-replicative adenovirus serotype 5 encoding a unique large fusion protein composed of a truncated HBV core, a modified HBV polymerase and two HBV envelope domains) have been shown to induce immunogenicity in mice and healthy subjects.116,117 However, results of clinical trials were disappointing.118–120 GS-4774 at 3 doses 2, 10 and 40 yeast units every 4 weeks for 24 weeks was compared to tenofovir among HBeAg positive and negative viremic patients without cirrhosis. The primary endpoint was HBsAg loss. No HBsAg loss was observed in any of the treatment arms and there were no significant differences in decline of HBsAg levels was observed between the GS-4774 dosing groups and the tenofovir group. HB-110 (a vaccine composed of three plasmids that encode for the HBV envelope proteins S and L, core protein, polymerase, and human IL-12) was evaluated I combination with adefovir and compared to adefovir alone.121 HBV-specific T-cell responses were induced in a portion of patients and a single patient who received high dose of HB-110 experienced HBeAg seroconversion.121 HepTcell (FP-02.2) another candidate vaccine, composed of nine synthetic peptides derived from the most conserved domains of HBV is currently in phase I trials.111 NASVAC (ABX203), a vaccine based on recombinant HBsAg and HBV core proteins, did not prevent viral relapse after discontinuing nucleos(t)ide analogues in HBeAg-negative patients.122 Further results of therapeutic vaccine trials are awaited but based on preliminary results it is unlikely that therapeutic vaccine alone would be effective and a combination approach will be required.
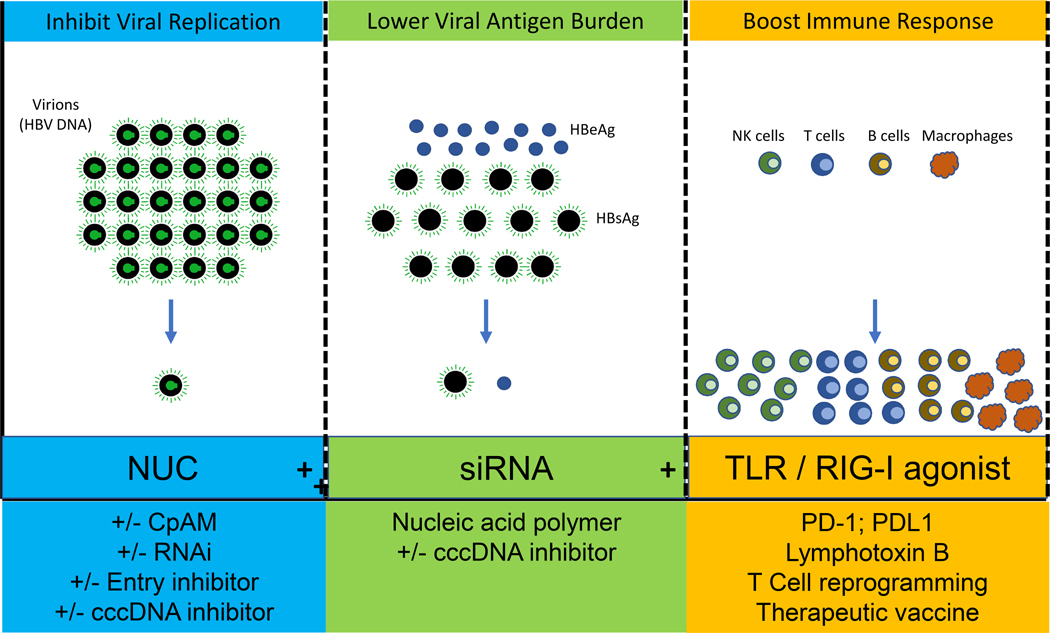
Combination therapy
Based on current management of other chronic viral infections, it is very likely that a cocktail of antivirals targeting multiple steps in the viral lifecycle or a combined antiviral/immunomodulatory approach will be needed to achieve functional cure. Which specific agents will need to be required and in what sequence in a therapeutic regimen are currently unknown as most of the drugs in the pipeline are in early phase development. Safety with monotherapy and then in combination therapy will need to be demonstrated before longer duration studies could be conducted. One therapeutic approach would be to use multiple direct acting antiviral agents with different mechanisms of action to achieve complete inhibition of intrahepatic HBV replication. A possible regimen might consist of a nucleos(t)ide analogue as a backbone plus 1 or 2 other agents such as a CpAM, siRNA, entry inhibitor or cccDNA inhibitor, Figure 2. A second approach might be to combine agents that inhibit viral replication with ones that specifically reduce viral antigen load, Figure 2. Such a strategy is based upon the rationale that a high viral antigen burden may be a contributor to the exhausted immune phenotype characteristic of chronic hepatitis B. Potential regimens might include a siRNA, a NAP or cccDNA inhibitor in combination with a direct acting antiviral. The sequence of administration of agents in such a regimen is unknown. Whether both agents would have to be used in combination initially or as an add-on approach will require future study. Yet another approach might be to use a combination antiviral / inhibitor of viral antigen burden with an immunemodulator to boost either innate immune immunity e.g. TLR-RIG-I agonist or to restore the HBV specific T-cell response such as a T-cell receptor gene transfer or CAR-T cells or therapeutic vaccines, Figure 2.
Figure 2: Possible Future Combination Regimens to Achieve Functional Cure.
NUC-nucleos(t)ide analogue, CpAM-core protein allosteric modulator, RNAi-RNA inhibitor, cccDNA-covalently closed circular DNA, siRNA-small interfering RNA, NAP-nucleic acid polymer, TLR-toll-like receptor, RIG-I- retinoic acid-inducible gene-I, PD-1- programmed cell death protein 1, PD-L1- Programmed death-ligand 1, CAR-T- chimeric antigen receptor T cells.
It is also likely that treatment may need to be tailored for different patient populations such as those who are HBeAg positive or negative, treatment naïve versus treatment experienced, those with high or low/suppressed viral replication, those with or without cirrhosis, high versus low viral antigen burden and perhaps based on genotype. These are issues that will need to be addressed in future trial designs.
Conclusion
Although this is an exciting time for drug development in chronic hepatitis B, it is important to remember that if we are to have an impact on the global burden of HBV disease, any future treatment regimen will have to be not only efficacious and safe but also tolerable, easy to administer, scalable and above all affordable. Current antiviral therapy satisfies most of these criteria but must be administered long term and is associated with very low rates of functional cure. There are safety concerns related to some of the therapeutic approaches in development such as the risk of hepatitis flares, hepatic decompensation, autoimmunity and drug toxicity that will need to be addressed. These are particularly relevant issues for resource limited areas of the world, where the majority of chronic carriers reside, where the infrastructure and cost of additional monitoring may be too burdensome. While we await newer therapies that can lead to functional cure, it is important to not forget some essential steps in the efforts to eliminate HBV: better vaccination coverage, reduce vertical transmission, and improve population-wide testing and linkage to care of patients who require treatment.
Key Points.
The number of persons with chronic HBV infection is high.
These persons are at risk for complications of cirrhosis and hepatocellular carcinoma.
There is a need for novel, finite therapy that can cure chronic HBV infection.
This review will highlight key developments in antiviral/immunomodulatory therapy, the rationale for choosing these approaches and possible therapeutic regimens.
Acknowledgments
Funding: This work was in part supported by the Intramural Research Program of NIDDK, NIH. Marc G. Ghany is an employee of the NIH.
Abbreviations
- ALT
alanine aminotransferase
- anti-HBs
hepatitis B surface antibody
- APOBEC
apolipoprotein B mRNA editing enzyme catalytic subunit
- CAR
chimeric antigen receptors
- cccDNA
covalently closed circular DNA
- CHB
Chronic HBV
- CpAMs
core protein assembly modulators
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeats associated nuclease 9
- CTLA
cytotoxic T-lymphocyte–associated antigen
- HBeAg
hepatitis B e antigen
- HBV
Hepatitis B virus
- HBIG
hepatitis B immunoglobulin
- HBsAg
hepatitis B surface antigen
- HBX
HBV X protein
- HCC
hepatocellular carcinoma
- HDV
hepatitis D
- HLA
human leukocyte antigens
- IFN-γ
interferon-γ
- IL
interleukin
- LAG-3
lymphocyte activation gene-3
- LTa
lymphotoxin-a
- LTb
lymphotoxin-b
- LTbR
Lymphotoxin-b receptor
- MAIT
mucosal associated invariant T cells
- NAPs
Nucleic acid polymers
- NK
natural killer cells
- NNRTI
non-nucleotide reverse transcriptase inhibitor
- NTCP
transporter sodium taurocholate co-transporting polypeptide
- PD
Programmed cell death protein
- pgRNA
pregenomic RNA
- rcDNA
relaxed circular DNA genome
- RIG-I
retinoic acid-inducible gene-I
- RNA
ribonucleic acid
- STING
stimulator of IFN genes
- TALENs
transcription activator-like effector nucleases
- TCR
T cell receptor
- TIM-3
T-cell immunoglobulin domain and mucin 3
- TLR
toll-like receptor
- TNF-α
Tumor Necrosis Factor alpha
- WHV
woodchuck hepatitis virus
- ZFNs
zinc-finger nucleases
Footnotes
Financial Disclosure: Elias Spyrou, nothing to disclose; Coleman I. Smith, nothing to disclose; Marc G. Ghany, nothing to disclose.
Other Disclosure: The manuscript has not been submitted to another journal and has not been published in whole or in part elsewhere previously.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.www.who.int/mediacentre/factsheets/fs164/en/ Last accessed September 30th, 2019.
- 2.Nassal M HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64(12):1972–1984. [DOI] [PubMed] [Google Scholar]
- 3.Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: From discovery to cure. Hepatology. 2015;62(6):1893–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wooddell CI, Yuen MF, Chan HL, et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017;9(409). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yip TC, Wong GL, Chan HL, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol. 2019;70(3):361–370. [DOI] [PubMed] [Google Scholar]
- 6.Ni Y, Lempp FA, Mehrle S, et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146(4):1070–1083. [DOI] [PubMed] [Google Scholar]
- 7.Verrier ER, Colpitts CC, Bach C, et al. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology. 2016;63(1):35–48. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko C, Michler T, Protzer U. Novel viral and host targets to cure hepatitis B. Curr Opin Virol. 2017;24:38–45. [DOI] [PubMed] [Google Scholar]
- 10.Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46(6):1759–1768. [DOI] [PubMed] [Google Scholar]
- 11.Zhong G, Yan H, Wang H, et al. Sodium taurocholate cotransporting polypeptide mediates woolly monkey hepatitis B virus infection of Tupaia hepatocytes. J Virol. 2013;87(12):7176–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lempp FA, Wiedtke E, Qu B, et al. Sodium taurocholate cotransporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes. Hepatology. 2017;66(3):703–716. [DOI] [PubMed] [Google Scholar]
- 13.Sankhyan A, Sharma C, Dutta D, et al. Inhibition of preS1-hepatocyte interaction by an array of recombinant human antibodies from naturally recovered individuals. Sci Rep. 2016;6:21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ying C, Van Pelt JF, Van Lommel A, et al. Sulphated and sulphonated polymers inhibit the initial interaction of hepatitis B virus with hepatocytes. Antivir Chem Chemother. 2002;13(3):157–164. [DOI] [PubMed] [Google Scholar]
- 15.Lucifora J, Esser K, Protzer U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral Res. 2013;97(2):195–197. [DOI] [PubMed] [Google Scholar]
- 16.Volz T, Allweiss L, Ben MM, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58(5):861–867. [DOI] [PubMed] [Google Scholar]
- 17.Di Stefano G, Colonna FP, Bongini A, Busi C, Mattioli A, Fiume L. Ribavirin conjugated with lactosaminated poly-L-lysine: selective delivery to the liver and increased antiviral activity in mice with viral hepatitis. Biochem Pharmacol. 1997;54(3):357–363. [DOI] [PubMed] [Google Scholar]
- 18.Krepstakies M, Lucifora J, Nagel CH, et al. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J Infect Dis. 2012;205(11):1654–1664. [DOI] [PubMed] [Google Scholar]
- 19.Petcu DJ, Aldrich CE, Coates L, Taylor JM, Mason WS. Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology. 1988;167(2):385–392. [PubMed] [Google Scholar]
- 20.Nkongolo S, Ni Y, Lempp FA, et al. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J Hepatol. 2014;60(4):723–731. [DOI] [PubMed] [Google Scholar]
- 21.Watashi K, Sluder A, Daito T, et al. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology. 2014;59(5):1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galun E, Eren R, Safadi R, et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology. 2002;35(3):673–679. [DOI] [PubMed] [Google Scholar]
- 23.Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984;52(2):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong HJ, Ryu CJ, Hur H, et al. In vivo neutralization of hepatitis B virus infection by an anti-preS1 humanized antibody in chimpanzees. Virology. 2004;318(1):134–141. [DOI] [PubMed] [Google Scholar]
- 25.Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329(25):1842–1847. [DOI] [PubMed] [Google Scholar]
- 26.Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147(1):48–64. [DOI] [PubMed] [Google Scholar]
- 27.Bogomolov P VN, Allweiss L et al. A proof-of-concept Phase 2a clinical trial with HBV/HDV entry inhibitor Myrcludex B. Hepatology. 2014;60. [Google Scholar]
- 28.Wedemeyer H, Schöneweis K, Bogomolov PO, et al. GS-13-Final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferon Alpha 2a in patients with chronic HBV/HDV co-infection. Journal of Hepatology. 2019;70(1):e81. [Google Scholar]
- 29.Zimmerman KA, Fischer KP, Joyce MA, Tyrrell DL. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. J Virol. 2008;82(16):8013–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyo B, Bloom K, Scott T, Ely A, Arbuthnot P. Advances with using CRISPR/Cas-mediated gene editing to treat infections with hepatitis B virus and hepatitis C virus. Virus Res. 2018;244:311–320. [DOI] [PubMed] [Google Scholar]
- 31.Makarova KS, Haft DH, Barrangou R, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9(6):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin SR, Yang HC, Kuo YT, et al. The CRISPR/Cas9 System Facilitates Clearance of the Intrahepatic HBV Templates In Vivo. Mol Ther Nucleic Acids. 2014;3:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ely A, Moyo B, Arbuthnot P. Progress With Developing Use of Gene Editing To Cure Chronic Infection With Hepatitis B Virus. Mol Ther. 2016;24(4):671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cradick TJ, Keck K, Bradshaw S, Jamieson AC, McCaffrey AP. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Mol Ther. 2010;18(5):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Zhang W, Lin J, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther. 2014;22(2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloom K, Ely A, Mussolino C, Cathomen T, Arbuthnot P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol Ther. 2013;21(10):1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343(6176):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isorce N, Testoni B, Locatelli M, et al. Antiviral activity of various interferons and proinflammatory cytokines in non-transformed cultured hepatocytes infected with hepatitis B virus. Antiviral Res. 2016;130:36–45. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y, Stadler D, Lucifora J, et al. Interferon-gamma and Tumor Necrosis Factor-alpha Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016;150(1):194–205. [DOI] [PubMed] [Google Scholar]
- 40.Tropberger P, Mercier A, Robinson M, Zhong W, Ganem DE, Holdorf M. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proc Natl Acad Sci U S A. 2015;112(42):E5715–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belloni L, Allweiss L, Guerrieri F, et al. IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122(2):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Yen TS, Wu L, et al. Enhancement of hepatitis B virus replication by its X protein in transgenic mice. J Virol. 2002;76(5):2579–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Decorsiere A, Mueller H, van Breugel PC, et al. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531(7594):386–389. [DOI] [PubMed] [Google Scholar]
- 44.Sekiba K, Otsuka M, Ohno M, et al. Pevonedistat, a Neuronal Precursor Cell-Expressed Developmentally Down-Regulated Protein 8-Activating Enzyme Inhibitor, Is a Potent Inhibitor of Hepatitis B Virus. Hepatology. 2019;69(5):1903–1915. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Cha EJ, Lim JE, et al. Structural characterization of an intrinsically unfolded mini-HBX protein from hepatitis B virus. Mol Cells. 2012;34(2):165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucifora J, Arzberger S, Durantel D, et al. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J Hepatol. 2011;55(5):996–1003. [DOI] [PubMed] [Google Scholar]
- 47.Streinu-Cercel A, Gane E, Cheng W, et al. A phase 2a study evaluating the multi-dose activity of ARB-1467 in HBeAg positive and negative virally suppressed subjects with hepatitis B. Journal of Hepatology. 2017;66(1):S688–S689. [Google Scholar]
- 48.Thi EP, Dhillon AP, Ardzinski A, et al. ARB-1740, a RNA Interference Therapeutic for Chronic Hepatitis B Infection. ACS Infect Dis. 2018. [DOI] [PubMed] [Google Scholar]
- 49.Mao T, Kao S-C, Cock T-A, et al. BB-HB-331, a DNA-Directed RNA Interference (ddRNAi) Agent Targeting Hepatitis B Virus (HBV), Can Effectively Suppress HBV In Vitro and In Vivo. Oligonucleotide Therapeutics. 2016;24(Supplement 1):S103. [Google Scholar]
- 50.Alnylam. Press release: Alnylam and Vir form strategic alliance to advance RNAi therapeutics for infectious diseases. 2017.
- 51.Yuen M, Chan H, Liu K, et al. Differential reductions in viral antigens expressed from cccDNAVS integrated DNA in treatment naive HBeAg positive and negative patients with chronic HBV after RNA interference therapy with ARC-520. 2016;64(suppl. 2):S390–S391. [Google Scholar]
- 52.Han K, Cremer J, Elston R, et al. A Randomized, Double-Blind, Placebo-Controlled, First-Time-in-Human Study to Assess the Safety, Tolerability, and Pharmacokinetics of Single and Multiple Ascending Doses of GSK3389404 in Healthy Subjects. Clin Pharmacol Drug Dev. 2019;8(6):790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soriano V, Barreiro P, Benitez L, Pena JM, de Mendoza C. New antivirals for the treatment of chronic hepatitis B. Expert Opin Investig Drugs. 2017;26(7):843–851. [DOI] [PubMed] [Google Scholar]
- 54.Mueller H, Wildum S, Luangsay S, et al. A novel orally available small molecule that inhibits hepatitis B virus expression. J Hepatol. 2018;68(3):412–420. [DOI] [PubMed] [Google Scholar]
- 55.Diab A, Foca A, Zoulim F, Durantel D, Andrisani O. The diverse functions of the hepatitis B core/capsid protein (HBc) in the viral life cycle: Implications for the development of HBc-targeting antivirals. Antiviral Res. 2018;149:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghany MG, Block TM. Disease Pathways and Mechanisms of Potential Drug Targets. Clin Liver Dis (Hoboken). 2018;12(1):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zlotnick A, Venkatakrishnan B, Tan Z, Lewellyn E, Turner W, Francis S. Core protein: A pleiotropic keystone in the HBV lifecycle. Antiviral Res. 2015;121:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang XY, Wei ZM, Wu GY, et al. In vitro inhibition of HBV replication by a novel compound, GLS4, and its efficacy against adefovir-dipivoxil-resistant HBV mutations. Antivir Ther. 2012;17(5):793–803. [DOI] [PubMed] [Google Scholar]
- 59.Delaney WEt, Edwards R, Colledge D, et al. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46(9):3057–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Billioud G, Pichoud C, Puerstinger G, Neyts J, Zoulim F. The main hepatitis B virus (HBV) mutants resistant to nucleoside analogs are susceptible in vitro to non-nucleoside inhibitors of HBV replication. Antiviral Res. 2011;92(2):271–276. [DOI] [PubMed] [Google Scholar]
- 61.Berke JM, Tan Y, Verbinnen T, et al. Antiviral profiling of the capsid assembly modulator BAY41–4109 on full-length HBV genotype A-H clinical isolates and core site-directed mutants in vitro. Antiviral Res. 2017;144:205–215. [DOI] [PubMed] [Google Scholar]
- 62.Yuen MF, Gane EJ, Kim DJ, et al. Antiviral Activity, Safety, and Pharmacokinetics of Capsid Assembly Modulator NVR 3–778 in Patients with Chronic HBV Infection. Gastroenterology. 2019;156(5):1392–1403 e1397. [DOI] [PubMed] [Google Scholar]
- 63.Zoulim F, Yogaratnam JZ, Vandenbossche JJ, et al. Safety, pharmakokinetics and antiviral activity of novel capsid assembly modulator (CAM) JNJ-56136379 (JNJ-6379) in treatmentnaive chronic hepatitis B (CHB) patients without cirrhosis. Journal of Hepatology. 2018;68:S102. [Google Scholar]
- 64.Gane E, Liu A, Yuen MF, et al. RO7049389, a core protein allosteric modulator, demonstrates robust anti-HBV activity in chronic hepatitis B patients and is safe and well tolerated. Journal of Hepatology. 2018;68:S101. [Google Scholar]
- 65.Corporation AB. Arbutus Announces Preliminary Phase 1a/1b Clinical Trial Results for AB-506, an Oral Capsid Inhibitor in Development for People with Chronic Hepatitis B. Company website announcement; 2019. [Google Scholar]
- 66.Ma JL j; Nguyen T; Bae H; Schiff ER; Fung S; Yuen MF; Hassanein T; Hann HW; Elkhashab M; Dieterich D; Sulkowski M; Kwo P; Nahass R; Agarwal K; Ramji A; Park J; Ravendhran N; Chan S; Weilert F; Han SH; Ayoub W; Gane E; Jacobson I; Bennett M; Huang Q; Yan R; Huey V; Ruby E; Liaw S; Colonno R; Lopatin U. Interim safety and efficacy results of the ABI-H0731 phase 2a program exploring the combination of ABI-H0731 with Nuc therapy in treatment-naive and treatment-suppressed chronic hepatitis B patients. Journal of Hepatology. 2019;70(1, Supplement):e130. [Google Scholar]
- 67.Lanier ER, Ptak RG, Lampert BM, et al. Development of hexadecyloxypropyl tenofovir (CMX157) for treatment of infection caused by wild-type and nucleoside/nucleotide-resistant HIV. Antimicrob Agents Chemother. 2010;54(7):2901–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahn SH, Kim W, Jung YK, et al. Efficacy and Safety of Besifovir Dipivoxil Maleate Compared With Tenofovir Disoproxil Fumarate in Treatment of Chronic Hepatitis B Virus Infection. Clinical Gastroenterology and Hepatology. 2019;17(9):1850–1859.e1854. [DOI] [PubMed] [Google Scholar]
- 69.Yuen MF, Kim J, Kim CR, et al. A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir Ther. 2006;11(8):977–983. [PubMed] [Google Scholar]
- 70.Yuen MF, Han KH, Um SH, et al. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology. 2010;51(3):767–776. [DOI] [PubMed] [Google Scholar]
- 71.Lai CL, Ahn SH, Lee KS, et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut. 2014;63(6):996–1004. [DOI] [PubMed] [Google Scholar]
- 72.Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One. 2016;11(6):e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noordeen F, Scougallp CA, Grosse A, et al. Therapeutic Antiviral Effect of the Nucleic Acid Polymer REP 2055 against Persistent Duck Hepatitis B Virus Infection. PLoS One. 2015;10(11):e0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quinet J, Jamard C, Burtin M, et al. Nucleic acid polymer REP 2139 and nucleos(T)ide analogues act synergistically against chronic hepadnaviral infection in vivo in Pekin ducks. Hepatology. 2018;67(6):2127–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jansen L, Vaillant A, Stelma F, et al. O114 : Serum HBV-RNA levels decline significantly in chronic hepatitis B patients dosed with the nucleic-acid polymer REP2139-CA. Journal of Hepatology. 2015;62:S250. [Google Scholar]
- 76.Bazinet M, Pantea V, Placinta G, et al. Preliminary safety and efficacy of REP 2139-Mg or REP 2165-Mg used in combination with tenofovir disoproxil fumarate and pegylated interferon alpha 2a in treatment naïve Caucasian patients with chronic HBeAg negative HBV infection. Paper presented at: Hepatology2016. [Google Scholar]
- 77.Bertoletti A, Le Bert N. Immunotherapy for Chronic Hepatitis B Virus Infection. Gut Liver. 2018;12(5):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng X, Xia Y, Serti E, et al. Hepatitis B virus evades innate immunity of hepatocytes but activates cytokine production by macrophages. Hepatology. 2017;66(6):1779–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol. 2016;64(1 Suppl):S60–S70. [DOI] [PubMed] [Google Scholar]
- 80.McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74(5):2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sato S, Li K, Kameyama T, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42(1):123–132. [DOI] [PubMed] [Google Scholar]
- 82.Lanford RE, Guerra B, Chavez D, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144(7):1508–1517, 1517 e1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menne S, Tumas DB, Liu KH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol. 2015;62(6):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gane EJ, Lim YS, Gordon SC, et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol. 2015;63(2):320–328. [DOI] [PubMed] [Google Scholar]
- 85.Janssen HLA, Brunetto MR, Kim YJ, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68(3):431–440. [DOI] [PubMed] [Google Scholar]
- 86.Daffis S, Chamberlain J, Zheng J, et al. Sustained efficacy and surface antigen seroconversion in the woodchuck model of chronic hepatitis B with the selective toll-like receptor 8 agonist GS-9688. Journal of Hepatology. 2017;66(1):S692–S693. [Google Scholar]
- 87.Walsh R, Hammond R, Jackson K, et al. Effects of SB9200 (Inarigivir) therapy on immune responses in patients with chronic hepatitis B. Journal of Hepatology. 2018;68:S89. [Google Scholar]
- 88.Yuen MC CY; Liu CJ; Jen RW; Elkhashab M; Coffin C; Kim W; Greenbloom S; Ramji A; Lim YS; Kim YJ; Fung S; Kim DJ; Jang JW; Lee KS; Afdhal N; Iyer R; Macfarlane C; Jackson K; Locarnini S; Chan H Ascending dose cohort study of inarigivir - A novel RIG I agonist in chronic HBV patients: Final results of the ACHIEVE trial. Journal of Hepatology 2019;70(1, Supplement):e45–e79. [Google Scholar]
- 89.Guo F, Han Y, Zhao X, et al. STING Agonists Induce an Innate Antiviral Immune Response against Hepatitis B Virus. Antimicrobial Agents and Chemotherapy. 2015;59(2):1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ji C, Sastry KS, Tiefenthaler G, et al. Targeted delivery of interferon-alpha to hepatitis B virus-infected cells using T-cell receptor-like antibodies. Hepatology. 2012;56(6):2027–2038. [DOI] [PubMed] [Google Scholar]
- 91.Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64(1 Suppl):S71–S83. [DOI] [PubMed] [Google Scholar]
- 92.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. [DOI] [PubMed] [Google Scholar]
- 93.Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61(6):1212–1219. [DOI] [PubMed] [Google Scholar]
- 94.Schurich A, Khanna P, Lopes AR, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53(5):1494–1503. [DOI] [PubMed] [Google Scholar]
- 95.Peppa D, Gill US, Reynolds G, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. 2013;210(1):99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. [DOI] [PubMed] [Google Scholar]
- 97.Pallett LJ, Gill US, Quaglia A, et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat Med. 2015;21(6):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Das A, Ellis G, Pallant C, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189(8):3925–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mann DA, Marra F. Fibrogenic signalling in hepatic stellate cells. J Hepatol. 2010;52(6):949–950. [DOI] [PubMed] [Google Scholar]
- 100.Fisicaro P, Valdatta C, Massari M, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138(2):682–693, 693 e681–684. [DOI] [PubMed] [Google Scholar]
- 101.Liu J, Zhang E, Ma Z, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10(1):e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balsitis S, Gali V, Mason PJ, et al. Safety and efficacy of anti-PD-L1 therapy in the woodchuck model of HBV infection. PLoS One. 2018;13(2):e0190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gane E, Gaggar A, Nguyen AH, et al. A phase1 study evaluating anti-PD-1 treatment with or without GS-4774 in HBeAg negative chronic hepatitis B patients. Journal of Hepatology. 2017;66(1):S26–S27. [Google Scholar]
- 105.Ilan Y, Nagler A, Adler R, et al. Adoptive transfer of immunity to hepatitis B virus after T cell-depleted allogeneic bone marrow transplantation. Hepatology. 1993;18(2):246–252. [PubMed] [Google Scholar]
- 106.Lau GK, Lok AS, Liang RH, et al. Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology. 1997;25(6):1497–1501. [DOI] [PubMed] [Google Scholar]
- 107.Gehring AJ, Xue SA, Ho ZZ, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55(1):103–110. [DOI] [PubMed] [Google Scholar]
- 108.Krebs K, Bottinger N, Huang LR, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145(2):456–465. [DOI] [PubMed] [Google Scholar]
- 109.Qasim W, Brunetto M, Gehring AJ, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62(2):486–491. [DOI] [PubMed] [Google Scholar]
- 110.Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. Hepatology. 2017;66(4):1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gehring AJ, Protzer U. Targeting Innate and Adaptive Immune Responses to Cure Chronic HBV Infection. Gastroenterology. 2019;156(2):325–337. [DOI] [PubMed] [Google Scholar]
- 112.ClinicalTrials.gov Phase I Study of INO-1800 With or Without INO-9112 + EP in Chronic Hepatitis B Subjects. https://clinicaltrials.gov/ct2/show/NCT02431312?term=ino-1800&draw=1&rank=1 Published May 1, 2015. Accessed.
- 113.ClinicalTrials.gov A First-In-Human Study to Evaluate Safety, Tolerability, Reactogenicity, and Immunogenicity of JNJ-64300535, a DNA Vaccine, Administered by Electroporation-Mediated Intramuscular Injection, in Participants With Chronic Hepatitis B Who Are on Stable Nucleos(t)Ide Therapy and Virologically Suppressed. https://clinicaltrials.gov/ct2/show/NCT03463369?term=JNJ-64300535&rank=1 Published March 13, 2018. Accessed2019.
- 114.Kosinska AD, Zhang E, Johrden L, et al. Combination of DNA prime--adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog. 2013;9(6):e1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu J, Kosinska A, Lu M, Roggendorf M. New therapeutic vaccination strategies for the treatment of chronic hepatitis B. Virol Sin. 2014;29(1):10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin P, Dubois C, Jacquier E, et al. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice. Gut. 2015;64(12):1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaggar A, Coeshott C, Apelian D, et al. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine. 2014;32(39):4925–4931. [DOI] [PubMed] [Google Scholar]
- 118.Vandepapeliere P, Lau GK, Leroux-Roels G, et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine. 2007;25(51):8585–8597. [DOI] [PubMed] [Google Scholar]
- 119.Mancini-Bourgine M, Fontaine H, Scott-Algara D, Pol S, Brechot C, Michel ML. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology. 2004;40(4):874–882. [DOI] [PubMed] [Google Scholar]
- 120.Lok AS, Pan CQ, Han SH, et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol. 2016;65(3):509–516. [DOI] [PubMed] [Google Scholar]
- 121.Yoon SK, Seo YB, Im SJ, et al. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015;35(3):805–815. [DOI] [PubMed] [Google Scholar]
- 122.Wedemeyer H, Hui AJ, Sukeepaisarnjaroen W, et al. Therapeutic vaccination of chronic hepatitis B patients with ABX203 (NASVAC) to prevent relapse after stopping NUCs: contrasting timing rebound between tenofovir and entecavir. Journal of Hepatology. 2017;66(1):S101. [Google Scholar]