Abstract
Objective
This study aimed to determine the effects of a novel biodegradable implant releasing platelet-derived growth factor (PDGF) at the fracture site on fracture healing in a rat tibia fracture model.
Methods
In this study, 35 male Sprague-Dawley rats weighing between 300 and 350 g were used. The rats were divided into four groups: Group A (control group without any treatment, n=10), Group B (spacer without PDGF Group, n=10), Group C (spacer with PDGF group, n=10), and Group D (healthy rat Group, n=5). Standardized fractures were created in the right tibias of rats, and then biodegradable implants made of poly-β-hydroxybutyrate-co-3-hydroxy valerate were implanted at the fracture sites in Groups B and C. In Group C, implants were loaded with 600 ng of PDGF. Animals were sacrificed 30 days after the operation, and fracture healing in each group was assessed radiologically based on the Goldberg score. Furthermore, the anteroposterior (AP) and mediolateral (ML) callus diameters were measured macroscopically, and fracture sites were mechanically tested.
Results
In the radiological assessment, Group C showed higher fracture healing rate than Groups A and B (p=0.001), whereas no significant difference was found between group C and Group D (p>0.05). In the macroscopic assessment, while Group C exhibited the thickest AP callus diameter (p=0.02), no significant differences in ML callus diameters existed among the groups (p>0.05). Mechanical testing revealed that Group C had higher torsional strength (p=0.001) and stiffness than Groups A and B (p=0.001) while there was no significant difference between Groups C and D (p>0.05).
Conclusion
Biodegradable implant releasing PDGF may have positive effects on fracture healing.
Keywords: Biodegradable, Continuous releasing systems, Implants, PDGF, Fracture healing
With the evolution of medical science, research on implant improvement has concentrated on biodegradable plastic materials (1, 2). The application of biopolymers has many advantages over most other materials, such as better biocompatibility and biodegradability and being environmentally friendly (3). Biodegradable polymers are being used in many areas of medicine, such as drug delivery systems, tissue engineering, and other applications (2).
Many conditions, such as trauma, tumor resection, deformity surgeries, infections, etc., may cause bone defects. Bone tissue has some regeneration and remodeling capacity provided by the natural healing process. However, the loss of bone tissue may exceed the repair capacity of the bone (4). As autologous bone grafting is still the gold standard procedure in such cases, the need for additional surgery and its possible complication risks are some of the limiting factors in its frequent use (5). Therefore, regenerative medicine using intracellular and extracellular signaling pathways has become important for overcoming these limitations (6). There are identified factors that have a role in fracture healing with their local or systemic effects. One of those factors is platelet-derived growth factor (PDGF), which has a powerful effect on mesenchymal cells (7–9). One of the known effects of PDGF on bone tissue is osteoblastic migration (10). In addition to the chemotactic feature, it is a mitogenic factor and has been shown to stimulate mesenchymal cells that initiate chondrogenesis and bone formation (11–13). The expression of PDGF at the fracture site also stimulates osteoprogenitor cell differentiation and collagen synthesis. In addition to these osteoblastic effects, PDGF also accelerates bone resorption by increasing the number of osteoclasts at the fracture site and provides remodeling (14, 15). PDGF is formed by two different polypeptide chains (A and B) and named after the combinations of these chains (PDGF-AA, PDGF-AB, PDGF-BB) (16). A multicenter study revealed that better success with comparable fusion rates, less pain and fewer complications can be achieved with a local PDGF-BB application after ankle joint fusions compared with autogenous bone grafting (17). The aim of our study was to research the effects of a localized PDGF release from poly-β-hydroxybutyrate-co-3-hydroxyvalerate (PHBV)-based implants at the fracture site. With the application of a successful growth factor-releasing biodegradable implant, the local application of a growth factor that has positive effects on fracture healing will noticeably improve treatment efficacy as healing occurs earlier.
Materials and Methods
Groups
Ethics approval for this study was obtained from the Experimental Animal Ethical Committee of Kırıkkale University School of Medicine. The study was performed on 35 rats, which were divided into 4 groups. A fracture model was performed on the right tibias of all rats. Group A (n=10) was the control group of this study, and these rats did not receive any treatment. Groups B (n=10) and C (n=10) were the treatment groups, and biodegradable spacers were implanted at the fracture sites in these animals. Group C implants were loaded with 600 ng PDGF-BB, enough for a 20 ng per day release for 30 days (9). Group D (n=5) included healthy rats.
Implant preparation
Polymer (PHBV) was mixed in a glass beaker with chloroform until the solution became a homogenous and viscous paste. This homogenous solution was injected into a 3 mm diameter cylindrical mold (Figure 1). After 12 hours of waiting time, the plastic was hardened enough to be cut clearly. Two parts of the prepared PHBV cylinder measuring 40 mm and 42 mm were cut and used in the study. The 40-mm part was divided into 20 empty 3×2 mm cylindrical implants. The 42-mm implant was solved with chloroform, and 12.5 μg of PDGF-BB was added to the solution. This solution (PHBV+PDGF) was injected into the mold. After the hardening of the plastic cylinder, it was divided into 21 PDGF-BB-loaded (600 ng each) 3×2 mm cylindrical implants (Figure 2).
Figure 1. a–c.
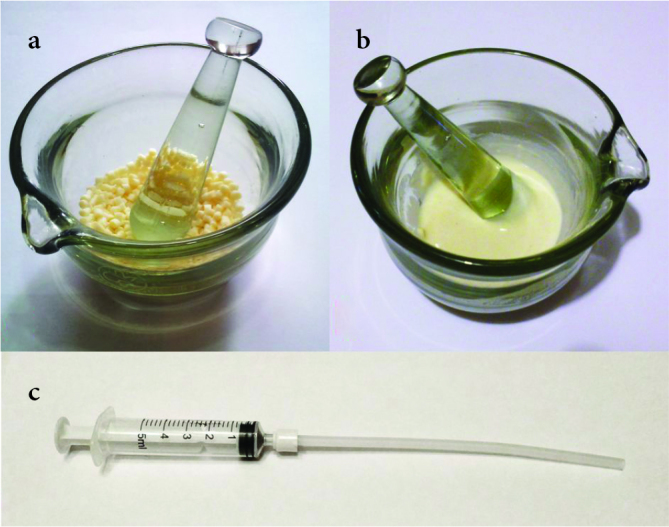
Sections of the implant preparation process; a. The polymer pellets inside the glass mortar before preparation, b. polymer paste after processing with chloroform, and c. the injection mold system
Figure 2. a, b.
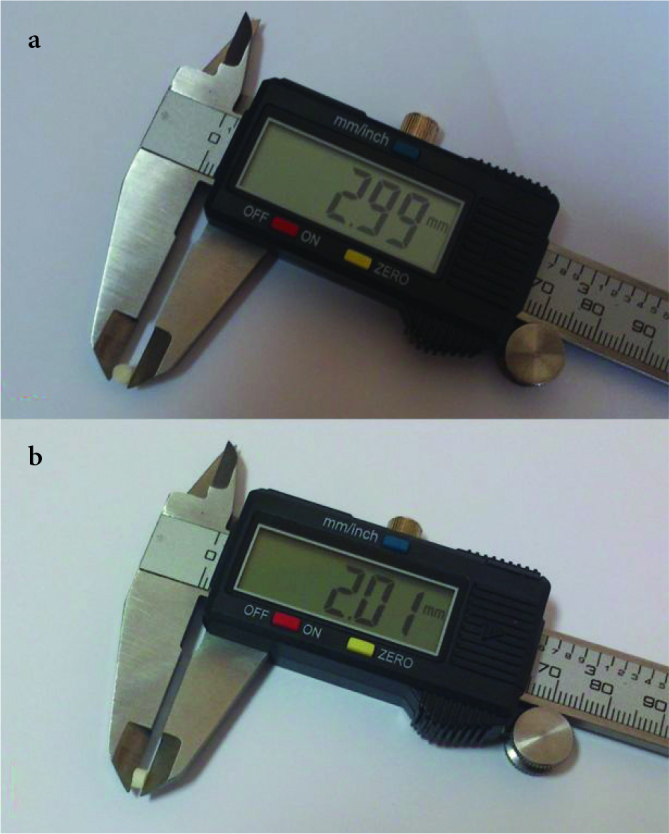
Polymer spacer measurements before application; a. the caliber of the spacers and b. the height of the spacers
Surgical procedure
All surgeries were performed under general anesthesia in sterile operating room conditions after proper surgical preparations. A combination of ketamine (Ketalar®, Pfizer, Turkey) 50 mg/kg and xylazine (Rompun®, Bayer, Turkey) 10 mg/kg was injected from the left inguinal area intraperitoneally to provide general anesthesia. The right legs of all the rats were shaved and prepared with 7.5% povidone-iodine (Batticon®, Adeka, Turkey) preoperatively to lessen the risk of infection. An anteromedially longitudinal incision of approximately 1 cm was made at the proximal cruris. The fracture model was produced at the proximal metaphysodiaphyseal region after the dissection of soft tissues (Figure 3a) with a 3.0 mm sterile drill at a speed of 120 rpm (18, 19) (Figure 3b). Incisions in Group A rats were closed after achieving the fracture model. Group B and C rat tibias were drilled, and implants were applied at the fracture sites (Figure 3. c, d). The incisions were sutured anatomically with 4/0 absorbable braided sutures and cleaned with a 7.5% povidone-iodine solution. The study ended with the sacrifice of all the rats on the 30th day postoperatively. No complications were observed at the time of the study. Rats were sacrificed by carbon dioxide inhalation. Tibias were harvested subsequent to the radiologic evaluation, and during preparation they were kept in a 10% formaldehyde solution.
Figure 3. a–d.
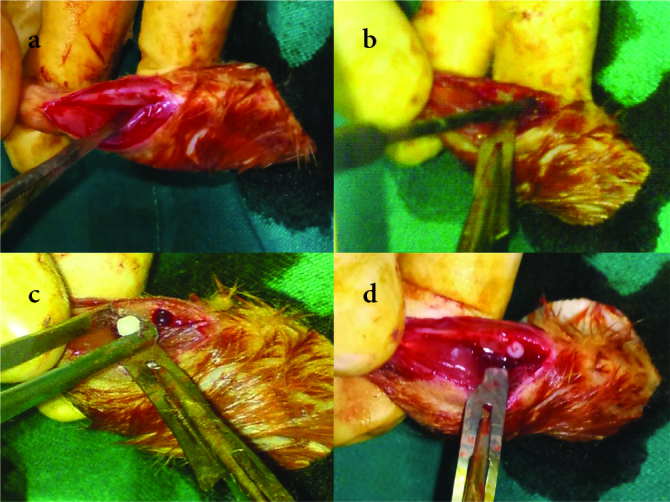
Intraoperative photos representing (a) the dissected medial tibial cortex, (b) the drilling of the tibia, (c) the implant placement, and (d) the tibial fracture model with an implant in place before skin closure
In this study, we examined the tibias radiologically, macroscopically, histologically, and mechanically.
Radiological evaluation
Group A (n=10), B (n=10), and C (n=10) rats were sacrificed on the 30th postoperative day. All the relevant x-rays were taken immediately after surgery (day 0) and sacrifice (day 30), and their Goldberg scores were evaluated (20). This scoring system gives 0 points for nonunion, 1 point for the possibility of union, and 2 points for completely united fractures. Three blinded researchers (MY, MT, MC) evaluated all the radiological images independently according to the scoring system. When there was a disagreement about the scores, reevaluation was made until a consensus was reached to prevent bias.
Macroscopic evaluation
All the tibias were harvested after the radiological evaluation. The AP and ML diameters of callosities were determined and noted at their largest site with a digital caliper (PMS 150, Conrad Electronic Gmbh., Germany).
Mechanical testing
All the tibias were examined mechanically with special torsion testing software (Partner™, Instron Inc., USA) using a low capacity torsion testing system (Instron 55MT2, Instron Inc., USA). A sliding mold frame was prepared to center the bones in the mold. This system ensured centralization of the bone, eliminating the potential bending and shear forces during the test (Figure 4). The bones were molded into acrylic cement applied to the dynamic and static chins of the system and only the callus was left outside the cement to undergo the torsional forces at the fracture site (Figure 5). Torsional tests were performed at a constant speed of 2.5 deg/sec. The last highest value in the load-degree graph was recorded as the maximum torsional strength (N.m) (Figure 6). This value was then divided by the degree at which the fracture occurred to calculate the torsional stiffness value. These two values were used to compare the groups.
Figure 4.

A sliding mold system that has been used to center the bones in the mold until satisfactory hardening of the cement is achieved
Figure 5.

Tibias buried inside cement molds before mechanical testing
Figure 6.
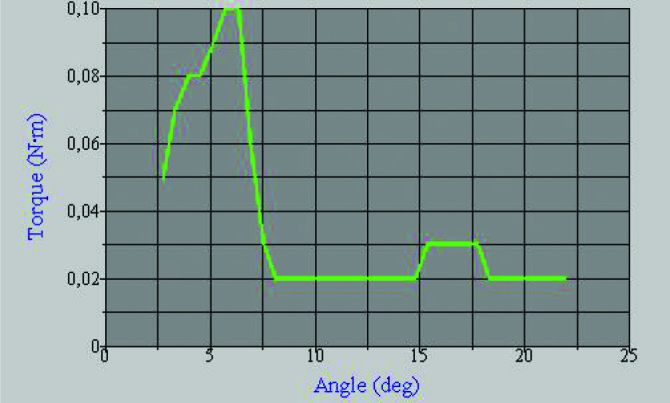
Sample torque/angle graph obtained from the mechanical testing software
Histological evaluation
Two random tibia samples of Groups A, B, and C that were eligible were evaluated histologically for fracture healing and callosities after mechanical tests. The bones were fixed in a 10% formaldehyde solution and stayed in 10% nitric acid/formalin solution for 24 hours for decalcification. They were cut into 1 mm thick sections and stained with hematoxylene/eosin. The evaluation was performed with a scoring system defined by Huo et al. (21) (Table 1).
Table 1.
Histological scoring system
| Score | Histological findings |
|---|---|
| 1 | Fibrous tissue |
| 2 | Mostly fibrous, less cartilaginous tissue |
| 3 | Equal amount of fibrous and cartilaginous tissue |
| 4 | Cartilaginous tissue |
| 5 | Mostly cartilaginous tissue, less immature (woven) bone |
| 6 | Equal amount of cartilaginous tissue and immature bone |
| 7 | Mostly immature (woven) bone, less cartilaginous tissue |
| 8 | Immature (woven) bone |
| 9 | Mostly immature, less mature bone |
| 10 | Mature (lamellar) bone |
Statistical analysis
One-way analysis of variance (ANOVA) was performed followed by Tukey post hoc tests to identify differences between treatment groups for quantitative values, and a Chi-square test was performed for qualitative values. (Statistical Package for Social Sciences version 17.0, SPSS Inc.; Chicago, IL, USA) Significance was established at p<0.05.
Results
Radiological healing
X-rays taken on the 30th day postoperatively showed significantly better radiological healing in Group C than in the other treatment groups. (p=0.001). The difference in radiological healing between Group A and Group B was not significant (p=0.293) (Table 2 and Figure 7).
Table 2.
Goldberg scores on the 30th postoperative day
| Goldberg scores | Ave± SD | Min | Max |
|---|---|---|---|
| Group A | 0.7±0.31 | 0 | 1 |
| Group B | 1.4±0.56 | 0 | 2 |
| Group C | 1.8±0.31 | 1 | 2 |
| p=0.293* | p=0.001** | ||
Ave: average; SD: standard deviation; Min: minimum; Max: maximum
p value of the radiological comparison between Group A and Group B Goldberg scores. (Chi-squared test)
p value of the radiological comparison between Group C and Group A Goldberg scores. p value of the radiological comparison between Group C and Group B Goldberg scores (Chi-squared test)
Figure 7. a–c.
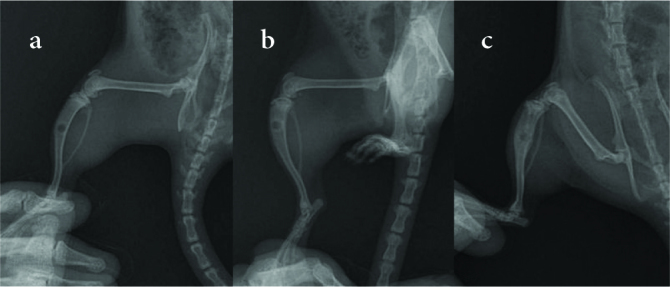
Sample radiographs of (a) the untreated group (Group A), (b) the treatment group with blank implants (Group B), and (c) the PDGF-releasing implant group (Group C)
Macroscopic evaluation
On the 30th day postoperatively, we found a statistically significant difference in the AP diameters of callosities between Group C and other treatment groups. (p=0.02). There was no significant difference in AP diameters between Group A and Group B (p>0.05). The comparison of ML callosity diameters showed no statistically significant difference between the groups (p>0.05) (Table 3).
Table 3.
Average anteroposterior (AP) and mediolateral (ML) callus diameters at postoperative 30th day
| Callus diameters | Ave± SD | Min | Max | |||
|---|---|---|---|---|---|---|
|
| ||||||
| AP | ML | AP | ML | AP | ML | |
| Group A | 5.0±1.13 | 3.7±1.48 | 3.70 | 2.40 | 6.80 | 6.30 |
| Group B | 5.5±0.85 | 4.0±0.75 | 4.50 | 3.30 | 7.10 | 6.10 |
| Group C | 6.8±1.09 | 4.6±0.91 | 5.40 | 3.20 | 8.70 | 5.80 |
|
| ||||||
| p=0.54* | p=0.02** | |||||
|
| ||||||
| p=0.75*** | p=0.52**** | p=0.18***** | ||||
Ave: average; SD: standard deviation; Min: minimum; Max: maximum; ANOVA: analysis of variance
p value of macroscopically evaluated AP callus diameters between Group A and Group B (One-way ANOVA with post hoc Tukey),
p value of macroscopically evaluated AP callus diameters between Group C and Group A and p value of macroscopically evaluated AP callus diameters between Group C and Group B (One-way ANOVA with post hoc Tukey),
p value of macroscopically evaluated ML callus diameters between Group A and Group B (One-way ANOVA with post hoc Tukey),
p value of macroscopically evaluated ML callus diameters between Group C and Group B (One-way ANOVA with post hoc Tukey),
p value of macroscopically evaluated ML callus diameters between Group C and Group A (One-way ANOVA with post hoc Tukey)
Mechanical testing
On comparing the maximum torsional strength, we found a statistically significant difference between the values for Group C and those of the other treatment groups (p=0.001). There was no statistically significant difference between the values for Group A and those for Group B (p=0.351). The maximum torsional strength of Group C tibias was higher than that of Group D tibias, but this difference was not statistically significant (p=0.831). A torsional stiffness comparison showed a statistically significant difference between Group C and Group A (p=0.001). Additionally, a statistical significance was found between the values for Group C and those for Group B (p=0.02). The results showed a statistically significant difference between the values for Group D and those for Group A (p=0.004). A significant difference between the values for Group D and Group B (p=0.009) was found. At the same time, the comparison between Group C and Group D showed no significant difference (p=0.999). The results of Groups A and Group B were not significantly different (p=0.985) (Table 4).
Table 4.
Average measured torsional strength and average calculated torsional stiffness measured at postoperatively 30th day
| Mechanical values | Ave± SD | Min | Max | |||
|---|---|---|---|---|---|---|
|
| ||||||
| T. strength | T. stiffness | T. strength | T. stiffness | T. strength | T. stiffness | |
| Group A (n=10) | 17.9±6.6 | 2.36±0.79 | 10.00 | 1.29 | 29.00 | 3.84 |
| Group B (n=10) | 23.4±4.6 | 2.55±1.22 | 17.00 | 1.26 | 31.00 | 4.75 |
| Group C (n=10) | 40.2±10.6 | 4.74±1.42 | 25.00 | 3.23 | 53.00 | 7.14 |
| Group D (n=5) | 36.8±3.7 | 4.82±2.91 | 31.00 | 2.85 | 41.00 | 6.57 |
|
| ||||||
| p>0.05* | p=0.001** | p=0.011*** | ||||
|
| ||||||
| p=0.004† | p=0.001†† | p=0.009††† | p=0.02†††† | |||
Ave: average; SD: standard deviation; Min: minimum; Max: maximum; ANOVA: analysis of variance
p value of mechanically measured torsional strength values between Group A and Group B, and p value of mechanically measured torsional strength values between Group C and Group D. (One-way ANOVA with post hoc Tukey) p value of calculated torsional stiffness values between Group A and Group B, and p value of mechanically measured torsional strength values between Group C and Group D. (One-way ANOVA with post hoc Tukey),
p value of mechanically measured torsional strength values between Group A and Group D, and p value of mechanically measured torsional strength values between Group C and Group A, and p value of mechanically measured torsional strength values between Group C and Group B. (One-way ANOVA with post hoc Tukey),
p value of mechanically measured torsional strength values between Group B and Group D (One-way ANOVA with post hoc Tukey),
p value of calculated torsional stiffness values between Group A and Group D (One-way ANOVA with post hoc Tukey),
p value of calculated torsional stiffness values between Group C and Group A (One-way ANOVA with post hoc Tukey),
p value of calculated torsional stiffness values between Group B and Group D (One-way ANOVA with post hoc Tukey),
p value of calculated torsional stiffness values between Group B and Group C (One-way ANOVA with post hoc Tukey)
Histological evaluation
On the 30th day postoperatively, cellular proliferation, vascularity and cellular maturity in the callus region were significantly higher in the PDGF-releasing implant group (Group C) than in the other treatment groups (Figure 8, 9). Microscopic evaluation revealed increased vascularization, undifferentiated periosteal cell, and osteoblastic proliferation in Group C. Additionally, as evaluated by the histological scoring system defined by Huo et al., the scores of Group C were greater than those of the other groups, indicating that trabecular and lamellar bone formation had occurred earlier in the growth factor-releasing treatment group (21) (Table 5). However, as most of the bones are not eligible to be evaluated histologically after mechanical tests, no statistical analysis was performed on histological data.
Figure 8.
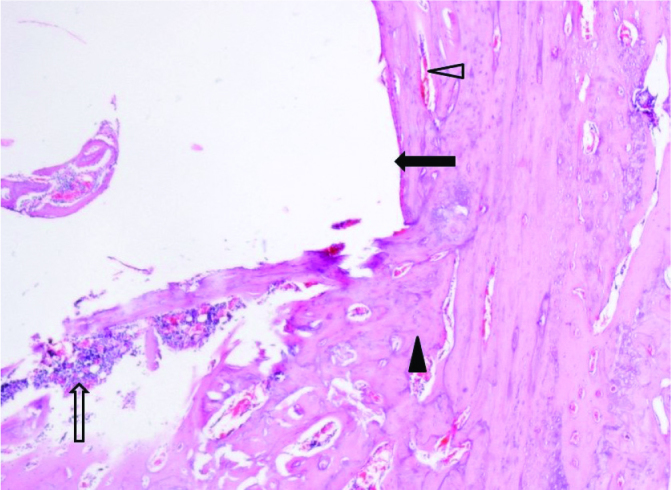
Mature lamellar bone trabeculae with congested vascular structures, 40× magnification of a hematoxylin-eosin stained section (black arrow: residual implant space, transparent arrow: fibro-collagenous connective tissue and mononuclear cell infiltration, black arrowhead: lamellar bone trabeculae, transparent arrowhead: congested vascular structures)
Figure 9.
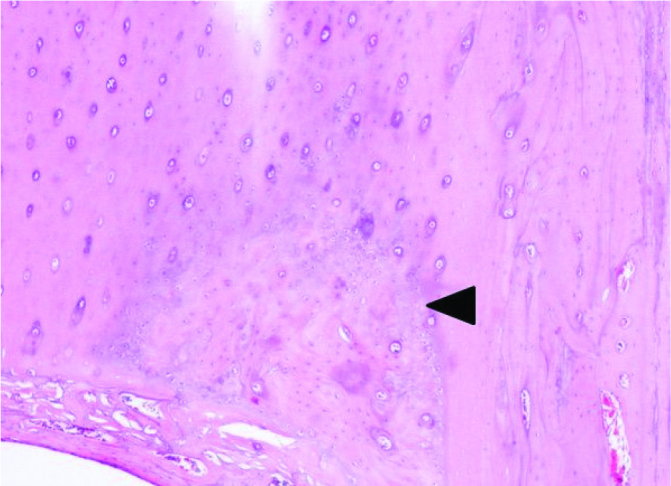
Dense mature lamellar bone tissue with surrounding healthy cortical bone, 100× magnification of a hematoxylin-eosin stained section (black arrowhead: lamellar bone trabeculae)
Table 5.
Histological scores of examined specimens
| Sample No. 1 | Sample No. 2 | |
|---|---|---|
| Group A | 5 | 6 |
| Group B | 7 | 8 |
| Group C | 10 | 10 |
Discussion
Previous studies have shown increased callus density and volume with PDGF applications (22). In our study, the macroscopic evaluation showed a statistically significant increase in callus diameters in PDGF-releasing implant-applied rats compared with other groups. The macroscopic increase in the amount of callus volumes showed that the growth factors supported healing with increased chondrogenic and osteoblastic chemotaxis and mitogenic activity that provided callus formation in the earlier healing phase. Therefore, one may assume that the biodegradable implant/spacer systems are capable of successfully releasing growth factors.
The local PDGF-releasing treatment group (Group C) of this study had significantly better radiological healing results than the other treatment groups. The significantly better radiographic scores of the PDGF-treated groups compared with the other groups support the findings of previous studies (23). The radiological evaluation of our study showed that a localized PDGF release was helpful in obtaining superior healing with earlier callus maturation compared with other treatment groups. The positive effects of a local PDGF application on early callus maturation support the use of biodegradable implants/spacers as growth factor-releasing systems.
The results of different studies on the effects of PDGF on fracture healing have suggested that the local or systemic administration of PDGF increases biomechanical strength and may contribute to improved bone mineral density in dual-energy X-Ray absorbsiometry (DEXA) (24, 25). This study showed a statistically significant increase in the maximum torsional strength and stiffness between the local release of PDGF-BB group and other treatment groups at the end of the 4th week. The superior torsional strength and stiffness results of PDGF-treated groups in this study were a result of increased callus maturation with osteoblastic differentiation and osteoclastic suppression potency of locally derived PDGF that supports enhanced fracture healing. An important finding of this study was that there was no statistically significant difference between the PDGF-releasing treatment group and healthy tibias in the 4th week with respect to torsional stiffness. The equal biomechanical results show biomechanical healing of the bone tissue with complete callus maturation with potentially positive local PDGF release effects. This may promote the overall healing process that lets the area become fully weight-bearing or allow the patient to functionally recover earlier.
In terms of the histological evaluation, the callus consisted of more lamellar bone substance in the PDGF-releasing treatment group. Additionally, cellular proliferation, vascularity and cellular maturity in the callus region were significantly higher in the PDGF-releasing implant group than in the other treatment groups. Cellular proliferation mostly consisted of undifferentiated periosteal and osteoblastic cells. Similar to other studies, the PDGF-treated rats in this study had enhanced histological fracture healing results, such as dense lamellar bone content in addition to an increased woven bone ratio at the early stages of healing (9, 26). Studies on growth factor-releasing biodegradable implants showed, histologically, that lamellar bone formation was increased at the callus site, similar to the results of our study (27). The histological effect of PDGF on healing tissue is another supportive finding of local growth factor release from the implant/spacer system.
This study supports many previous findings and provides an opportunity to improve fracture treatment-assisting devices, such as growth factor-releasing implants or spacers. The results of our study showed that fracture treatment with a local PDGF-releasing biodegradable implant provides radiologically, mechanically, and histologically superior results with earlier healing, which may allow rehabilitation to be initiated in a shorter time period. With a simple production process, these implant/spacers can be produced in the operating room and customized for the patient. This study showed that a successful growth factor-releasing implant/spacer system can be easily manufactured from biodegradable polymers. When combined with a bone-healing supportive local growth factor, such as PDGF, this implant/spacer system may provide better and earlier fracture healing. The similar mechanical results between the PDGF-releasing implant treatment group and healthy bones support the idea of an enhanced fracture healing process with this treatment. This may lead to better functional results and fewer complications by shortening the time to after-treatment procedures, such as physical rehabilitation. Considering the many advantages of biodegradable polymers, we believe that local PDGF-releasing biodegradable implants and spacers would be a good choice for fracture treatment strategies in the future.
This study has some limitations. First, the total dosage of 600 ng PDGF was calculated according to previous studies, but the total and continuous PDGF-releasing capacity of the PHBV spacers is still unknown. However, this study shows that local PDGF-releasing biodegradable systems have positive effects on fracture healing, and further studies to determine the growth factor-releasing capacity of these systems and the PDGF concentration at the microenvironment of the fracture site are needed. The second limitation is the lack of statistical analysis of the histological results because of an inadequate number of histological samples. This study was designed as a pilot study, and the researchers had a limited number of rats. Therefore, no satisfactory histomorphometric analysis could be performed. The third limitation of this study pertains to the mechanical properties of the above-described implant/spacer systems.
There should be enough data about the mechanical properties of these implant/spacer systems to define and recommend them as a treatment technique. Further histomorphometric and histochemical studies are needed to define the therapeutic capabilities of the PHBV-based biodegradable growth factor-releasing implant/spacer systems as well as mechanical studies to prove their feasibility for treatment at the fracture site.
HIGHLIGHTS.
Platelet derived growth factors have qualitative and quantitative positive effects on fracture callus which supports the bone mechanically.
Biodegradable polymers can be successfully used in fracture healing surgery without any local foreign body reaction.
With an earlier maturation of fracture callus tissue local growth factor releasing systems made from biodegradable polymers may shorten the fracture healing process.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Local Committee on Animal Research Ethics of Kırıkkale University School of Medicine.
Author Contributions: Concept - M.Y.; Design - M.Y., M.T., M.Ç.; Supervision - M.T.; Materials - M.Y.; Data Collection and/or Processing - M.Y.; Analysis and/or Interpretation - M.Y., M.Ç.; Literature Search - M.Y.; Writing Manuscript - M.Y.; Critical Review - M.T., M.Ç.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Claes L, Ignatius A. Development of new, biodegradable implants. Chirurg. 2002;73:990–6. doi: 10.1007/s00104-002-0543-0. [DOI] [PubMed] [Google Scholar]
- 2.Elmowafy E, Abdal-Hay A, Skouras A, Tiboni M, Casettari L, Guarino V. Polyhydroxyalkanoate (PHA): applications in drug delivery and tissue engineering. Expert Rev Med Devices. 2019;16:467–82. doi: 10.1080/17434440.2019.1615439. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Zhang J, Li Y, Moran S, Khang G, Ge Z. Poly (l-lactide-co-caprolactone) scaffolds enhanced with poly (beta-hydroxybutyrate-co-beta-hydroxyvalerate) microspheres for cartilage regeneration. Biomed Mater. 2013;8 doi: 10.1088/1748-6041/8/2/025005. doi: 10.1088/1748-6041/8/2/025005. Epub 2013 Feb 5. [DOI] [PubMed] [Google Scholar]
- 4.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9 doi: 10.1186/1741-7015-9-66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichte P, Pape HC, Pufe T, Kobbe P, Fischer H. Scaffolds for bone healing: concepts, materials and evidence. Injury. 2011;42:569–73. doi: 10.1016/j.injury.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233:2937–48. doi: 10.1002/jcp.26042. [DOI] [PubMed] [Google Scholar]
- 7.Pacifici L, Casella F, Ripari M. The principles of tissue engineering: role of growth factors in the bone regeneration. Minerva Stomatol. 2002;51:351–9. [PubMed] [Google Scholar]
- 8.Li A, Xia X, Yeh J, et al. PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRalpha and derepressing BMP-Smad1/5/8 signaling. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113785. doi: 10.1371/journal.pone.0113785. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Zube L, Breitbart EA, O’Connor JP, et al. Recombinant human platelet-derived growth factor BB (rhPDGF-BB) and beta-tricalcium phosphate/collagen matrix enhance fracture healing in a diabetic rat model. J Orthop Res. 2009;27:1074–81. doi: 10.1002/jor.20842. [DOI] [PubMed] [Google Scholar]
- 10.Hengartner NE, Fiedler J, Ignatius A, Brenner RE. IL-1beta inhibits human osteoblast migration. Mol Med. 2013;19:36–42. doi: 10.2119/molmed.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollinger JO, Onikepe AO, MacKrell J, et al. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-BB and an injectable beta-tricalcium phosphate/collagen matrix. J Orthop Res. 2008;26:83–90. doi: 10.1002/jor.20453. [DOI] [PubMed] [Google Scholar]
- 12.Canalis E. Growth factor control of bone mass. J Cell Biochem. 2009;108:769–77. doi: 10.1002/jcb.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29:1795–803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 14.Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent. 2004;13:301–9. doi: 10.1097/01.id.0000148555.91063.06. [DOI] [PubMed] [Google Scholar]
- 15.Thoma DS, Jung RE, Hanseler P, Hammerle CHF, Cochran DL, Weber FE. Impact of recombinant platelet-derived growth factor BB on bone regeneration: a study in rabbits. Int J Periodontics Restorative Dent. 2012;32:195–202. [PubMed] [Google Scholar]
- 16.Andre J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiGiovanni CW, Lin SS, Baumhauer JF, et al. Recombinant human platelet-derived growth factor-BB and beta-tricalcium phosphate (rhPDGF-BB/beta-TCP): an alternative to autogenous bone graft. J Bone Joint Surg Am. 2013;95:1184–92. doi: 10.2106/JBJS.K.01422. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Kim HW. Rat defect models for bone grafts and tissue engineered bone constructs. Tissue Eng Regen Med. 2013;10:310–6. doi: 10.1007/s13770-013-1093-x. [DOI] [Google Scholar]
- 19.Bernabe PFE, Melo LGN, Cintra LTA, Gomes JE, Dezan E, Nagata MJH. Bone healing in critical-size defects treated with either bone graft, membrane, or a combination of both materials: a histological and histometric study in rat tibiae. Clin Oral Implants Res. 2012;23:384–8. doi: 10.1111/j.1600-0501.2011.02166.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg VM, Powell A, Shaffer JW, Zika J, Bos GD, Heiple KG. Bone grafting: role of histocompatibility in transplantation. J Orthop Res. 1985;3:389–404. doi: 10.1002/jor.1100030401. [DOI] [PubMed] [Google Scholar]
- 21.Huo MH, Troiano NW, Pelker RR, Gundberg CM, Friedlaender GE. The influence of ibuprofen on fracture repair: Biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J Orthop Res. 1991;9:383–90. doi: 10.1002/jor.1100090310. [DOI] [PubMed] [Google Scholar]
- 22.Kılıçoğlu SS. Mikroskobi düzeyinde kırık iyileşmesi. Ankara Üniversitesi Tıp Fakültesi Mecmuası. 2002;55:143–50. doi: 10.1501/Tipfak_0000000021. [DOI] [Google Scholar]
- 23.Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm. 2004;58:197–208. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Mitlak BH, Finkelman RD, Hill EL, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11:238–47. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 25.Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203–8. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 26.Terzioğlu A, Aslan G, Tuncalı D, Elagöz Ş, Hasırcı V, Gürsel İ. Transforming Growth Factor β-1 Incorporating Biodegradable Polyhydroxybutyrate-co-Hydroxyvalerate Rods: Effects of Controlled Delivery System on Bone Healing. Türk Plast Rekonstr Est Cer Derg. 2005;13:4. [Google Scholar]
- 27.Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. J Oral Maxillofac Surg. 2002;60:1176–81. doi: 10.1053/joms.2002.34994. [DOI] [PubMed] [Google Scholar]
- 28.Kumarasuriyar A, Jackson RA, Grondahl L, Trau M, Nurcombe V, Cool SM. Poly (beta-hydroxybutyrate-co-beta-hydroxyvalerate) supports in vitro osteogenesis. Tissue Eng. 2005;11:1281–95. doi: 10.1089/ten.2005.11.1281. [DOI] [PubMed] [Google Scholar]
- 29.Kumarasuriyar A, Grondahl L, Nurcombe V, Cool SM. Osteoblasts up-regupregulate the expression of extracellular proteases following attachment to Poly (beta-hydroxybutyrate-co-beta-hydroxyvalerate) Gene. 2009;428:53–8. doi: 10.1016/j.gene.2008.09.020. [DOI] [PubMed] [Google Scholar]


