Abstract
Objective
The aim of this study was to compare the effects of local administrations of platelet-rich plasma (PRP) with autologous conditioned serum (ACS) on Achilles tendon healing in a rat model.
Methods
In this study, 40 male Sprague-Dawley rats, aged 12 months and weighing 350 to 400 g were used. The rats were divided into three groups (n=10 in each group): a control group and two treatment groups (PRP vs ACS). A standardized procedure was applied for the complete rupture and repair of the Achilles tendon in each group. The PRP group received one dose of PRP on the operative area, and ACS group received ACS at 24, 48, and 72 hours after the surgery. The control group received no injection. Animals were sacrificed 30 days after the operation, and tendon healing in each group was assessed histopathologically based on Bonar’s semi-quantitative score and Movin’s semi-quantitative grading scale. For the biomechanical analyses, unoperated Achilles tendons of all rats in the control and ACS groups were also harvested, and pulling tests were applied to the specimen to measure the longitudinal axis strength. The highest force value among the data obtained was defined as the maximum strength value (Fmax).
Results
The mean Bonar’s score was significantly lower in the PRP group (3.8±0.8) than in the ACS (4.8±0.45) and control groups (5.2±0.837) (p=0.0028). The mean Movin’s score was significantly lower in the PRP group (7.80±1.49) than in the ACS (9.8±1) and control groups (11.2±2.4) (p=0.029). The ratio of type I collagen was significantly higher in the PRP group (60±6) than in the ACS (52±4.5) and control groups (42±9) (p=0.005). Biomechanical results obtained from operated sites were comparable in terms of Fmax among groups (PRP, 33.93±2.61; ACS, 35.24±3.26; control, 35.69±3.62) (p=0.674). Similarly, the results obtained from unoperated sites were comparable among groups (PRP, 47.71±1.21; ACS, 48.14±2; control, 49.14.69±1.88) (p=0.395).
Conclusion
In terms of histopathological results, PRP seems to be more effective than ACS for Achilles tendon healing in rats.
Keywords: Achilles tendon, Experimental model, Platelet-rich plasma, Autologous conditioned serum, Biological agents, Growth factors
Achilles tendon is the thickest and the strongest tendon of the human body; however, its rupture is the most common (1). Degeneration or insufficient perfusion of the tendon, usage of fluocinolone or steroids, and history of contralateral Achilles tendon rupture are the risk factors for primary acute Achilles tendon rupture (2). Its prevalence is 18/100,000 and is most common in males aged between 30 and 50 years and during recreational sports (3). Conservative and surgical treatment methods are available; recent studies usually recommend surgical procedures (4). Regardless of advancements and novel techniques of the treatments, recovery time is long and re-rupture rates are high (5). Considering the aforementioned problems, additive approaches like utilization of biological agents comprising growth factors are becoming more prevalent. Growth factors enhance the tendon healing with their crucial role in angiogenesis, cellular proliferation, extracellular matrix production, remodeling, and maturation (6, 7). Numerous experimental studies have shown that these approaches comprising growth factor utilization accelerate autologous recovery capacity (6, 8).
In the recent years, platelet-rich plasma (PRP) has frequently been used in the treatment of orthopedic injuries (8, 9). PRP is derived from blood via specific procedures. It comprises increased amounts of thrombocytes and growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin-like growth factor 1 and 2 (IGF-1, IGF-2), and hepatocyte growth factor (HGF) (7, 10). It has been shown that as a result of high concentration of growth factors in it, administration of PRP on the injury area enhances postoperative recovery and functionality (10).
Autologous conditioned serum (ACS) is a biological basis treatment agent developed by Wehling et al. during the mid-1990s. It is used locally in musculoskeletal diseases. Specific procedures conducted on whole blood yield macrophage and monocyte-based production of IL-1 receptor antagonist (IL-1Ra) and other anti-inflammatory cytokines (11). IL-1Ra is the natural inhibitor of IL-1, which is active during inflammation (11). ACS also comprises a high concentration of IGF-1, HGF, and FGF (12). ACS has been shown to be effective on diseases, the pathogenesis of which relies on inflammation (6, 13, 14).
We aimed to compare the histopathological and biomechanical outcomes of local administration of ACS and PRP on day 30 after Achilles tendon rupture surgery. To our knowledge, this is the first study comparing ACS and PRP. We hypothesize that both ACS and PRP would lead to better outcomes than the control group. It is not possible to anticipate the superiority of ACS or PRP according to the current literature.
Materials and Methods
Local ethics committee approval was obtained before the study on 11/30/2015 (2015–46). Forty 12-month-old adult male Sprague-Dawley rats with a mean body weight of 350–450 g, including 10 rats as PRP and ACS donors, were used in the study. The animals were kept five rats to a cage at a temperature of 22°C under a 12 hr:12 hr light-dark cycle. They were fed ad libitum with standard rat feed and had free access to water.
Thirty rats were divided into three groups with group 1 as the PRP group (n=10), group 2 as the ACS group (n=10), and group 3 as the control group (n=10). Prophylactic gentamycin (8 mg/kg) was administered to the rats 30 min before the surgical procedure. The surgery was initiated with administration of inhaled anesthesia, which started with 4% isoflurane (Forane) as an induction dose and continued with 2% as a maintenance dose. The posterior side of the right cruris was shaved, iodine was applied under aseptic surgical conditions, and the area was covered using a sterile green cover; a standard posterior longitudinal incision of 2 cm was applied, and the Achilles tendon was revealed. A complete transverse incision was performed using a no. 11 scalpel (Plusmed, İstanbul, Turkey) at 4–5 mm on the proximal side of the Achilles tendon-calcaneus junction. The end of the Achilles tendon was non-traumatically resutured using the modified Kessler method with PDO II 4/0 (BOZ, Ankara, Turkey). The incision site was sutured with four 3/0 propylene (Dogsan, İstanbul, Turkey) sutures placed at equal distances under sterile conditions, and dressing was applied with povidone iodine (Batticon R, Adeka, Istanbul, Turkey). No immobilization method was applied to the rats during the postoperative period.
Preparation of ACS
The five rats included in the donor group were decapitated after the collection of 5–6 cc of blood under anesthesia. The blood samples collected from the donor group were transferred into special Orthokine (Orthogen AG, Düsseldorf, Germany) injectors containing glass spheres, with a surface area of 21 mm2, under a temperature of 37°C. The samples were centrifuged using a centrifugation device (Megafuge, Kendro, Langenselbold, Germany) at a rate of 3,500 revolution/min (rpm) for 10 min. Following this, the concentrated serum was collected in 0.2-mL quantities and kept at −20°C. The samples were melted and brought up to room temperature before injection, and they were injected into the surgery area of the Achilles tendon at 170 uL using a 1-mL insulin injector at 24, 48, and 72 hours after the operation. This dose was calculated according to a previous study based on the rats’ body weight (6). Each injection was applied to sutures 2 and 3.
Preparation of PRP
On an average, 5 cc of intracardiac blood was taken from the five rats in the donor group under high dose anesthesia, and then they were decapitated. The blood was taken by injectors with 3.8% sodium citrate, diluted to 1/10. Using a Smith and Nephew Prosys PRS (PRP) biokit (Prodizen, Inc., Seoul, South Korea) and table-type VS-5000i2 centrifuge device, one cc of PRP was obtained after being centrifuged for 3 min at 3.000 rpm. The obtained PRP was left at +4°C for 2 hours, and when the platelets were created, they were put into 96-uL tubes with the help of a micropipette. Before administration, 16 uL of 10% CaCl2 was added to each tube, and the platelets were activated. Finally, with the help of an injector, they were applied to the repair region percutaneously between the suture 2 and 3 after the surgery area was closed. According to the previous study, PRP was applied as one step to the wound site (15). The control group did not receive any such injections. Injections of phosphate-buffered saline alone were not considered as this has previously been shown to not have any effect (16). Similarly, injection of unconditioned serum is of questionable value because platelet activation during its preparation cannot be avoided. Both the PRP and control groups were injected using injectors at 24, 48, and 72 hour to create the same trauma as the ACS group.
On day 30, all the rats of both groups were euthanized under anesthesia for histopathological (n=5) and biomechanical (n=5) testing. The right Achilles tendon and one part of the calcaneus were removed with the femur condyle. Care was taken to leave the plantaris tendon in place during the removal of the Achilles tendon to prevent the biomechanical measurements from being affected. For the biomechanical analyses, the left Achilles tendons of all rats in the control and ACS groups were also removed.
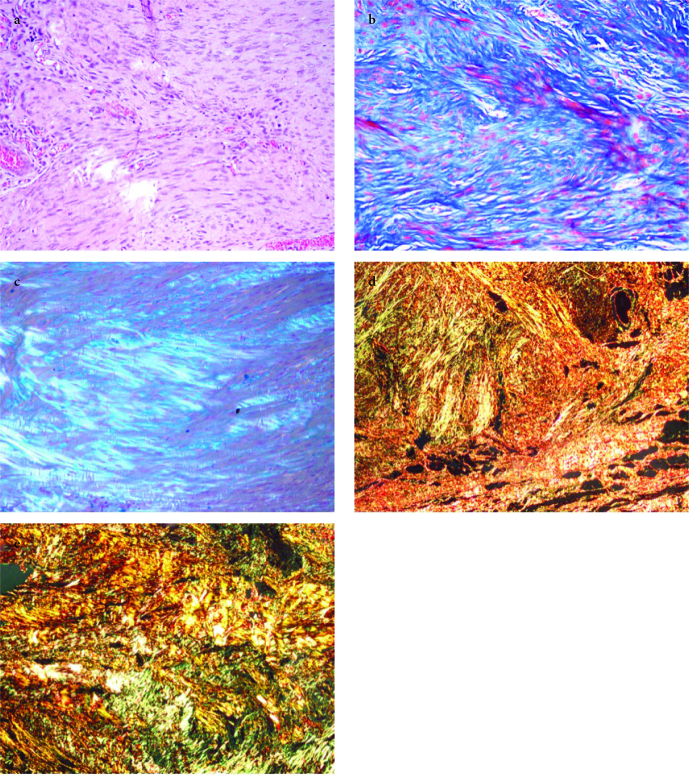
Samples were obtained from the tendon repair area for histopathological evaluation, which was made by a blinded evaluator. Tendon tissue samples were fixed using 10% neutral formaldehyde solution and kept in 5% formic acid. After the histopathological preparation processes, the materials were embedded in paraffin blocks and sliced. The sections were stained with hematoxylin and eosin (H&E) (Abcam, Cambridge, UK), Masson’s trichrome (BIOGNOST, Zagreb, Crotia), and Alcian blue (pH 2.5) (Sigma-Aldrich, St. Louis, MO, USA) Figure 1. a–c. The samples were examined using a BX51TF (Olympus, Tokyo, Japan) light microscope. Bonar’s semi-quantitative score and Movin’s semi-quantitative grading scale were used for evaluation.
Figure 1. a–e.
(a) The nuclei in tenocytes becomes more ovoid to round in shape without conspicuous cytoplasm; 1–2 clusters of capillaries seen per 10 high power fields. Separation of fibers with loss of demarcation of bundles and clear loss of normal polarization pattern. Control 5, H&E, ×200; (b) Separation of individual fibers with maintenance of demarcated bundles. PRP 4, Masson Trichrom, ×200; (c) Stainable mucin between fibers but bundles still discrete. ACS 4, Alcian blue, polarized light, ×200; (d) Type I/III collagen, PRP 1, Picrosirius Red, polarized light, ×100; (e) Type I/III collagen, ACS 2, Picrosirius Red, polarized light, ×100
Bonar’s scale includes the analysis of the following components: 1) tenocytes, 2) ground substance, 3) collagen, and 4) vascularity. Each variable was scored on a 4-point scale of 0–3 as follows: 0, normal; 1, slightly abnormal; 2, abnormal; and 3, markedly abnormal. The samples were scored according to whether there was a significant abnormal appearance. The total score varied between 0 (normal tendon) and 12 (severest abnormality) (17). Movin’s semi-quantitative scale includes analysis of eight variables as follows: 1) fiber structure, 2) fiber arrangement, 3) rounding of the nuclei, 4) regional variations in cellularity, 5) increased vascularity, 6) decreased collagen stainability, 7) hyalinization, and 8) glycosaminoglycan (GAG) content. The first seven variables were evaluated using the slides stained with H&E, whereas the eighth variable—the GAG content—was examined using the slides stained with Alcian blue (pH 2.5). Each variable was scored between 0 and 3 as follows: 0, normal; 1, slightly abnormal; 2, abnormal; and 3, markedly abnormal. The total semi-quantitative histological score varied between 0 (normal tendon) and 24 (severest abnormality) (18).
Evaluation of the type I/III collagen-staining pattern by Sirius Red (Chondrex, Redmond, WA, USA) was performed using an Olympus BX51 (Olympus, Tokyo, Japan) polarized light microscope under ×400 magnification. Type III collagen staining was evaluated using an Olympus BX51 light microscope under ×400 magnification (Figure 2. d, e).
For the biomechanical evaluations, which were made by a blinded evaluator, Achilles tendon samples were kept at −20°C and defrosted to room temperature on the study day; measurements were performed by connecting the Achilles tendons from the origin and insertion sites. The pulling test was applied to measure the longitudinal axis strength, and this was carried out using a Mecmesin Multitest 5-i mechanical test device (Mecmesin, Slinfold, West Sussex, United Kingdom) and software (Emperor, Mecmesin). Unnotched grasper jaws, which were specifically designed and manufactured for the study, were employed. The tests were performed with a 10 mm/min pulling rate for each sample. Loading values measured with a sensitivity of 0.1% by the device’s load cell were entered into the software (Emperor), and force values (F) were obtained. The tests were terminated after the detection of a decrease in the force and rupture of the sample. The highest force value among the data obtained was determined as the maximum strength value, Fmax.
Statistical analysis
The Kolmogorov-Smirnoff test was used to explore normal distribution. Group comparisons were provided by one-way ANOVA and Student’s t-test. The computer package SPSS Complex Samples Statistics version 21.0 (IBM SPSS Corp.; Armonk, NY, USA) was conducted to run statistical analyses.
Results
PRP group scores were significantly lower than those of the ACS and control groups for both Bonar’s and Movin’s scales (p<0.05). ACS group scores were similar to those of the control group for Bonar’s and Movin’s scales (p>0.05) (Table 1, 2).
Table 1.
Group comparisons according to Bonar’s and Movin’s Scale
| n | Mean | SD | p | |
|---|---|---|---|---|
| Bonar’s Scale | ||||
|
| ||||
| ACS | 5 | 4.80 | 0.447 | |
| PRP | 5 | 3.80 | 0.837 | *0.029 |
| Control | 5 | 5.20 | 0.837 | |
| Total | 15 | 4.60 | 0.910 | |
|
| ||||
| Movin’s Scale | ||||
|
| ||||
| ACS | 5 | 9.80 | 1.095 | |
| PRP | 5 | 7.80 | 1.483 | *0.028 |
| Control | 5 | 11.20 | 2.387 | |
| Total | 15 | 9.60 | 2.165 | |
ACS: autologous conditioned serum; PRP: platelet-rich plasma; SD: standard deviation
One-way ANOVA test
p<0.05
Table 2.
Group comparisons according to collagen type I ratio at Sirius Red staining
| n | Mean (%) | SD | p | |
|---|---|---|---|---|
| ACS | 5 | 52.00 | 4.472 | *0.005 |
| PRP | 5 | 60.00 | 6.124 | |
| Control | 5 | 42.00 | 9.083 | |
| Total | 15 | 51.33 | 9.904 |
ACS: autologous conditioned serum; PRP: platelet-rich plasma; SD: standard deviation
One-way ANOVA test
p<0.05
According to Sirius Red staining analyses, the PRP group had significantly higher type I collagen ratio than the control and ACS groups (p<0.05). Type I collagen ratio of the ACS group was not significantly different from the control group (p>0.05) (Table 3).
Table 3.
Group comparisons according to biomechanical results
| n | Mean | SD | p | |
|---|---|---|---|---|
| ACS-operated | 5 | 35.24 | 3.26 | 0.674 |
| PRP-operated | 5 | 33.93 | 2.61 | |
| Control-operated | 5 | 35.69 | 3.62 | |
| ACS-healthy | 5 | 48.14 | 2.00 | 0.395 |
| PRP-healthy | 5 | 47.61 | 1.21 | |
| Control-healthy | 5 | 49.14 | 1.88 |
ACS: autologous conditioned serum; PRP: platelet-rich plasma; SD: standard deviation
One-way ANOVA test
Biomechanical analyses did not reveal significant differences between operated groups (p>0.05). Group comparisons were conducted between healthy tendons, and there was no difference between Fmax values of either healthy side of each group (p>0.05) (Table 4).
Discussion
As a result of frequent and severe complications of treatments for Achilles tendon rupture, postoperative additive biological procedures are usually applied (5). In this study, we aimed to compare the efficacy of two procedures, which are utilized after Achilles tendon surgery: ACS and PRP. This is the first study comparing the efficacy of ACS and PRP treatments. In our study, it was demonstrated that PRP treatment is significantly more effective than ACS treatment and no treatment (control group) on the basis of the histopathological healing outcomes. Histopathological outcomes were analyzed via Bonar’s and Movin’s scales and Sirius staining type I/III collagen ratio; PRP outcomes were significantly better at all three analyses. ACS treatment histopathological outcomes were similar to those of the control group, and the difference was not statistically significant. PRP and ACS treatments’ biomechanical outcomes were similar on day 30.
PRP comprises concentrated autologous growth factors (7, 19). Thrombocyte concentration, leukocyte count, and erythrocyte presence in PRP may differ owing to its preparation method (20). Furthermore, dosage and frequency of treatment may also alter the efficacy of PRP (21). PRP treatment has been used in various areas as oral surgery, maxillofacial surgery, chronic skin and soft tissue ulcerations, and open fractures (22). PRP utilization in soft tissue injuries has been increasing, although the results vary across clinical and experimental studies (19, 23–25). There are a limited number of clinical studies; however, PRP utilization was shown to accelerate tendon and ligament healing in athletes (15).
There are numerous experimental and clinical studies evaluating PRP treatment after Achilles tendon rupture. Majority of the animal studies demonstrated the beneficial effect of PRP on tendon healing, although some studies found no significant impact (15, 26, 27). Thus, the effect of PRP treatment on tendon healing is yet undetermined. As a result of their clinical study, Sanchez et al. showed that PRP administration in addition to surgical repair of Achilles tendon accelerated healing and functional recovery (19). Aspenberg et al. found that percutaneous PRP treatment after Achilles tendon surgery increased callus amount and thickness (25). Lyras et al. investigated the effect of PRP on angiogenesis during the early phase of healing and found that it enhanced neovascularization, and they argued that this effect resulted in the enhancement of tendon healing and histological quality of scar tissue (22). Sadoghi et al. reviewed 14 experimental and clinical studies together for a meta-analysis in 2012 and investigated the effect of PRP on treatment efficacy of Achilles tendon rupture and tendinopathy (8). They found that PRP had a significant impact on the maturation of scar tissue after Achilles tendon rupture surgery but did not have a significant effect on the treatment of Achilles tendinopathy. Experimental studies of Murray et al. (28) and Majewski et al. (6) found no biomechanical effects of PRP. In our study, we demonstrated the beneficial effect of PRP on histopathological analysis on day 30 after Achilles tendon rupture surgery. Our biomechanical findings were not significantly different from the control group.
ACS comprises anti-inflammatory cytokines and growth factors; thus, it has been used for muscle injuries, lumbar back pain, sciatic pain, and particularly osteoarthrosis. Its beneficial effects have been shown in randomized controlled studies (29). Baltzer et al. established that ACS usage for knee osteoarthritis reduced symptoms; ACS had chondro-protective and chondro-regenerative effects in their clinical research with 376 cases (13). Darabos et al. (30) showed that local ACS usage for muscle injuries improved healing time according to clinical and magnetic resonance imaging findings. It has been shown that tunnel enlargement development after anterior cruciate ligament reconstruction was less with ACS usage.
There are various experimental studies investigating the effects of ACS usage after Achilles tendon surgery (6, 31–33). In their experimental study with 80 rats, Majewski et al. evaluated ACS usage after Achilles tendon surgery with histopathological, biomechanical, and immunohistochemical analyses and found that the use of ACS had a significant effect on collagen composition, histological appearance, and mechanical resistance during tendon regeneration (6). They observed the biomechanical effects earliest at the fourth week. In our study, although we did find ACS as histopathologically effective on day 30, we did not observe a biomechanically significant difference.
One of the valuable results of our study is that we have better histopathological results in the PRP group than the control group after Achilles tendon surgery. Although both ACS and PRP have more cytokine and growth factor density than whole blood, their inclusion rates are different. ACS comprises IL-1Ra and anti-inflammatory cytokines (IL-4, IL-10, IL-13), whereas PRP contains VEGF, IGF-1, IGF-2, FGF, and HGF (7, 11, 22, 31, 34). We regard VEGF as being the main factor behind the better results obtained from the PRP application. VEGF, which is a crucial factor in angiogenesis during the early phases of tendon healing, has a significant role in a low-perfusion region like Achilles tendon. Lyras et al. showed that PRP administration accelerated Achilles tendon healing via stimulating angiogenesis by its high concentration of VEGF (22). They showed an increase of angiogenesis in the first 2 weeks, including proliferative and inflammation phases of the tendon healing. These findings are supported by various PRP studies conducted on rabbit patellar tendon defect and sheep Achilles tendon models (33, 35).
Biomechanical outcomes of ACS and PRP administration after Achilles tendon surgery vary between studies. Kaux et al. (35) and Saughi et al. (8) claimed that PRP had a biomechanically significant effect, although Yuksel et al. (15) found no significant effect on biomechanical results. Majewski et al. showed that ACS had a significant effect on biomechanical findings in the eighth week. In our study, we observed no significant biomechanical effect of either PRP or ACS on day 30. We deduced that the reason for that result was that collagen cross-linkage and remodeling were not completed by day 30 despite accelerated tendon healing with the help of PRP and ACS.
The results of our study should be viewed in the context of its limitations. The first limitation is that owing to the experimental design, a minimum number of subjects sufficient for statistical validity were included in this study. However, a posthoc power analysis revealed a power of 75.3% and 77.4% for Bonar and Movin scores’ analyses, respectively. Another limitation of our study is that the histopathological evaluations, Bonar’s and Movin’s scales, are semi-quantitative assessments, which may be subjective, although the histopathological evaluations were conducted by a pathologist who was blinded to the groups to which the rats belonged. Lastly, the rupture was made iatrogenically and did not occur by degeneration. We aimed to obtain the effect of biological methods on healthy tendons and not on tendon rupture pathophysiology. Tendon healing is a complex process consisting of biomolecular and biomechanical stimulants and initiates, perpetuates, and terminates on a broad spectrum (36). We refrained iatrogenic tendinopathy so as to not make that process more complicated.
In conclusion, we observed that PRP treatment after Achilles tendon surgery had better histopathological results than both the ACS and control groups. We did not find a significant effect on biomechanical results obtained from either treatment by ACS or by PRP. We deduced that PRP administration was an effective treatment for Achilles tendon repair after surgery. Nevertheless, further clinical and experimental studies investigating the efficacy of PRP and ACS are needed. We conclude that biological methods such as PRP and ACS are promising treatments for tendon healing.
HIGHLIGHTS.
Application of PRP following Achilles tendon repair surgery is efficient in rat model.
Application of ACS is superior to control condition, however, ACS is not as efficient as PRP.
PRP is a promising adjuvant treatment option with its efficiency and easy technique after Achilles tendon repair surgery.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Bağcılar Training and Research Hospital Management and the Bağcılar Training and Research Hospital Experimental Research and Skill Development Center (BADABEM).
Author Contributions: Concept - E.G.; Design - E.G, S.Y.; Supervision - M.A.G.; Materials - E.G., S.Y., O.B.; Data Collection and/or Processing - E.G., A.Ç.; Analysis and/or Interpretation - E.G., S.Y., A.Ç.; Literature Search - E.G., S.Y., O.B.; Writing Manuscript - E.G., S.Y.; Critical Review - M.A.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Maquirriain J. Achilles tendon rupture: Avoiding tendon lengthening during surgical repair and rehabilitation. Yale J Biol Med. 2011;84:289–300. [PMC free article] [PubMed] [Google Scholar]
- 2.Seeger JD, West WA, Fife D, Noel GJ, Johnson LN, Walker AM. Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol Drug Saf. 2006;15:784–92. doi: 10.1002/pds.1214. [DOI] [PubMed] [Google Scholar]
- 3.Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports Med Arthrosc Rev. 2018;26:16–30. doi: 10.1097/JSA.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 4.Deng S, Sun Z, Zhang C, Chen G, Li J. Surgical treatment versus conservative management for acute achilles tendon rupture: A systematic review and meta-analysis of randomized controlled trials. J Foot Ankle Surg. 2017;56:1236–43. doi: 10.1053/j.jfas.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Majewski M, Widmer KH, Steinbruck K. Achilles tendon ruptures: 25 year’s experience in sport-orthopedic treatment. Sportverletz Sportschaden. 2002;16:167–73. doi: 10.1055/s-2002-37065. [DOI] [PubMed] [Google Scholar]
- 6.Majewski M, Ochsner PE, Liu F, Fluckiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37:2117–25. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- 7.Alsousou J, Thompson M, Harrison P, Willett K, Franklin S. Effect of platelet-rich plasma on healing tissues in acute ruptured Achilles tendon: A human immunohistochemistry study. Lancet. 2015;385(Suppl 1):S19. doi: 10.1016/S0140-6736(15)60334-8. [DOI] [PubMed] [Google Scholar]
- 8.Sadoghi P, Rosso C, Valderrabano V, Leithner A, Vavken P. The role of platelets in the treatment of Achilles tendon injuries. J Orthop Res. 2013;31:111–8. doi: 10.1002/jor.22199. [DOI] [PubMed] [Google Scholar]
- 9.Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966–71. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 10.Zou J, Mo X, Shi Z, et al. A prospective study of platelet-rich plasma as biological augmentation for acute achilles tendon rupture repair. Biomed Res Int. 2016;2016 doi: 10.1155/2016/9364170. doi: 10.1155/2016/9364170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans CH, Chevalier X, Wehling P. Autologous conditioned serum. Phys Med Rehabil Clin N Am. 2016;27:893–908. doi: 10.1016/j.pmr.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Rutgers M, Saris DB, Dhert WJ, Creemers LB. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res Ther. 2010;12:R114. doi: 10.1186/ar3050. doi: 10.1186/ar3050. Epub 2010 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baltzer AWA, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:152–60. doi: 10.1016/j.joca.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Becker C, Heidersdorf S, Drewlo S, de Rodriguez SZ, Kramer J, Willburger RE. Efficacy of epidural perineural injections with autologous conditioned serum for lumbar radicular compression: An investigator-initiated, prospective, double-blind, reference-controlled study. Spine (Phila Pa 1976) 2007;32:1803–8. doi: 10.1097/BRS.0b013e3181076514. [DOI] [PubMed] [Google Scholar]
- 15.Yuksel S, Adanir O, Gultekin MZ, et al. Effect of platelet-rich plasma for treatment of Achilles tendons in free-moving rats after surgical incision and treatment. Acta Orthop Traumatol Turc. 2015;49:544–51. doi: 10.3944/AOTT.2015.15.0028. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwagi K, Mochizuki Y, Yasunaga Y, Ishida O, Deie M, Ochi M. Effects of transforming growth factor-beta 1 on the early stages of healing of the Achilles tendon in a rat model. Scand J Plast Reconstr Surg Hand Surg. 2004;38:193–7. doi: 10.1080/02844310410029110. [DOI] [PubMed] [Google Scholar]
- 17.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–8. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand. 1997;68:170–5. doi: 10.3109/17453679709004002. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–51. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 20.Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344–52. doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 21.Circi E, Akman YE, Sukur E, Bozkurt ER, Tuzuner T, Ozturkmen Y. Impact of platelet-rich plasma injection timing on healing of Achilles tendon injury in a rat model. Acta Orthop Traumatol Turc. 2016;50:366–72. doi: 10.3944/AOTT.2015.15.0271. [DOI] [PubMed] [Google Scholar]
- 22.Lyras DN, Kazakos K, Verettas D, et al. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30:1101–6. doi: 10.3113/FAI.2009.1101. [DOI] [PubMed] [Google Scholar]
- 23.Orrego M, Larrain C, Rosales J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008;24:1373–80. doi: 10.1016/j.arthro.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Sarikaya B, Yumusak N, Yigin A, Sipahioglu S, Yavuz U, Altay MA. Comparison of the effects of human recombinant epidermal growth factor and platelet-rich plasma on healing of rabbit patellar tendon. Eklem Hastalik Cerrahisi. 2017;28:92–9. doi: 10.5606/ehc.2017.55396. [DOI] [PubMed] [Google Scholar]
- 25.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75:93–9. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 26.Parafioriti A, Armiraglio E, Del Bianco S, Tibalt E, Oliva F, Berardi AC. Single injection of platelet-rich plasma in a rat Achilles tendon tear model. Muscles Ligaments Tendons J. 2011;1:41–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Fukawa T, Yamaguchi S, Watanabe A, et al. Quantitative assessment of tendon healing by using MR T2 mapping in a rabbit Achilles tendon transection model treated with platelet-rich plasma. Radiology. 2015;276:748–55. doi: 10.1148/radiol.2015141544. [DOI] [PubMed] [Google Scholar]
- 28.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27:639–45. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wehling P, Moser C, Frisbie D, et al. Autologous conditioned serum in the treatment of orthopedic diseases: The orthokine therapy. BioDrugs. 2007;21:323–32. doi: 10.2165/00063030-200721050-00004. [DOI] [PubMed] [Google Scholar]
- 30.Darabos N, Haspl M, Moser C, Darabos A, Bartolek D, Groenemeyer D. Intraarticular application of autologous conditioned serum (ACS) reduces bone tunnel widening after ACL reconstructive surgery in a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2011;19(Suppl 1):S36–46. doi: 10.1007/s00167-011-1458-4. [DOI] [PubMed] [Google Scholar]
- 31.Heisterbach PE, Todorov A, Fluckiger R, Evans CH, Majewski M. Effect of BMP-12, TGF-beta1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. Knee Surg Sports Traumatol Arthrosc. 2012;20:1907–14. doi: 10.1007/s00167-011-1772-x. [DOI] [PubMed] [Google Scholar]
- 32.Genc E, Beytemur O, Yuksel S, et al. Investigation of the biomechanical and histopathological effects of autologous conditioned serum on healing of achilles tendon. Acta Orthop Traumatol Turc. 2018;52:226–31. doi: 10.1016/j.aott.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anitua E, Sanchez M, Nurden AT, et al. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A. 2006;77:285–93. doi: 10.1002/jbm.a.30585. [DOI] [PubMed] [Google Scholar]
- 34.Baria M, Vasileff WK, Miller M, et al. Cellular components and growth factor content of platelet-rich plasma with a customizable commercial system. Am J Sports Med. 2019;47:1216–22. doi: 10.1177/0363546519827947. [DOI] [PubMed] [Google Scholar]
- 35.Kaux JF, Drion PV, Colige A, et al. Effects of platelet-rich plasma (PRP) on the healing of Achilles tendons of rats. Wound Repair Regen. 2012;20:748–56. doi: 10.1111/j.1524-475X.2012.00826.x. [DOI] [PubMed] [Google Scholar]
- 36.Jung HJ, Fisher MB, Woo SL. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med Arthrosc Rehabil Ther Technol. 2009;1 doi: 10.1186/1758-2555-1-9. doi: 10.1186/1758-2555-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]