Abstract
Olmesartan-induced spruelike enteropathy is a rare clinical entity that is characterized by unexplained chronic diarrhea and weight loss. Prompt recognition of this adverse event may be challenging due to clinical and histologic similarities with other small intestinal pathologies. We hereby delineate the case of an elderly female with a 14-month clinical history of non-bloody diarrhea and weight loss. After extensive diagnostic workup and exclusion of probable etiologies, the patient was diagnosed with olmesartan-associated enteropathy. A dramatic clinical and histologic recuperation was achieved after discontinuation of olmesartan. This paper illustrates the overarching need for a detailed clinical history focusing on medication review in patients presenting with chronic diarrhea with no obvious cause. The spruelike enteropathy associated with olmesartan is an emerging cause of small bowel injury. Clinicians should maintain a high index of suspicion for this adverse drug reaction. Early and correct diagnosis carries paramount importance in sparing these patients from unnecessary diagnostic investigations and therapeutic delays.
Keywords: olmesartan, diarrhea, spruelike enteropathy, adverse drug reaction, malabsorption
Introduction
Rubio-Tapia et al. first described olmesartan-associated enteropathy in 2012 [1]. Since then, several case reports and small case series have been documented. While villous atrophy has also been reported with the use of other angiotensin II receptor blockers, olmesartan has been reported to have the highest incidence of such complications. Prior reports of similar side effects with telmisartan, irbesartan, valsartan, losartan, and eprosartan are also available [2]. This entity shares predominant symptoms and histologic characteristics with celiac disease. However, negative celiac serology, coupled with no response to a gluten-free diet, clearly denotes the distinct nature of this adverse drug reaction [1]. Even though the Food and Drug Administration (FDA) issued a warning for the risk of enteropathy, olmesartan remains a popular antihypertensive drug. It demonstrates a promising response in lowering blood pressure and bears a favorable drug-drug interaction profile [3]. In these patients, the symptoms of chronic diarrhea and weight loss are particularly disabling. These presentation patterns frequently prompt clinicians to conduct extensive diagnostic workup [4]. Therefore, this enteropathy not only impacts the quality of life in such patients but also leads to exhaustion of valuable hospital resources. Interestingly, the treatment of this abnormality is relatively simple, mainly consisting of discontinuation of olmesartan therapy [4,5]. The present paper adds to the existing clinical evidence pertaining to this severe adverse reaction of olmesartan and serves the purpose of community awareness for early etiology establishment in affected patients. This case study has previously been presented as an abstract (Abstract: Malik A, Alsabbak H, Samreen A, Siddique K, Ashraf H, Zakharia K, Azrak M. Drug-Induced Flu-Like Enteropathy. Annual Scientific Meeting, American College of Gastroenterology; October 05-10, 2018, Philadelphia, Pennsylvania).
Case presentation
A 75-year-old Caucasian female presented to our medical center with frequent nocturnal, non-bloody, loose stools for the past 14 months. The diarrhea was also associated with nausea, vomiting, and weight loss (100 lbs). Conservative management resulted in only temporary relief. Esophagogastroduodenoscopy (EGD) was performed 13 months ago, which revealed hiatal hernia with chronic active gastritis. Her colonoscopy was normal. A trial of a gluten-free diet failed to resolve her symptoms. In addition, the patient’s medical history was significant for hypertension, hyperlipidemia, osteoarthritis, chronic obstructive pulmonary disease, hypothyroidism, and diabetes mellitus. At presentation, she had been adhering to antihypertensive therapy with olmesartan medoxomil (Benicar; Daiichi Sankyo, Inc., Basking Ridge, New Jersey) 40 mg/day over the past several years. Her other home medications included albuterol, budesonide, fluticasone, levothyroxine, insulin glargine, potassium fiber capsules, and vitamin B12. On admission, her blood pressure was 80/49 mm Hg, and heart rate was 100 beats per minute. Abdominal examination was unremarkable for abnormalities.
Investigations
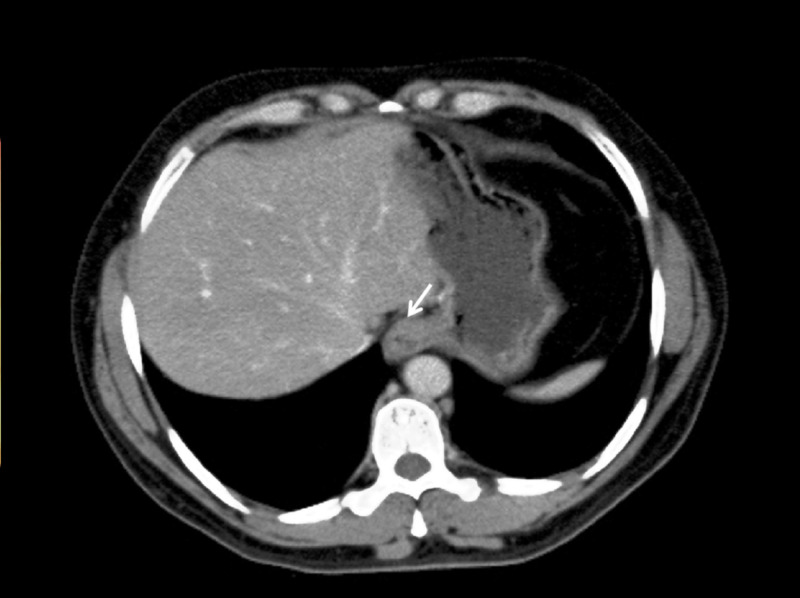
Laboratory evaluation revealed mild anemia, hyperchloremia (120 mEq/L), hypoalbuminemia (2.6 g/dL), and low serum bicarbonate levels (16 mEq/L). The findings of initial testing showed that the patient was in metabolic acidosis. Vitamin B12 186 ng/mL (seven months ago), vasoactive intestinal polypeptide <13 pmol/L, and gastrin was <25 pg/mL. Her serum creatinine level was mildly elevated to 1.2 mg/dL (baseline: 1.1 mg/dL). Erythrocyte sedimentation rate (ESR), serum lipase and amylase, and liver function tests were within normal limits. Stool leukocyte and fecal occult blood tests were positive. Stool cultures for ova, parasites, and Clostridium difficile toxins were negative. Fecal fat testing, Cryptosporidium and Giardia antigens, celiac panel, and human immunodeficiency virus testing came out negative. Contrast-enhanced computed tomography showed herniation of the part of the stomach through the esophageal hiatus into the thoracic cavity, consistent with hiatal hernia (Figure 1).
Figure 1. Contrast-enhanced computed tomography axial section (soft tissue window) at the level of diaphragm showing herniation of the part of the stomach through the esophageal hiatus into the thoracic cavity (arrow).
Computed tomography scan further divulged enlarged mesenteric lymph nodes, measuring 9-12 mm in the short axis (Figure 2).
Figure 2. Computed tomography post-intravenous contrast axial section (soft tissue window) at the level of aortic bifurcation showing enlarged mesenteric lymph nodes (arrows).
EGD showed scalloped duodenal folds and a mosaic mucosal pattern, consistent with cracked-mud appearance (Figure 3).
Figure 3. Upper endoscopy showing scalloped duodenal folds (black arrow) and mosaic mucosal pattern, consistent with cracked-mud appearance (blue arrow).
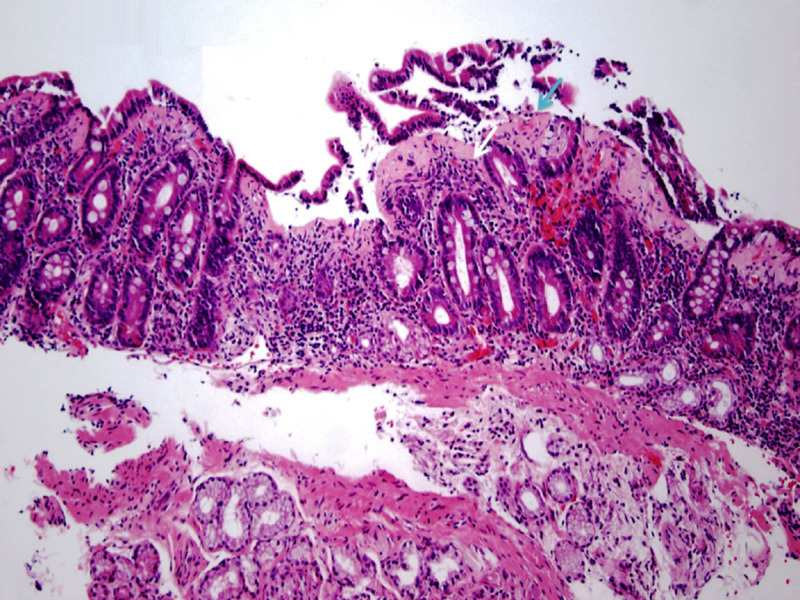
Minimal endoscopic changes were also noted in the colon and terminal ileum. Pathologic examination of the biopsy specimens from the duodenum showed chronic partial villous atrophy with increased intraepithelial lymphocytes (Figure 4).
Figure 4. Pathologic examination of the duodenal biopsy specimen showing partial villous atrophy (blue arrow) with associated increased intraepithelial lymphocytes (white arrow). (Hematoxylin and eosin staining; 100x).
A thickened subepithelial collagen table was another prominent histologic finding (Figure 5).
Figure 5. Duodenal biopsy showing a thickened subepithelial collagen band (arrows). (Hematoxylin and eosin staining; 100x).
Increased gastric submucosal lymphocytes and collagen deposits were also noted. Furthermore, chronic colitis with focal collagenous deposits was evident but random colonic biopsies ruled out microscopic colitis.
Differential diagnosis
The differentials included drug-induced and autoimmune enteropathies, hypogammaglobulinemia-associated sprue, tropical sprue, small intestinal bacterial overgrowth, Giardiasis, microscopic colitis, intestinal lymphoma, Clostridium difficile colitis, Whipple's disease, collagenous sprue or unclassified sprue. On the basis of workup findings and exclusion of probable etiologies, she was eventually diagnosed with olmesartan-induced spruelike enteropathy.
Treatment
The patient was educated about her disease and was directed to immediately discontinue olmesartan. She was managed conservatively for gastrointestinal complaints and was prescribed alternate medication for hypertension.
Outcome and follow-up
After six weeks of discontinuing olmesartan, she showed clinical remission of her symptoms and signs. She began thriving, as reflected by her weight gain (5 lbs). The complete clinical resolution of her gastrointestinal symptoms was achieved in three months. Repeat EGD performed after six months showed macroscopically normal duodenal folds and mucosa (Figure 6).
Figure 6. Repeat upper endoscopy performed after six months showing normal duodenal folds (black arrow) and macroscopically normal mucosa (blue arrow).
Pathologic examination of the biopsy specimens also showed histological recovery (Figure 7).
Figure 7. Repeat duodenal biopsy showing normal duodenal villous architecture (arrows). (Hematoxylin and eosin staining; 100x).
The patient has been symptom-free and continues to do well to date.
Discussion
Olmesartan is a widely used angiotensin II receptor blocker for hypertension. According to one estimate, around 45.3 million prescriptions were dispensed in the United States through the year 2011 [6]. The overall tolerability profile of this drug has been good, with only a few minor side effects such as dizziness, flu-like symptoms, or headache [7]. However, clinicians at Mayo Clinic identified a novel entity of severe spruelike enteropathy secondary to the use of olmesartan in 2012 [1]. Several case reports and small case series of this adverse drug reaction have been described thus far. Published medical literature demonstrates that this reaction shows no clear gender predominance. It mostly involves patients in their seventh and eighth decades of life [8]. The clinical presentation of olmesartan-related spruelike enteropathy is routinely characterized by chronic non-bloody diarrhea, vomiting, crampy abdominal pain, and weight loss [9]. Occasionally, the aforementioned symptoms may lead to dehydration, electrolyte imbalance, or acute kidney injury [10]. While it remains extremely rare, gastrointestinal perforation can also occur in such patients [11].
The exact pathogenesis of this adverse drug reaction is unknown. However, severe intestinal inflammation and mucosal damage following the chronic use of olmesartan implicate cell-mediated immune response [12]. In a majority of patients with severe disease, the presence of either HLA-DQ2 or DQ8 haplotypes has been identified. This observation further endorses the altered immune response as the likely causal mechanism [12]. Furthermore, increased levels of transforming growth factor-beta (TGF-β) have been considered as causative factors for intestinal epithelial injury. The elevated levels of TGF-β have been thought to be triggered by the increased levels of angiotensin II in such patients [12]. The delay between the initiation of olmesartan treatment and disease activity also supports immune-mediated pathophysiology. The length of the time period between olmesartan exposure and symptom-onset is variable, ranging from a few months to several years. Based on existing clinical data, the mean duration of this time interval has been 3.1 years [9].
Laboratory evaluation predominantly reveals a severe malabsorption process. Anemia, hypoalbuminemia, electrolyte imbalance, and vitamin deficiencies develop after long-term use of olmesartan. Given the overlapping clinical and histological patterns with celiac disease, negative celiac serology is imperative in the detection of olmesartan-associated disease [13]. The endoscopic appearance of this lesion is mostly non-specific. Nodular mucosal changes in the duodenum with partial or complete villous atrophy and ulcerations can be found [13]. It is notable that concurrent involvement of the entire gastrointestinal tract has also been observed. Endoscopic biopsy is an important diagnostic investigation. Pathologic examination of biopsy specimens commonly shows total or partial duodenal villous atrophy, mucosal granulocytic infiltration, and a thickened subepithelial collagen layer [9,12,14]. Conversely, a remarkable histologic variation may exist in these patients. In that context, endoscopic biopsy findings alone cannot be considered as reliable diagnostic markers due to the lack of statistically significant data [15]. Olmesartan cessation results in prompt clinical improvement in most patients. Therefore, an olmesartan-free interval can also help in the identification of this etiology in difficult-to-diagnose cases. It is notable that the definitive diagnosis can only be made after the resolution of clinical and histological findings following olmesartan cessation.
With regard to treatment, immediate olmesartan discontinuation is imperative. The median time to resolution of individual symptoms varies from case to case. It may require a few weeks to several months in order to achieve symptomatic relief [16]. The small bowel mucosal changes mostly disappear in three to six months of the drug withdrawal. Therefore, a follow-up endoscopic biopsy is warranted to gauge the mucosal improvement. Notably, some patients may only show a partial response to olmesartan cessation alone [17]. In such cases, additional treatment with budesonide may be employed. The monitoring of the progression of severe and recurrent olmesartan-induced spruelike enteropathy is important after the cessation of the implicated drug [17].
The patient involved in this study demonstrated clinical symptoms related to celiac disease. However, no response to a gluten-free diet and negative serology provided a vital diagnostic clue that precluded a diagnosis of celiac disease. The endoscopic biopsy was remarkable for partial villous atrophy, suggesting a possible drug-induced injury. After a careful review of the medications, olmesartan was considered the culprit drug. It was immediately discontinued and her symptoms showed improvement. Chronic diarrhea and weight loss were particularly disabling in this patient. Therefore, if the diagnosis had been made early in the course of the disease, she could have been spared from these significant problems. Furthermore, extensive diagnostic workup was performed, which turned out to be negative for all probable etiologies. It not only delayed the diagnosis but also exhausted valuable hospital resources. Thus, physicians should remain vigilant and include olmesartan-induced spruelike enteropathy in the differentials of chronic diarrhea to avoid such predicaments.
Conclusions
This article highlights the pathologic association between olmesartan and spruelike enteropathy. This adverse drug reaction may closely mimic several intestinal disorders warranting extreme clinical vigilance in such patients. Although it is a relatively rare clinical entity, physicians should be aware of this adverse event to avoid extensive workup and treatment delays. Prospective studies evaluating the serological and histological profiles of olmesartan-induced spruelike enteropathy are required to further elucidate the associated diagnostic and therapeutic conundrums.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Severe spruelike enteropathy associated with olmesartan. Rubio-Tapia A, Herman ML, Ludvigsson JF, Kelly DG, Mangan TF, Wu TT, Murray JA. Mayo Clin Proc. 2012;87:732–738. doi: 10.1016/j.mayocp.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angiotensin II receptor blockers and gastrointestinal adverse events of resembling sprue-like enteropathy: a systematic review. Kamal A, Fain C, Park A, Wang P, Gonzalez-Velez E, Leffler DA, Hutfless SM. Gastroenterol Rep (Oxf) 2019;7:162–167. doi: 10.1093/gastro/goz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA Drug Safety Communication: FDA approves label changes to include intestinal problems (sprue-like enteropathy) linked to blood pressure medicine olmesartan medoxomil. [Feb;2020 ];https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-include-intestinal-problems-sprue 2013
- 4.Angiotensin-II inhibitor (olmesartan)-induced collagenous sprue with resolution following discontinuation of drug. Nielsen JA, Steephen A, Lewin M. World J Gastroenterol. 2013;19:6928–6930. doi: 10.3748/wjg.v19.i40.6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drug-induced Sprue-like intestinal disease. Freeman HJ. Int J Celiac Dis. 2014;2:49–53. [Google Scholar]
- 6.Use of olmesartan and enteropathy outcomes: a multi-database study. Dong YH, Jin Y, Tsacogianis TN, He M, Hsieh PH, Gagne JJ. Aliment Pharmacol Ther. 2018;47:792–800. doi: 10.1111/apt.14518. [DOI] [PubMed] [Google Scholar]
- 7.Olmesartan medoxomil: a review of its use in the management of hypertension. Scott LJ, McCormack PL. Drugs. 2008;68:1239–1272. doi: 10.2165/00003495-200868090-00005. [DOI] [PubMed] [Google Scholar]
- 8.Systematic review: sprue-like enteropathy associated with olmesartan. Ianiro G, Bibbò S, Montalto M, Ricci R, Gasbarrini A, Cammarota G. Aliment Pharmacol Ther. 2014;40:16–23. doi: 10.1111/apt.12780. [DOI] [PubMed] [Google Scholar]
- 9.Olmesartan-associated enteropathy: a review of clinical and histologic findings. Choi EY, McKenna BJ. Arch Pathol Lab Med. 2015;139:1242–1247. doi: 10.5858/arpa.2015-0204-RA. [DOI] [PubMed] [Google Scholar]
- 10.Five cases of sprue-like enteropathy in patients treated by olmesartan. Théophile H, David XR, Miremont-Salamé G, Haramburu F. Dig Liver Dis. 2014;46:465–469. doi: 10.1016/j.dld.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Olmesartan associated sprue-like enteropathy and colon perforation. Abdelghany M, Gonzalez L, Slater J, Begley C. Case Rep Gastrointest Med. 2014;2014:3. doi: 10.1155/2014/494098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olmesartan-associated sprue-like enteropathy: a systematic review with emphasis on histopathology. Burbure N, Lebwohl B, Arguelles-Grande C, Green PH, Bhagat G, Lagana S. Hum Pathol. 2016;50:127–134. doi: 10.1016/j.humpath.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Olmesartan-associated enteropathy: results of a national survey. Marthey L, Cadiot G, Seksik P, et al. Aliment Pharmacol Ther. 2014;40:1103–1109. doi: 10.1111/apt.12937. [DOI] [PubMed] [Google Scholar]
- 14.Olmesartan-induced enteropathy: an unusual cause of villous atrophy. Eusébio M, Caldeira P, Antunes AG, Ramos A, Velasco F, Cadillá J, Guerreiro H. GE Port J Gastroenterol. 2016;23:91–95. doi: 10.1016/j.jpge.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olmesartan associated enteropathy: a rare underdiagnosed cause of diarrhea and weight loss. Gonakoti S, Khullar S, Rajkumar A. Am J Case Rep. 2019;20:111–116. doi: 10.12659/AJCR.913207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olmesartan-associated enteropathy: an unexpected cause of chronic diarrhoea. Teixeira M, Macedo S, Martins A, Batista T, Novais A, Costa M, Cancela E. Eur J Case Rep Intern Med. 2019;6:1093. doi: 10.12890/2019_001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprue-like enteropathy associated with olmesartan: a new kid on the enteropathy block. Hujoel IA, Rubio-Tapia A. GE Port J Gastroenterol. 2016;23:61–65. doi: 10.1016/j.jpge.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]