Summary
Two major legislative actions since 2015, the 21st Century Cures Act of 2016 and the U.S. Food and Drug Administration (FDA) Reauthorization Act of 2017, contain significant provisions that potentially streamline drug development times, and by extension, may reduce costs. Evidence suggests, however, that development times have already been significantly affected by previous legislation and FDA programs, through accelerated approval pathways and adoption of more flexible definitions of clinical evidence of efficacy. The COVID-19 pandemic is pushing researchers and commercial entities to further test the limits of drug and vaccine development times and approvals, at an as yet unknown level of risk to patients. COVID-19 drug and vaccine trials are even now making use of accelerated drug approval programs, blended trials, and adaptive trial design to accelerate approval of therapeutics in the pandemic.
Key Words: COVID-19, drug approval, drug legislation, emergency use, expanded access, pandemic, vaccine approval
Abbreviations and Acronyms: AA, Accelerated Approval; BT, Breakthrough Therapy; DAB, drugs and biologics; EUA, Emergency Use Application; FDA, U.S. Food and Drug Administration; FDARA, Food and Drug Administration Reauthorization Act; IND, Investigational New Drug; NDA, New Drug Application; PDUFA, Prescription Drug User Fee Act; RMAT, Regenerative Medicine Advanced Therapy
After the Federal Food, Drug, and Cosmetics Act of 1938, all drugs marketed in the United States have been required to pass safety approval by the U.S. Food and Drug Administration (FDA) (1). The 1962 Kefauver-Harris Amendments to the act further required that drugs have proven efficacy for their intended use (2). However, the complex regulatory environment for approving safety and efficacy of new drugs and biologics (DABs) has been blamed for delays in both DAB development and deployment of critical therapies to patients in need, a progressive decline in annual drug approvals, and burgeoning costs of DAB development. Basic processes of FDA approval of new DABs were covered in a previous review in 2016 (3). Those are briefly summarized here, followed by a review of the changes in the drug development legislation in the last 5 years and their effects on the timeline of new DAB development, a followed by brief summary of some strategies being employed for DAB approval in the setting of the COVID-19 pandemic.
Pathways to Drug Approval
Following preclinical development, including in vitro and in vivo (animal) studies, and before proceeding to testing in any human subjects, the FDA must be involved in all drug development in the United States. There are 3 common pathways that developers can pursue (3).
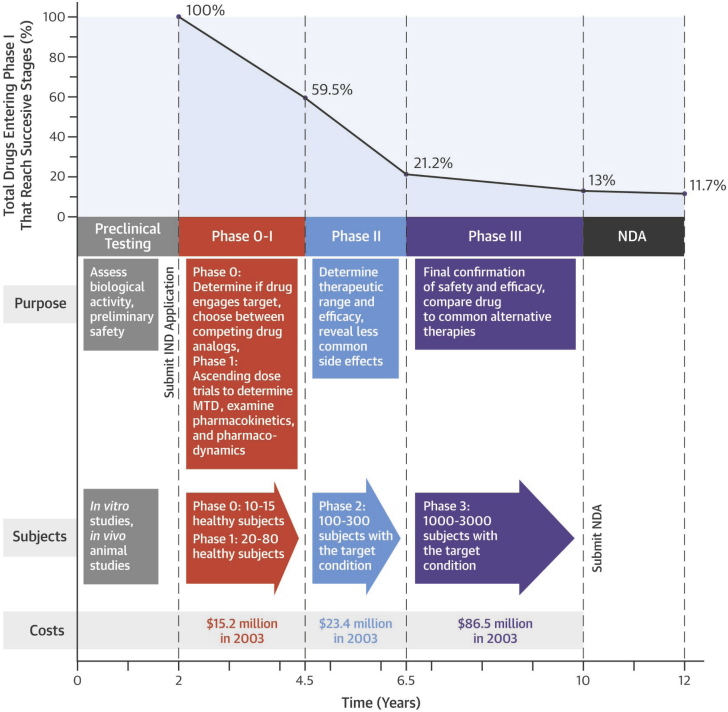
In a standard pathway for common drugs, this starts when the researcher or sponsor (usually a commercial entity) files an Investigational New Drug (IND) application—either an Investigator IND or Commercial IND—that, once approved, will allow the investigational drug to be transported from the manufacturer to and among interstate research entities for clinical studies. The application includes information about the drug, the researcher’s qualifications, the manufacturing process, the clinical study protocols, and commitments to obtain informed consent and institutional review board approval. If after 30 days from filing there is no objection from the FDA, clinical studies can commence in what is generally divided into 3 phases of testing, progressing from small studies in healthy volunteers to large studies in targeted patient populations. Timelines, characteristics, and success rates of the standard clinical phases are summarized in Figure 1.
Figure 1.
Approximate Timelines, Characteristics, and Success Rates of the Standard Clinical Phases of Human Trials for Common Drugs
See Van Norman (3). MTD = maximum tolerated dose; NDA = New Drug Application.
Before progressing from either Phase I or II, the investigator must pause and provide information on the safety of the new drug, any new information discovered in each of those phases, and any changes in manufacturing or drug preparation. If there is no objection from the FDA at each of these stages, the drug can proceed to the next clinical phase. Upon completion of successful Phase III studies, a process that takes around 8 to 10 years for most common drugs, the investigator or sponsor pays an application fee and files a New Drug Application (NDA) that includes extensive information on the drug, the results of all phases of testing, manufacturing and facilities, quality control, labeling, and risk evaluation and mitigation. For an NDA, the FDA requires “substantial evidence” of a drug’s safety and efficacy, a requirement that until recently was interpreted to mean at least 2 adequate and well-controlled Phase III trials with convincing demonstration of efficacy (4). The FDA has 60 days to file the application, unless it raises questions, and after the filing, it must review the application within 180 days. Once the FDA review has occurred, if there are no objections, the manufacturer can make and market the drug for its approved clinical use.
A second pathway to drug approval is available when an emergency situation does not permit sufficient time for a standard IND process or institutional review board approval. These generally involve individual patients who have a serious or life-threatening illness for which delay of clinical treatment would be devastating, or for which no treatment protocols exist. The treating physician can apply for an Emergency IND by directly contacting the FDA, which if immediate therapy is needed, can approve such applications over the phone. For less emergent conditions, a 30-day FDA review period applies (5). The investigator can proceed with treatment, but must still complete a full IND in a timely fashion.
In a third pathway, a Treatment IND can be sought for approval for use of an experimental drug that is showing promise in clinical studies, but has not completed them. These are also termed “Expanded Use INDs” (3). These can be issued for a drug that is already under investigation or has completed clinical studies and is awaiting an NDA approval that the sponsor is actively pursuing. The drug must be intended to treat a serious or life-threatening condition for which there is no satisfactory alternative treatment (6). Treatment INDs are often used to bridge the period of time between Phase III efficacy demonstration and NDA approval for those patients who are trial participants and who have benefitted from the drug, but they can also be used to allow treatment of nonstudy patients and populations.
Two other pathways for approval exist to be used only under very extraordinary circumstances. In some situations, such as a nuclear accident or terrorism attack involving a biological or radiation weapon, DABs can be approved and deployed in the absence of human trials altogether—primarily because to carry out human trials that, required, for example, exposure to lethal doses of radiation, would be unethical (7). Additionally, in the face of an officially declared public health emergency, the FDA can provide Emergency Use Authorizations (EUAs) (8) that permit public release of the DAB after Phase II efficacy has been shown. Phase III human clinical studies are deferred, and once the emergency has abated, confirmatory studies must be carried out after market approval (they are then usually termed Phase 4 studies). This latter “pathway” is of particular interest in the COVID-19 pandemic.
Recent Legislation Affecting FDA Approvals
Between 2015 and the present, 2 major legislative initiatives address long DAB development times: the 21st Century Cures Act (9), and the FDA Reauthorization Act (FDARA) of 2017 (10), part of which contains the sixth revision of the Prescription Drug User Fee Act (PDUFA VI). Most of the provisions in these laws do not change the basic steps in drug development reviewed previously, but concentrate on FDA funding and staffing to facilitate FDA review; establish programs in which the FDA works more closely with the DAB development entity to reduce reiterative reviews and delays; and encourage reinterpretation of existing FDA rules to allow more innovative clinical trial design and use of biomarkers and “real-world” data in evaluating efficacy. Basic provisions of these legislative efforts are summarized in Table 1 (9, 10, 11).
Table 1.
Relevant Features of Major U.S. Drug Development Legislation, 2015 to 2019
| Year | Legislation | Provisions |
|---|---|---|
| 2016 | 21st Century Cures Act |
|
| 2017 | FDARA of 2017 | In addition to miscellaneous systems reporting and organizational improvements:
|
| 2017 | PDUFA VI (a section of the FDARA) |
|
FDA = U.S. Food and Drug Administration; FDARA = Food and Drug Administration Reauthorization Act; PDUFA = Prescription Drug User Fee Act; RACE = Research to Accelerate Cures and Equity; RMAT = Regenerative Medicine Advanced Therapy.
Changing DAB Development Times
Driven largely by budget limitations that prevented the FDA from employing sufficient personnel to provide timely processing of NDAs, FDA review times ran around 33 months in 1987 (12). In the face of a growing AIDS crisis, the pharmaceutical industry offered funding to the FDA in the form of “user fees” in exchange for commitments to accelerate reviews, with the intention that these fees would in large part be used to hire sufficient staffing to accomplish this goal (13). The proposed fees caused great discomfort among many policy makers, because it would create a dependence for essential funding by the FDA on the pharmaceutical companies it regulates. Nevertheless, user fees were adopted and have remained an important funding source for FDA reviews, providing about $1.22 billion (79%) of the FDA’s regulatory spending for DABs in 2017 (14). The PDUFA required that these funds only be used to expedite reviews of human DAB applications. Although the PDUFA included a 5-year sunset provision, it was so successful (the FDA reviewed 90% of DAB applications within 6 or 12 months after submission) [13]), Congress reauthorized the PDUFA in successive years (PDUFA II to VI) requiring further reductions in review times. Subsequent provisions authorized the use of user fees to shorten clinical trial times, funded the FDA program for evaluating post-market DAB safety—a provision that was later removed—and reduced the number of review cycles for DAB approvals. The PDUFA section of the FDARA of 2017 raised these user fees and provided for annual increases through 2022. The FDARA also imposed new fees for generic drug applications ($171,823), generic drug manufacturing facilities ($211,087 annually), medical device pre-market notifications ($10,566), pre-market authorization submission ($310,764), and biosimilar applications ($1,746,745) (13). In 2020, the NDA user fee is $2,942,965 (15), compared with the original user fee of $100,000 in 1992 (13). The FDARA sets the goals for complete reviews of 90% of all applications within 6 to 10 months for biosimilars, 8 to 10 months for generic drug applications, and 180 days for medical devices (16). Under the FDA’s PDUFA VI commitment letter, the FDA agreed to undertake initiatives to explore the use of “real-world data,” hire new staff to support increased use of biomarker and surrogate endpoints, and promote use of adaptive, Bayesian, and other novel clinical trial designs (14,17). DABs that meet an important public health need will be fast-tracked with an aim for the FDA to act at least 1 month before the standard PDUFA deadline.
As a result of the FDARA, user fees have risen faster than the FDA budget as a whole, and now make up 75% of the scientific review budgets for brand name and generic drugs, and over 40% of the total 2016 FDA budget. The FDA’s dependence on industry funding has put pressure on Congress to maintain the FDARA to avoid substantial FDA layoffs (estimated as up to 5,000 full-time positions) (13). Many observers express concern that there has been a weakening of the regulatory independence of the FDA, and that this may have a substantial impact on the FDA’s efficacy and safety standards. Such issues call to mind the industry/regulatory interdependence between the Federal Aviation Administration and the airline industry that has been strongly implicated in a failure of oversight and fatal design flaws of the Boeing 737 Max (18).
Under the PDUFA, total review times at the FDA decreased from 1.2 years for the period 2006 to 2017, to 10.1 months in 2018 for a standard application and 7.6 months for a priority application (i.e., DABs that met FDA criteria as a therapeutic advance and received priority attention) (14). In 2017, Hwang et al. (19) reported that novel therapeutics enrolled in at least 1 accelerated development program at the FDA from 2012 to 2016 experienced decreased median development time of nearly 1 year compared with nonaccelerated therapeutics (7.1 vs. 8.0 years, respectively). DABs with Breakthrough Therapy (BT) designation (i.e., new therapies representing a substantial improvement over existing therapies for a serious or life-threatening condition) shaved over 3 years off development time compared with non-BT drugs (4.8 vs. 8.0 years, respectively) (19). However, despite this decline in review times, the total time for IND effective date to final approval appears to have increased in the last 12 years, from an average of 7.0 years for the period of 1997 to 2007, to 9.1 years from 2008 to 2017, due in large part to increased trial times that offset reduced FDA review times (14).
Evolving Interpretation of FDA Requirements
Controlled trials
The overall roadmap for pre-approval clinical drug testing at the FDA is largely unchanged over the last several decades, even by recent legislation. Current law requires that efficacy claims in the NDA be supported by “adequate and well-controlled trials” (4,20); however, under various legislative actions, including those in the last 5 years, what constitutes “adequate and well-controlled” is being interpreted with increasing flexibility. Although at least 2 adequate, controlled trials were originally required by FDA regulations, later legislation encouraged the FDA to accept a single pivotal trial under some circumstances—such as when data from other populations than the target 1 provide supportive evidence. From 2015 to 2017, the proportion of NDAs that included at least 2 controlled trials decreased from the previous level of 80% to 52.8%. Furthermore, the number of NDAs approved during that period that relied on at least 1 Phase III study using an active comparator rather than historic controls or placebo fell from 44% to 29%, and the proportion of NDA approvals based on nonrandomized, uncontrolled studies increased from 4% to 17%. Despite these changes, however, the actual length of clinical trials themselves for NDAs increased from 2015 to 2017, with almost one-half (46%) including at least 1 pivotal trial of 6 months’ duration or more, compared with just 26% from 1995 to 1997 (21).
Drugs for rare diseases
The FDA has for some time now also emphasized flexibility in its approval criteria with respect to treatments of rare diseases. This has far-reaching implications in a new era of precision medicine in which subtypes of common conditions, such as cancers, can be defined by genotyping and pharmacogenomics. Such genetic conditions qualify as rare diseases in regulatory language if they affect fewer than 200,000 cases in the United States, and qualify under the Orphan Drug Act of 1983 for more flexible FDA testing standards, research grants, tax benefits, and 7 years of nonpatent exclusivity (22,23). DAB development for rare diseases is also able to access specific FDA pathways for approval.
Accelerated pathways and DAB designations
The FDA has instituted several designations to facilitate the development and review cycles of new drugs that meet an unmet medical need in treating serious or life-threatening conditions, all but 1 of which were legislated before 2012 (Table 2) (14,24,25).
Table 2.
FDA Designations to Accelerate Drug Development Times
| Designation | Year/Legislation | Criteria |
|---|---|---|
| Accelerated Approval | FDA instituted in 1992, but legislation in 2012 (FDASIA) allowed use of surrogate endpoints. | DAB that treats a serious condition∗ that fills an unmet medical need can be approved on the basis of a surrogate endpoint: for example, lab marker, radiographic images, physical sign, or other finding that is thought to predict clinical benefit. Surrogate or intermediate clinical endpoints are allowed. |
| Priority Review | 1992 PDUFA | DAB would be a significant improvement in the safety or efficacy of the treatment, diagnosis, or prevention of serious conditions when compared to standard therapy. |
| Fast Track | FDA Modernization Act of 1997 | DABs that treat a serious condition must fulfill an unmet medical need or provide therapy that is substantially better in safety and efficacy than existing ones. Fast-Track DABs may also be eligible for Accelerated Approval and Priority Review if criteria are met, |
| Breakthrough Therapy | 2012 FDASIA | DABs that are intended to treat a serious condition, and preliminary clinical evidence indicates that it may demonstrated substantial improvement over available therapy on a clinically significant endpoint. Surrogate endpoints allowed. |
| Regenerative Medicine Advanced Therapy | 21st Century Cures Act, 2016 | Regenerative medicine advanced therapy (cell therapy therapeutic tissue engineering product, human cell and tissue product, or any combination product using these) used to treat, modify, reverse, or cure a serious or life-threatening condition, and preliminary clinical evidence indicates that it has the potential to address an unmet medical need |
DAB = Drugs and Biologics; FDASIA = U.S. Food and Drug Administration Safety Innovations Act; other abbreviations as in Table 1.
The FDA defines a serious condition as one that will have an impact on day-to-day survival, functioning or the likelihood that if left untreated a condition will progress from a less serious one to a serious one.
The Orphan Drug Act of 1983 was followed by the Accelerated Approval (AA) program in 1992 that allows approval on the basis of surrogate endpoints that are seen as “reasonably likely” to predict clinical benefit, rather than demonstration of improvement of clinical endpoints per se. Completion of post-approval studies to verify the clinical benefit (Phase 4 confirmatory trials) is required to maintain market approval (25). “Fast Track” designation authorized by the FDA Modernization Act of 1997 allowed more frequent reviews with the FDA and expedited rolling reviews (26). DABs that are fast-tracked are also eligible for AA and Priority Review designations, which commits the FDA to act on an NDA within 6 months (compared with the standard review of 10 months) (27). The BT program essentially formalized FDA review processes in the AA program. FDA guidance then indicated that BT drugs might be approved on the basis of studies with alternative clinical designs that could be smaller in number of subjects and scope, and use surrogate endpoints or biomarkers to determine efficacy (28,29). The 21st Century Cures Act mandated that the FDA maximize use of these existing programs, including the use of alternative measures, such as radiographic imaging and biomarkers, as determinants of therapeutic efficacy rather than clinical outcomes alone (9). In addition, the 21st Century Cures Act authorized a new drug designation, the Regenerative Medicine Advanced Therapies (RMAT) designation, that also qualifies for accelerated approval pathways.
Drugs carrying an Orphan Drug designation can access these accelerated pathways, requiring smaller trials (median participants n = 96 vs. 290 for common diseases), avoiding randomization or double-blinding (30% vs. 80% and 4% vs. 33%, respectively, compared with trials for common diseases), and achieving approval on the basis of interim effects (e.g., disease response) rather than mortality/survival clinical endpoints. Between 2008 and 2018, the proportion of DABs approved under the Orphan Drug Act increased to 22% from a previous level of 18% (30). The number of DABs that qualified for FT designation tripled between 1989 and 2018, with most of that increase occurring between 2009 and 2018. Over one-quarter of new DABs approved between 2014 and 2018 were granted BT designation (31).
Too few new cardiovascular drugs have been approved in the last 5 years to comment with confidence about changing drug approval times, but increased utilization of accelerated pathways has occurred in other drug categories, particularly oncology. Around 95% of all new anticancer DABs approved between 2012 and 2017 in the United States used 1 or more of these expedited programs (32). These changes decreased development times significantly (4.8 vs. 8 years) (19). Yamashita et al. (24) found that for anticancer therapies, pursuit of any of the expedited programs was associated with reduced review times (Table 3), by a range of 2.0 to 3.4 years. This finding is similar to a previous study by Hwang et al. (32). Most DAB approvals that they examined in the AA program (88%) used a noncomparative study design (24). As more and more diseases are genetically subclassified, more drugs qualify for AA tracts. Almost two-thirds of all INDs (64%) now qualify for such programs (14).
Table 3.
Drug Approval Times in Years in FDA Accelerated Pathways
| Track Designation∗ | Major Cancer | Minor Cancer | Decrease in Review Time |
|---|---|---|---|
| Fast Track | 7.2 | 9.2 | 2.0 |
| Breakthrough Therapy | 6.4 | 9.6 | 3.2 |
| Accelerated Approval | 6.2 | 9.6 | 3.4 |
| Priority Review | 8.0 | 10.2 | 2.2 |
Average times in years from Investigational New Drug application to U.S. Food and Drug Administration (FDA) drug approval for anticancer drugs and biologicals achieving accelerated track designation at the FDA from 2012 to 2017 compared with overall anticancer drug approval times for the same period (median of 8.3 ys, n = 115 drugs), and separated by categorization as a drug for “minor” versus “major” cancer. (24).
Regenerative Medicine Advanced Therapy designation was created too recently to comment on review times.
The RMAT drug classification went live in March of 2017 and is too new to determine whether it has been associated with reduced development timelines (33).
Is patient safety being compromised?
Accelerated drug approval pathways shave time off of the approval process and may theoretically reduce DAB development costs, but they raise concerns of whether, in the interest of saving time and money, sacrifices are made in patient safety. One study of approvals of BT and non-BT approvals of cancer drugs failed to show differences in response rates, new mechanisms of action, mortality, or serious side effects (32), suggesting that BT designation may not be accompanied by greater efficacy, and may not present increased immediate risks to patients. Another study, however, found that BT and AA approvals were associated with fewer randomized controlled trials in the approval phase, and that entities without supporting randomized controlled trials were significantly more likely to be associated with post-approval modifications in common adverse events (71% vs. 29%) and had higher odds of post-approval major modifications in warnings and precautions (88% vs. 62%) (34), highlighting the need for clinician vigilance when prescribing an FDA-approved treatment that is a graduate from an accelerated pathway (35).
How Regulatory Changes Have Impacted the Development of DABS in the COVID-19 Pandemic
Efforts to reach accelerated approval of drug treatments and preventative vaccines in fighting the COVID-19 pandemic have included substantial use of accelerated pathways to FDA approval. COVID-19 was declared a public emergency by the Secretary of Health and Human Services on February 4th, 2020, who also confirmed that circumstances existed to justify EUAs for drugs and biological products (8,36). EUAs can be issued by the FDA very quickly: the regulations call for an automatic authorization if the FDA does not object within 30 days of application.
As of May 11, 2020, EUAs have authorized the use of hydroxychloroquine and chloroquine, convalescent plasma, hyperimmune globulin, remdesivir, and fresenius propoven (propofol) 2% in the treatment of COVID-19 (37). Although some of these therapies represent new advances, the EUAs also approve new uses for already established drugs—under an EUA, a new use for a therapeutic agent is not considered unapproved use of drugs off label (38). One of the most controversial examples has been the EUA for hydroxychloroquine for treatment of COVID-19 infections. Hydroxychloroquine is an approved therapy for malaria, rheumatoid arthritis, and lupus, with significant toxicity and as yet unproven efficacy against COVID-19 (39). Hydroxychloroquine received FDA approval for treatment of COVID-19 infections via EUA despite very limited and controversial clinical evidence (40). On June 15th, 2020, the FDA revoked its EUA for hydroxychloroquine due to lack of efficacy.
The U.S. “Operation Warp Speed Vaccine Initiative”—a public–private partnership among government agencies and private entities to produce a vaccine by early 2021 (40)—will clearly take advantage of many of the established accelerated pathways, (e.g., AA, Priority Review, and BT designations). In addition, the indirect effects of PDUFA VI funding for FDA staffing and other resource needs are likely to be of help. Provisions of both the 21st Century Cures Act and FDARA require the FDA to encourage the development of innovative trial design, the use of Bayesian adaptive trials and blended trials, the use of surrogate endpoints, and the incorporation of real-world data in determining efficacy of therapeutics (9,10,41). Examples of entities that have announced studies employing such strategies include Johnson and Johnson’s (New Brunswick, New Jersey) vaccine trial (1 of 5 candidate vaccines supported by Operation Warp Speed), which announced on June 10th, 2020, that it would begin a Phase I/II study (42). Oxford University has also indicated that trials of their vaccine will proceed along a process blending Phases I and II, and will certainly be applying for EUAs if efficacy is shown (43). With reportedly around 100 companies and academic institutions competing to develop a COVID-19 vaccine (44), it is a virtual given that they will all try to make full use of the innovative trial designs and accelerated programs combined with early EUAs to race for approval.
Footnotes
Dr. Van Norman has received financial support from the American College of Cardiology.
The author attests she is in compliance with human studies committees and animal welfare regulations of the author's institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.U.S. Food and Drug Administration FDA’s Origins and Functions: Part II: 1938, Food, Drug, Cosmetic Act. https://www.fda.gov/about-fda/fdas-evolving-regulatory-powers/part-ii-1938-food-drug-cosmetic-act Available at:
- 2.U.S. Food and Drug Administration FDA’s Origins and Functions: Part III. Drugs and Foods Under the 1938 Act and Its Amendments. https://www.fda.gov/about-fda/fdas-evolving-regulatory-powers/part-iii-drugs-and-foods-under-1938-act-and-its-amendments Available at:
- 3.Van Norman G.A. Drugs, devices and the FDA: part 1. An overview of approval processes for drugs. J Am Coll Cardiol Basic Trans Science. 2016;1:170–179. doi: 10.1016/j.jacbts.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabrowska A., Thaul S. Congressional Research Service; May 8, 2018. How FDA Approves Drugs and Regulates Their Safety and Effectiveness.https://fas.org/sgp/crs/misc/R41983.pdf Available at: [Google Scholar]
- 5.Van Norman G. Expanding patient access to investigational drugs. Single patient investigational new drug, and the “right to try”. J Am Coll Cardiol Basic Trans Science. 2018;3:280–293. doi: 10.1016/j.jacbts.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration IND Applications for Clinical Treatment (Expanded Access): Overview. https://www.fda.gov/drugs/investigational-new-drug-ind-application/ind-applications-clinical-treatment-expanded-access-overview Available at:
- 7.U.S. Food and Drug Administration Animal Rule Information. https://www.fda.gov/emergency-preparedness-and-response/mcm-regulatory-science/animal-rule-information Available at:
- 8.U.S. Food and Drug Administration Emergency Use Authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization Available at:
- 9.U.S. Food and Drug Administration 21st Century Cures Act. https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act Available at.
- 10.Public Law 115-52. 115th Congress. HR2430. August 18, 2017. The FDA Reauthorization Act of 2017. https://www.congress.gov/115/plaws/publ52/PLAW-115publ52.pdf Available at.
- 11.U.S. Food and Drug Administration Completed PDUFA VI Deliverables. June 5, 2020. https://www.fda.gov/industry/prescription-drug-user-fee-amendments/completed-pdufa-vi-deliverables Available at:
- 12.Government Accountability Office FDA Approval Times Have Decreased in Recent Years. GAO/PEMD-96-1. October 20, 1995. https://www.gao.gov/assets/230/221919.pdf Available at:
- 13.Darrow J.J., Avorn J., Kesselheim A.S. Speed, safety and industry funding—from PDUFA I to PDUFA VI. N Engl J Med. 2017;377:2278–2288. doi: 10.1056/NEJMhle1710706. [DOI] [PubMed] [Google Scholar]
- 14.Darrow J.J., Avorn J., Kesselheim A.S. FDA approval and regulation of pharmaceuticals, 1983-2018. JAMA. 2020;323:164–176. doi: 10.1001/jama.2019.20288. [DOI] [PubMed] [Google Scholar]
- 15.Federal Register Prescription Drug User Fee Rates for Fiscal Year 2020. https://www.federalregister.gov/documents/2019/08/02/2019-16435/prescription-drug-user-fee-rates-for-fiscal-year-2020 Available at:
- 16.U.S. Food and Drug Administration PDUFA Reauthorization Performance Goals and Procedures Fiscal Year 2018 Through 2022. https://www.fda.gov/media/99140/download Available at:
- 17.Sharfstein J.M. Reform at the FDA—in need of reform. JAMA. 2020;323:123–124. doi: 10.1001/jama.2019.20538. [DOI] [PubMed] [Google Scholar]
- 18.Gates D. Seattle Times; March 21, 2019. Flawed Analysis, Failed Oversight: How Boeing, FAA Certified the Suspect 737 MAX Flight Control System.https://www.seattletimes.com/business/boeing-aerospace/failed-certification-faa-missed-safety-issues-in-the-737-max-system-implicated-in-the-lion-air-crash/ Available at: [Google Scholar]
- 19.Hwang T., Darrow J.J., Kesselheim A.S. Research letter: the FDA’s expedited programs and clinical development times for novel therapeutics, 2012-2016. JAMA. 2017;318:2137–2138. doi: 10.1001/jama.2017.14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration Guidance for Industry. Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products. May 1998. https://www.fda.gov/media/71655/download Available at:
- 21.Zhang A.D., Puthumana J., Downing N.S., Shah N.D., Krumholz H., Ross J.S. Clinical Trial Evidence Supporting FDA Approval of Novel Therapeutics Agents Over Three Decades, 1995-2017. Cross-Sectional Analysis. https://www.medrxiv.org/content/10.1101/19007047v1 Available at:
- 22.Orphan Drug Act, Pub L No. 97-414, 96 Stat 2049 97th Congress (1983) https://www.govtrack.us/congress/bills/97/hr5238/text Available at:
- 23.U.S. Food and Drug Administration Orphan Drug Act—Relevant Excerpts. https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/orphan-drug-act-relevant-excerpts Available at:
- 24.Yamashita K., Kaneko M., Narukawa M. Regulatory characteristics and pivotal study design of US Food and Drug Administration approval of drugs for major vs. minor cancer. Eur J Clin Pharmacol. 2019;75:1193–1200. doi: 10.1007/s00228-019-02695-0. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration Accelerated Approval. https://www.fda.gov/drugs/information-healthcare-professionals-drugs/accelerated-approval-program Available at:
- 26.U.S. Food and Drug Administration Fast Track. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track Available at:
- 27.U.S. Food and Drug Administration Priority Review. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/priority-review Available at:
- 28.U.S. Food and Drug Administration Breakthrough Therapy. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy Available at:
- 29.U.S. Food and Drug Administration Guidance for Industry. Expedited Programs for Serious Conditions—Drugs and Biologics. May, 2014. https://www.fda.gov/media/86377/download Available at:
- 30.Kesselheim A.S., Meyers J.A., Avorn J. Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. JAMA. 2011;305:2320–2326. doi: 10.1001/jama.2011.769. [DOI] [PubMed] [Google Scholar]
- 31.Darrow J.J., Avorn J., Kesselheim A.S. The FDA breakthrough drug designation—four years of experience. N Engl J Med. 2018;378:1444–1453. doi: 10.1056/NEJMhpr1713338. [DOI] [PubMed] [Google Scholar]
- 32.Hwang T.J., Franklin J.M., Chen C.T. Efficacy, safety and regulatory approval of Food and Drug Administration-designated breakthrough and nonbreakthrough cancer medicines. J Clin Oncol. 2018;36:1805–1812. doi: 10.1200/JCO.2017.77.1592. [DOI] [PubMed] [Google Scholar]
- 33.Marks P. This Is Not a Test. RMAT Designation Goes Live. March 21, 2017. U.S. Food and Drug Administration. https://www.fda.gov/news-events/fda-voices/not-test-rmat-designation-goes-live Available at:
- 34.Shepshelovich D., Tibau A., Goldvaser H. Postmarketing modifications of drug labels for cancer drugs approved by the U.S. Food and Drug Administration between 2006 and 2106 with and without supporting randomized controlled trials. J Clin Oncol. 2018;36:1798–1804. doi: 10.1200/JCO.2017.77.5593. [DOI] [PubMed] [Google Scholar]
- 35.Kuderer N.M., Lyman G.H. Evolving landscape of US Food and Drug Administration drug approval in the era of precision oncology: finding the right balance between access and safety. J Clin Oncol. 2018;36:1773–1776. doi: 10.1200/JCO.2018.78.5592. [DOI] [PubMed] [Google Scholar]
- 36.Van Norman G.A. Expanding patient access to investigational new drugs: overview of intermediate and widespread treatment investigational new drugs, and emergency authorization in public health emergencies. J Am Coll Cardiol Basic Trans Science. 2018;3:403–414. doi: 10.1016/j.jacbts.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration . May 11, 2020. FDA Combating COVID-19 With Therapeutics.https://www.fda.gov/media/136832/download Available at: [Google Scholar]
- 38.U.S. Food and Drug Administration Understanding Use of Approved Drugs “Off Label”. https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label Available at: Accessed June 14, 202.
- 39.Van Norman G. “Warp Speed” operations in the COVID-19 pandemic: moving too quickly? J Am Coll Cardiol Basic Trans Science. 2020;5:730–734. doi: 10.1016/j.jacbts.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services. Trump Administration Announces Framework and Leadership for ‘Operation Warp Speed’. May 15, 2020. https://www.hhs.gov/about/news/2020/05/15/trump-administration-announces-framework-and-leadership-for-operation-warp-speed.html Available at:
- 41.Lewis R.J. An Overview of Bayesian Adaptive Clinical Trial Design. http://www.berryconsultants.com/wp-content/uploads/2012/09/An-Overview-of-Bayesian-Adaptive-Clinical-Trial-Design.pdf Available at:
- 42.Johnson & Johnson Johnson & Johnson Announces Acceleration of Its COVID-19 Vaccine Candidate; Phase I/2a Clinical Trial to Begin in Second Half of July. June 10, 2020. https://www.jnj.com/johnson-johnson-announces-acceleration-of-its-covid-19-vaccine-candidate-phase-1-2a-clinical-trial-to-begin-in-second-half-of-july Available at:
- 43.The Oxford Vaccine Group Oxford COVID-19 Vaccine to Begin Phase II/III Human Trials. May 22, 2020. Oxford University, Oxford, UK. https://www.ovg.ox.ac.uk/news/oxford-covid-19-vaccine-to-begin-phase-ii-iii-human-trials Available at:
- 44.Sanger D.E. The New York Times; April 29, 2020. Trump Seeks Push to Speed Vaccine Despite Safety Concerns.https://www.nytimes.com/2020/04/29/us/politics/trump-coronavirus-vaccine-operation-warp-speed.html?searchResultPosition=1 Available at: [Google Scholar]