Abstract
In the retina, modulation of the amplitude of dim visual signals primarily occurs at axon terminals of rod bipolar cells (RBCs). GABA and glycine inhibitory neurotransmitter receptors and the excitatory amino acid transporter 5 (EAAT5) modulate the RBC output. EAATs clear glutamate from the synapse, but they also have a glutamate-gated chloride conductance. EAAT5 acts primarily as an inhibitory glutamate-gated chloride channel. The relative role of visually evoked EAAT5 inhibition compared with GABA and glycine inhibition has not been addressed. In this study, we determine the contribution of EAAT5-mediated inhibition onto RBCs in response to light stimuli in mouse retinal slices. We find differences and similarities in the two forms of inhibition. Our results show that GABA and glycine mediate nearly all lateral inhibition onto RBCs, as EAAT5 is solely a mediator of RBC feedback inhibition. We also find that EAAT5 and conventional GABA inhibition both contribute to feedback inhibition at all stimulus intensities. Finally, our in silico modeling compares and contrasts EAAT5-mediated to GABA- and glycine-mediated feedback inhibition. Both forms of inhibition have a substantial impact on synaptic transmission to the postsynaptic AII amacrine cell. Our results suggest that the late phase EAAT5 inhibition acts with the early phase conventional, reciprocal GABA inhibition to modulate the rod signaling pathway between rod bipolar cells and their downstream synaptic targets.
NEW & NOTEWORTHY Excitatory amino acid transporter 5 (EAAT5) glutamate transporters have a chloride channel that is strongly activated by glutamate, which modulates excitatory signaling. We found that EAAT5 is a major contributor to feedback inhibition on rod bipolar cells. Inhibition to rod bipolar cells is also mediated by GABA and glycine. GABA and glycine mediate the early phase of feedback inhibition, and EAAT5 mediates a more delayed inhibition. Together, inhibitory transmitters and EAAT5 coordinate to mediate feedback inhibition, controlling neuronal output.
Keywords: EAAT5, glucose transporter, retina, rod bipolar cell
INTRODUCTION
The retina responds to visual stimuli over more than 12 orders of brightness magnitude (Mills and Massey 1995; Shapley and Enroth-Cugell 1984), necessitating substantial gain modulation of neural signals to avoid response saturation and maintain sensitivity. In the rod signaling pathway, gain modulation occurs primarily at the synapse between the rod bipolar cell (RBC) and the AII amacrine cell (Dunn et al. 2006). In response to light stimuli, a variety of amacrine cell populations release GABA and glycine onto RBC axon terminals, which activate GABAA, GABAC, and glycine receptors, evoking an inhibitory chloride conductance (Eggers and Lukasiewicz 2006a, 2011; Eggers et al. 2007). The inhibitory transmitters GABA and glycine mediate both feedback inhibition, in which excitation of the rod bipolar cell leads directly to reciprocal inhibition back onto itself, and lateral inhibition, in which inhibition is driven by other bipolar cells and occurs independently of the activity of the RBC of interest. GABA-mediated feedback inhibition of the RBC is mediated via the A17 amacrine cell reciprocal synapse while GABA- and glycine-mediated lateral inhibition occurs via various amacrine cell pathways (Chávez et al. 2010).
In addition to GABAA, GABAC and glycine receptors, RBCs are also subject to inhibition mediated by excitatory amino acid transporter 5 (EAAT5) (Arriza et al. 1997; Ichinose and Lukasiewicz 2012; Veruki et al 2006; Wersinger et al. 2006). Transporters in the EAAT family can remove glutamate from synapses following neurotransmission. Glutamate binding to EAATs also opens a chloride conductance through the transporter that suppresses synaptic activity (Eliasof and Werblin 1993; Fairman et al. 1995; Ichinose and Lukasiewicz 2012). EAAT5, in particular, plays a significant role in presynaptic inhibition but little role in glutamate uptake (Pow et al. 2000; Schneider et al. 2014). In the retina, the axon terminals of photoreceptors and RBCs express EAAT5 (Wersinger et al. 2006).
Overall, there are two classes of inhibition in rod bipolar cells: the conventional GABAA, GABAC, and glycine receptors and the less well understood EAAT5 glutamate transporter. There are two forms of inhibition, which either class could mediate: GABA receptors mediate both feedback and lateral inhibition, while EAAT5 could potentially mediate either feedback or a localized spillover inhibition from neighboring cells, or both. Note that spillover is a local form of lateral inhibition, which is distinguished from feedback or autoinhibition. Wersinger et al. (2006) and Veruki et al. (2006) demonstrated that RBC EAAT5 mediates feedback inhibition, since glutamate release from the RBC can evoke EAAT5-mediated currents at its own synaptic terminal. However, both of these studies likely enhanced the probability of glutamate release, using either electrical stimulation of cells beyond their normal physiological range or bath-applied antagonists of GABA and glycine receptor-mediated inhibition, exaggerating the role of EAAT5.
Why might cells need both GABA- and EAAT5-mediated feedback inhibition? In response to light, the depolarizing response of RBCs is limited by inhibitory neurotransmitters (Euler and Masland 2000), and blockade of GABA and glycine signaling is known to enhance glutamate release from bipolar cells (Eggers and Lukasiewicz 2011; Sagdullaev et al. 2011). Previous work from our group suggested that light-evoked EAAT5-mediated presynaptic inhibition also modulates RBC output (Ichinose and Lukasiewicz 2012). However, because blockade of GABA- and glycine-receptor mediated inhibition may enhance the activation of EAAT5 by glutamate, the role of the EAAT5 component may be overestimated. The role of feedback inhibition by EAAT5 is particularly unclear because, in an earlier study, glutamate spillover from neighboring RBCs mediated most of the light-evoked EAAT5 inhibition (Ichinose and Lukasiewicz 2012). Thus the relative contributions of EAAT5-mediated and GABA- mediated feedback inhibition to light-evoked responses remain unknown. In this study, we estimate the relative roles of GABA and EAAT5 inhibition (autoinhibition), attributed to direct feedback to the recorded RBC.
In this paper, we used focal puff application of inhibitory blockers to minimize network effects and found that GABA and EAAT5 forms of feedback inhibition shape the RBC output. Both forms of feedback inhibition, EAAT5 and GABA mediated, occurred at all light intensities. We also found that EAAT5 is not a significant contributor to lateral inhibition but is a primary source of feedback inhibition to RBCs. Finally, in silico modeling of the RBC-AII amacrine cell synapse showed that EAAT5-mediated and conventional feedback inhibition both have a substantial effect on synaptic transmission and may be important in preventing response saturation and in maintaining RBC signaling sensitivivity.
MATERIALS AND METHODS
Animal protocols.
Animal protocols were approved by the Washington University School of Medicine Animal Studies Committee.
Retinal dissection.
Mice of either sex (28–60 days of age; C57BL/6J strain; The Jackson Laboratory) were dark-adapted overnight and euthanized using carbon dioxide. The retina was isolated, and 400-µm slice preparations were made from the dorsal retina. All dissection procedures were performed in complete darkness under infrared illumination. Dissection medium (see below, Solutions and drugs) was continuously oxygenated. Retinal preparations were stored in an oxygenated dark box at room temperature until experimental use. Dissections were performed from 10 to 11 AM, and recordings were always performed in the afternoon.
Whole cell recordings.
Whole cell patch recordings were made from bipolar cell somata in retinal slices by viewing them with an upright microscope under infrared illumination. The sample chamber was curtained to prevent light leakage from infrared eyepieces reaching the samples. Extracellular media were continuously applied to the samples via a preheated perfusion system positioned near the slice preparation. Electrodes were made from borosilicate glass with a P97 Flaming/Brown puller (Sutter Instruments) and had resistances of ~5 MΩ. All recordings were made at 30°C. Signal software (Cambridge Electronic Design) was used to generate electrical command outputs and acquire and store data. Recordings were sampled at 5 kHz and filtered at 2 kHz with the four-pole Bessel filter on the Axopatch 200B (Molecular Devices). Liquid junction potentials were corrected at the beginning of each recording. Depending on the experiment, cells were either voltage clamped at 0 mV, the reversal potential for nonselective cation channels, isolating Cl− currents, or were held at −60 mV with ECl at 0 mV. In the latter case, the mGluR6-mediated cation currents ran down quickly and did not contaminate the Cl− currents.
Morphological identification.
Sulforhodamine B (0.005%) was included in the intracellular solution for all recordings to identify the cell type and to confirm correct placement of puff pipettes at the RBC axon terminal. After electrophysiological recordings were finished, cells were morphologically identified by their axon terminal ramification morphology and location in the inner plexiform layer (IPL) (Ghosh et al. 2004a, 2004b; Pignatelli and Strettoi 2004).
Solutions and drugs.
Retinal dissections were performed in HEPES-buffered extracellular Ringer’s solution containing (in mM): 137 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl, 28 glucose, 10 HEPES, 4 Na-l-lactate, 2 Na-pyruvate, and 0.5 l-glutamine, adjusted to pH 7.4 by NaOH. Physiological recordings were performed in bicarbonate-buffered extracellular solution containing (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 20 glucose, 1.25 NaH2PO4, 4 Na-l-lactate, 2 Na-pyruvate, and 0.5 l-glutamine. Extracellular solutions were continuously aerated with 95% O2-5% CO2 and the pH was 7.4 at 30°C. For experiments in which cells were held at 0 mV, the intracellular solution was (in mM): 120 CsOH, 120 d-gluconic acid, 1 CaCl2, 1 MgCl2, 11 EGTA, 10 Na-HEPES, 10 TEA-Cl, 5 ATP-Mg, and 1 GTP-Na, adjusted to pH 7.2 by CsOH. For experiments in which cells were held at −60 mV, the intracellular solution was (in mM): 125 CsCl, 25 HEPES, 10 TEA-Cl, 0.5 EGTA, 2.5 ATP-Mg, and 2.1 GDPβS, adjusted to pH 7.2 by CsOH.
When bath applied, conventional inhibitory receptor antagonists were the GABAA receptor antagonist (–)-bicuculline methobromide (50 µM), the GABAC receptor antagonist 1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid hydrate (TPMPA; 50 µM), and the glycine receptor antagonist strychnine (1 µM). When puff applied, the concentrations used were 250 µM bicuculline, 250 µM TPMPA, and 5 µM strychnine. The puff-applied glutamate concentration was 2.5 mM. EAATs were blocked with dl-threo-β-benzyloxyaspartic acid (TBOA; 50 µM extracellularly or 3 mM intracellularly). Excitatory glutamate receptors were generally not targeted pharmacologically, except in Fig. 1D, when N-methyl-d-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors were blocked by bath application of 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate (CNQX; 5 µM) and d-(–)-2-amino-5-phosphonopentanoic acid (d-AP5; 50 µM), respectively.
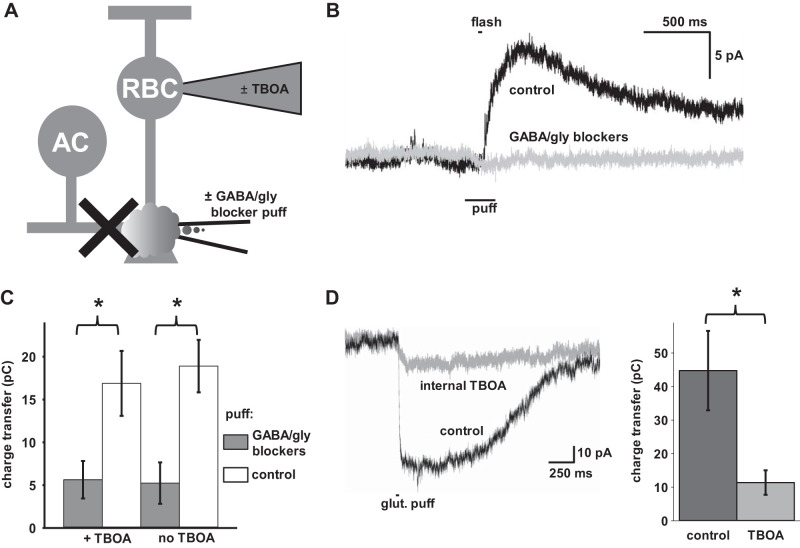
Fig. 1.
Intracellular and puff application of receptor antagonists. A: diagram illustrating experimental paradigm. Rod bipolar cells (RBCs) were recorded in the presence and absence of intracellular dl-threo-β-benzyloxyaspartic acid (TBOA) (3 mM) in the recording pipette, while a second puff pipette with extracellular solution with or without conventional inhibitor antagonists [250 µM bicuculline, 250 µM 1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid hydrate (TPMPA), 5 µM strychnine] was targeted at the axon terminal. B: puff application of GABA/glycine blockers successfully blocks the light-evoked IPSC. An RBC was voltage clamped at 0 mV and a 30 ms flash of light (106 photon·µm−2·s−1) was given with a puff of blocker (gray) or vehicle (black) starting 100 ms before the onset and ending 100 ms after the offset of light. C: Puff application of GABA/glycine antagonists (dark bars) blocks light-evoked Inhibitory postsynaptic currents (IPSCs) compared with controls (white bars) while intracellular TBOA (left) has no significant effect compared with controls (right). RBCs were voltage clamped at 0 mV (n = 12 cells). D: intracellular application of TBOA blocks the response to glutamate. RBCs with (gray) and without (black) intracellular TBOA were voltage clamped at −60 mV with ECl = 0 and 5 µM 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate (CNQX) and 50 µM d-AP5 bath-applied to block N-methyl-d-aspartic acid (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors, then subjected to a brief 2.5 mM glutamate puff. Left: 2 example traces. Right: charge transfer for each category. (n = 8 cells). Error bars indicate means ± SE. *P < 0.05.
Puff-applied drugs were dissolved in a HEPES-buffered extracellular solution identical to the one used for retinal dissections, loaded into pipettes similar to those used for patch recordings, and were puffed onto RBC terminals using a Picospritzer II (Parker Hannifin). To control for puffing artifacts, the same HEPES-buffered solution without drugs was also loaded into pipettes and applied via the same system as a control. Bath-applied drugs were dissolved into the recording solution described above and aerated using the same source of gas and perfused into the recording chamber using the same delivery system as the control solution. Drugs were superfused for at least 4 min into the recording chamber and then washed out of the chamber for at least 4 min before the next round of recordings. The order of experiments was randomized.
Light stimulation.
Full-field light stimuli were generated using a 505 nm LED projected through the optical path of the microscope, passing through a diffusion filter to ensure even lighting over the entire field and a wide enough lens to illuminate the entire retinal slice. Coarse control of the light intensity was achieved by inserting neutral-density filters of varying strength into the optical path. Fine control of light intensity was achieved by varying the current through the LED.
Data analysis.
Charge transfer was measured using the area function in Signal, and difference in charge transfer was calculated by simple subtraction. All data are presented as means ± SE. P values were calculated using two-tailed t tests. In experiments comparing the same conditions across multiple light intensities, corrections for multiple comparisons were made using the Holm-Bonferroni method.
Modeling.
The rod-bipolar to AII-amacrine cell synapse was simulated in NeuronC (Smith 1992). The RBC was modeled as a cell with a spherical soma (diameter: 11 µm), a cylindrical axon (length: 30 µm; diameter: 0.75 µm), and a spherical terminal (diameter: 4 µm), with the cell membrane modeled as a first-order resistor-capacitor circuit with a specific membrane resistance of 24 kOhm (Oltedal et al. 2009). The RBC soma included voltage-dependent potassium channels (0.14 mS/cm2), and the RBC terminal included voltage-dependent calcium channels (L-type, noninactivating, 8 mS/cm2) and calcium pumps (Vmax = 20 nA/cm2, Km = 20 µM, nonelectrogenic) (Oltedal et al. 2009). Calcium diffused through radial shells (n = 10) to/from an internal core. The excitatory input was added to the soma membrane as a conductance with reversal potential at 0 mV. Inhibitory inputs were added to the terminal membrane as conductances with reversal potential at −70 mV. Inhibitory conductances were varied over time to match isolated inhibitory conductances calculated from the experiments on real RBCs in the presence of bath-applied blockers (either GABA/glycine or TBOA), and the excitatory conductance was calibrated to generate a standard RBC voltage response to light in the presence of these inhibitory conductances. That excitatory conductance was then maintained as the standard excitatory input in further modeling experiments that varied inhibition.
The AII cell was represented as a small sphere clamped at −70 mV. The synapse was simulated by a transfer function for vesicle release and a temporal impulse function (release rate proportional to Ca2+ concentration, τ = 2 ms), as well as the size of the readily releasable pool (either 7 or 20) and its replenishment rate (50/s). For simplicity, release was set to be noiseless, i.e., only the average rate was simulated. The postsynaptic currents were generated by binding of the simulated glutamate release to a sequential-state Markov model of a nondesensitizing AMPA receptor/channel.
RESULTS
EAAT5-mediated glutamate spillover versus conventional lateral inhibition onto RBCs.
Light stimuli evoke inhibitory Cl− currents on RBC axon terminals, which are mediated by GABA and glycine receptors through amacrine cell activation and EAAT5 driven by released glutamate (Eggers and Lukasiewicz 2006a; Ichinose and Lukasiewicz 2012). To compare the relative contributions of EAAT5 spillover versus GABA and glycine lateral inhibitory pathways under physiologically relevant conditions, we measured the light-evoked inhibitory response in dark-adapted preparations in the presence and absence of EAAT5 antagonist TBOA (Shigeri et al. 2001) applied through the recording pipette (Ichinose and Lukasiewicz 2012) and in the presence or absence of a puff application of GABA and glycine receptor blockers on the axon terminal (Fig. 1A). Rod bipolar cells were voltage clamped at 0 mV and full-field light stimuli activated both GABA lateral inhibitory and EAAT5 spillover inhibitory inputs. In the presence of puff-applied GABA and glycine receptor antagonists, RBCs demonstrated a reduction of inhibition in response to full field light stimuli (Fig. 1B). In fact, conventional inhibitory blockers reduced inhibitory input between 66 and 73% when puff applied, regardless of the presence or absence of intracellular TBOA, suggesting that GABA- and glycine-mediated inhibition was dominant with our experimental conditions (Fig. 1C) (P < 0.02, n = 12 cells). In contrast, internal application of TBOA had no significant effect on the amount of inhibition onto RBCs in response to full field light stimuli, consistent with the notion that EAAT5 spillover inhibition was not a significant contributor to RBC lateral inhibition.
To confirm that intracellular TBOA in our preparation was capable of blocking glutamate-mediated inhibition, we tested the responses of control and TBOA-filled RBCs to glutamate puff application at the axon terminal. We demonstrated that internal TBOA reduced the responses to puffs of glutamate by 74% (Fig. 1D) (P = 0.01, n = 8 cells), in agreement with earlier results. Overall, these results indicate that, in the absence of direct electrical stimulation or network effects attributable to disinhibition, lateral inhibition evoked by full field light stimuli was largely mediated by GABA and glycine inputs onto RBCs but not by EAAT5 (Ichinose and Lukasiewicz 2012).
EAAT5 mediates feedback inhibition onto RBCs.
When the rod bipolar was voltage clamped at 0 mV, glutamate output from the cell was given time to reach a steady state and deplete the readily-releasable pool of vesicles, so any additional change in voltage-dependent glutamate release to a light stimulus was blocked, preventing us from recording any type of feedback inhibition. Thus these experiments did not test the extent of EAAT5-mediated feedback inhibition. To examine EAAT5 feedback under physiologically relevant conditions, we recorded RBCs held at a starting resting potential of −60 mV and then applied a voltage input modeled to duplicate the excitation pattern of an RBC in response to a light stimulus of matching intensity and duration as our prior experiment (Euler and Masland 2000) (Fig. 2E). By comparing responses from the same cell in the presence and absence of a given inhibitory antagonist, the difference in charge transfer between the two conditions allowed us to measure the relevant level of inhibition. For these experiments, ECl was 0 mV. Thus inhibitory blockers enhanced the outward current because they block inhibitory, inward currents.
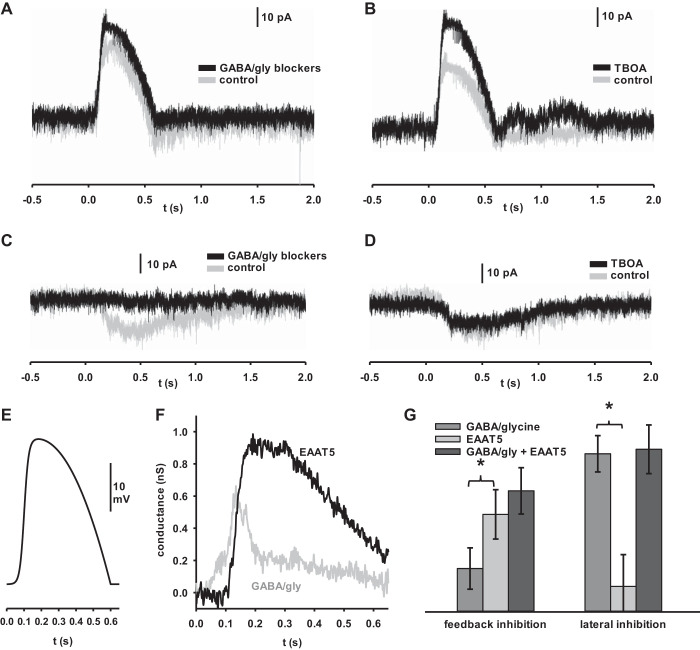
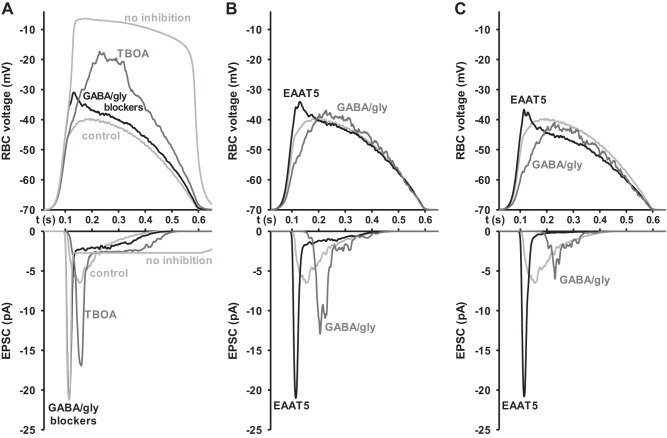
Fig. 2.
Excitatory amino acid transporter 5 (EAAT5) is the primary mediator of feedback inhibition in rod bipolar cells (RBCs) at ECl = 0 mV. A: RBC stimulated from a baseline of −60 mV with the voltage pulse pictured in Fig. 2E without (gray) and with (black) 2 s puff application of GABA/gly blockers. GABA/gly blockers elicited a small but measurable difference that could be attributed to feedback from conventional inhibitory sources. B: RBC stimulated from a baseline of −60 mV with the voltage pulse pictured in Fig. 2E without (gray) and with (black) extracellular application of dl-threo-β-benzyloxyaspartic acid (TBOA). TBOA elicited a larger difference that could be attributed to feedback inhibition from EAAT5. C: RBC held at −60 mV and flashed for 100 ms at 105 photon·µm−2·s−1 without (gray) and with (black) 2 s puff application of GABA/gly blockers. GABA/gly blockers showed a substantial blunting of the light response that could be attributed to lateral inhibition from conventional sources. D: RBCs held at −60 mV and flashed for 100 ms at 105 photon·µm−2·s−1 without (gray) and with (black) TBOA in the intracellular media. Very little difference between traces suggests that EAAT5 plays little role in lateral inhibition. E: voltage pulse input modeled to mimic the excitatory profile of an RBC in response to the light stimuli used here (Euler and Masland 2000). F: kinetics of conventional and EAAT5 inhibition. Control traces were subtracted from blocker traces for the experiments depicted in A and B, and then, each data point was divided by the holding potential at the moment it was recorded to calculate the inhibitory conductance. The inhibition masked by GABA and glycine blockers is shown in gray (GABA/gly), while the inhibition masked by TBOA is shown in black (EAAT5). Traces are averages (n = 24 cells). Overall, EAAT5 contributed more to feedback inhibition than GABA and glycine receptors, but GABA- and glycine-mediated feedback inhibition had a more rapid onset and peak than EAAT5-mediated feedb A and B ack inhibition. G, left: summary data for feedback inhibition experiments depicted in A and B. Data represent the difference in charge transfer between 2 comparitor conditions in individual cells. GABA/glycine- and EAAT5-mediated feedback were significantly different from each other and were also each significantly different from zero. (n = 24 cells). G, right: summary data for lateral inhibition experiments depicted in C and D. Data represent the difference in charge transfer between populations of cells. GABA/glycine mediated lateral inhibition was significantly different from both EAAT5-mediated lateral inhibition and from zero. EAAT5-mediated lateral inhibition was not significantly different from 0 (n = 10 cells) Error bars indicate SE. *P < 0.05.
We compared the feedback inhibitory charge transfer of RBCs in the presence and absence of a cocktail of synaptic inhibitory blockers and observed around 4.19 pC of GABA- and glycine-mediated inhibition (Fig. 2A), consistent with the findings reported by Chávez et al. (2006), who also demonstrated that there is measurable feedback inhibition to rod bipolar cells that can be blocked by GABA receptor antagonists. Meanwhile, the difference between TBOA and TBOA-free responses demonstrated that EAAT5 plays a larger role in feedback inhibition; on average around 9.41 pC (Fig. 2B). Overall, these results allow us to make direct comparisons of the extent to which each source of inhibition impacts RBCs in response to physiologically relevant stimuli. They demonstrate that feedback onto RBCs is mediated by both conventional GABA- and glycine-gated channels and by EAAT5, but, under our conditions, that EAAT5 is the larger mediator of feedback inhibition (Fig. 2G, left) (2.25-fold greater charge difference, P = 0.049, n = 24 cells). The two types of feedback inhibition also differed in their kinetics, with conventional feedback inhibition, mediated by GABA and glycine, demonstrating a faster onset and time to peak than EAAT5-mediated feedback (Fig. 2F). We also measured the lateral inhibitory responses of these cells while stimulated with light to compare lateral inhibition under similar conditions of resting membrane potential and ECl (Fig. 2, C and D). In agreement with our earlier findings, these results also confirm that lateral glutamate spillover activation of EAAT5 does not play any significant role in inhibition. Instead, lateral inhibition is almost entirely mediated by conventional GABA and glycine receptors (Fig. 2G, right) (6.19-fold greater charge difference, P = 0.003, n = 10 cells).
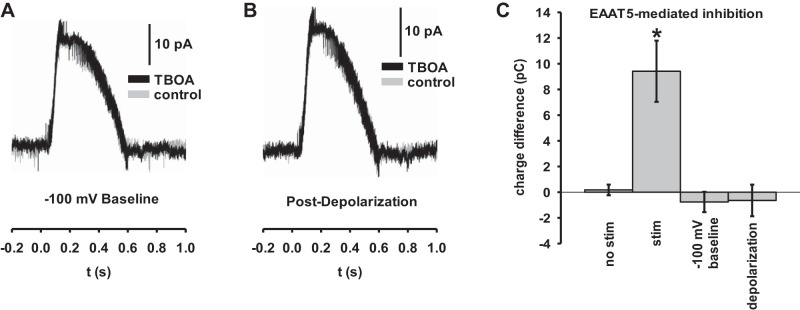
To demonstrate that the difference between the TBOA and TBOA-free traces in our feedback inhibition experiments were strictly attributable to glutamate release from the rod bipolar cell of interest, we performed two experiments that reduced or eliminated glutamate release. In the first, we held the cell at a reduced baseline of −100 mV and applied an electrical stimulus of the same amplitude, duration, and shape as before, to keep the peak stimulus potential below the threshold for vesicular release (Fig. 3A). In the second, we depolarized the cell to zero potential for an extended period of time to deplete the readily-releasable vesicle pool before stimulation, and then briefly returned the cell to −60 mV and applied the standard stimulus (Fig. 3B). In both cases, the TBOA-blockable effect was abolished (Fig. 3C) (n = 24 cells), confirming that the observed role of EAAT5 in feedback inhibition is dependent on synaptic vesicle release.
Fig. 3.
Excitatory amino acid transporter 5 (EAAT5)-mediated feedback inhibition is dependent on synaptic activation and glutamate release. A: rod bipolar cells (RBCs) stimulated from a baseline of −100 mV with the same voltage pulse as above, without (gray) and with (black) extracellular application of dl-threo-β-benzyloxyaspartic acid (TBOA). A lack of TBOA-sensitivity confirms that the feedback inhibition attributed to EAAT5 is dependent on synaptic activation. B: RBCs stimulated from a baseline of −60 mV with the same voltage pulse as above immediately following depolarization to −30 mV for 60 s. Lack of TBOA-sensitivity confirms that EAAT5-mediated feedback is dependent on vesicle release. C: summary data representing the difference in charge transfer between two comparitor conditions in individual cells. No stim indicates a cell held at −60 mV without applying a voltage stimulus. Stim, −100 mV baseline, and depolarization indicate experiments depicted in Fig. 2C and here in A and B, respectively. (n = 24 cells). Error bars indicate SE. *P < 0.05.
RBC inhibition is mediated by both conventional transmitters and EAAT5 at all light intensities.
Our work here suggests that spillover activation of EAAT5 is minimal, and previous work from our laboratory has suggested that in the dark-adapted retina, EAAT5-mediated inhibition is only elicited by high-intensity light stimuli (Ichinose and Lukasiewicz 2012). However, Veruki et al. (2006) showed that there was spontaneous activation of EAAT5, consistent with low intensity light activation of EAAT5. To reconcile these differences, we speculated that in prior work, when GABA- and glycine-mediated inhibition was blocked throughout the retina, downstream excitatory postsynaptic currents were enhanced (Eggers and Lukasiewicz 2011; Sagdullaev et al. 2011), causing increased EAAT5-mediated inhibition via greater glutamate release. To test this hypothesis, we used bath-applied GABA and glycine antagonists to characterize the level of EAAT5-mediated inhibition in response to light stimuli across a wide range of intensities. We found that EAAT5-mediated inhibition occurred at both dim and bright light stimulus intensities (Fig. 4, A and B) (n = 43 cells). Interestingly, GABA and glycine receptor blockers did not alter the light-evoked inhibitory currents at dim stimuli (10 and 100 photons·µm−2·s−1, suggesting they did not enhance the probability of glutamate release. These results differ from earlier work that showed that EAAT-mediated inhibition only occurred at bright light intensities (Ichinose and Lukasiewicz 2012).
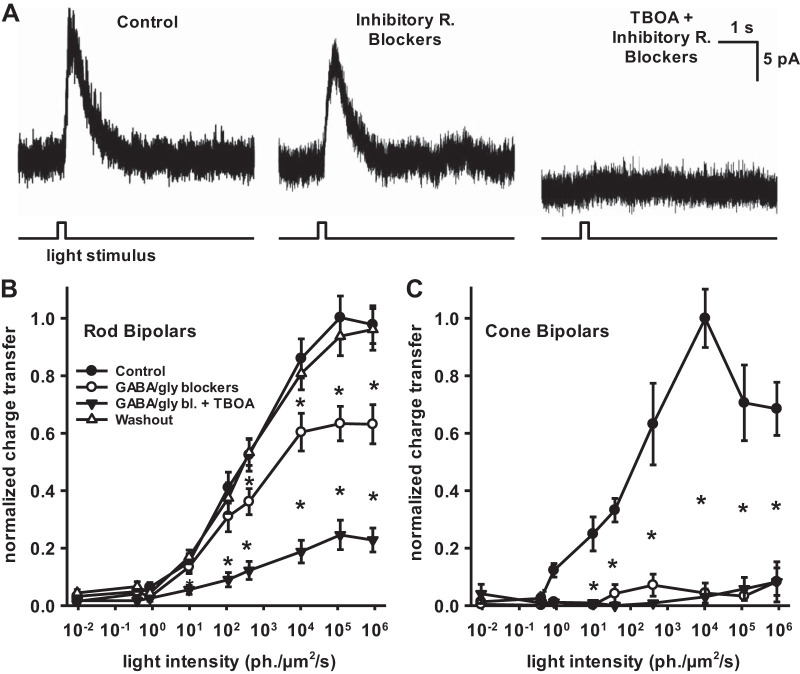
Fig. 4.
The mode of light-evoked inhibition to rod bipolar cells does not depend on light intensity. Inhibitory postsynaptic currents (IPSCs) evoked by a brief (200 ms) flash of light from bipolar cells voltage clamped at 0 mV and bath-applied inhibitory receptor antagonists. A: example rod bipolar cell (RBC) IPSCs evoked by a dim flash of light (400 photon·µm−2·s−1) from a black background were blocked very little by a mixture of GABA and glycine (gly) blockers [50 µM bicuculline, 50 µM 1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid hydrate (TPMPA), and 1 µM strychnine]. Subsequent addition of the EAAT antagonist dl-threo-β-benzyloxyaspartic acid (TBOA) (50 µM) blocked the current. B: normalized inhibitory response by light intensity for RBCs. All stimuli were presented in the context of a zero-light background. Conventional inhibitory receptor blockers (open circles) decreased inhibition at brighter light intensities compared with controls (filled circles), but TBOA (filled triangles) caused a larger reduction in inhibition that was significant at all light intensities above 10 photon·µm−2·s−1 (n = 43 cells). C: normalized inhibitory response by light intensity for ON cone bipolar cells. Conventional inhibitory receptor blockers alone (open circles) completely ablated the inhibitory response compared with controls (filled circles) (n = 4 cells) Error bars indicate SE. *P < 0.05.
The EAAT5-mediated current was unique to RBCs, as conventional inhibitory blockers eliminated all inhibition to cone bipolar cells (Fig. 4C) (n = 4 cells). This is consistent with previous work showing that EAAT5 is found in rod but not cone bipolar cells (Ichinose and Lukasiewicz 2012; Wersinger et al. 2006). All of these experiments were performed in dark-adapted retinas, and the dimmest stimuli that elicited an inhibitory response in either rod or cone bipolar cells were below cone sensitivity, suggesting that rod input can contribute inhibition onto cone pathways in response to dim stimuli (Bloomfield and Dacheux 2001; Sharpe and Stockman 1999; Trexler et al. 2005).
EAAT5 and synaptic feedback inhibition operate synergistically.
Feedback inhibition in general is important to ensure that signal transmission does not saturate, acting as a gain control to keep a synapse within the sensitive range of activation. We wondered why the RBC-AII synapse requires two separate feedback systems to accomplish this task. Previous work has established that RBC synapses are biphasic, with a transient excitatory component that encodes increases in contrast, and a sustained excitatory component that encodes luminance, mediated by depletion of the readily-releasable vesicle pool (Oesch and Diamond 2011). We speculated that two types of feedback inhibition with differing kinetics could be more efficient at tailoring fine control of the complex RBC synapse. To investigate this question, we compared the outputs of an in silico model of the RBC-AII amacrine cell synapse when given inputs modulated by the inhibitory conductances equal to the average isolated conductance from our experiments with real cells in the presence of bath-applied blockers (see Fig. 2F). An excitatory conductance was calibrated so that, in the presence of normal levels of both EAAT5 and GABA feedback inhibition, the RBCs would have a normal depolarization (see Fig. 2E), and moderate signaling to the AII resulted in an unsaturated excitatory postsynaptic current (EPSC) (Fig. 5A, “control”). When that same excitatory conductance was used to stimulate the RBC in the absence of either EAAT5 or conventional GABA and glycine feedback inhibition, our model RBC unsurprisingly demonstrated an increased depolarization. In the absence of conventional inhibitors, the response of the RBC had an exaggerated early peak (Fig. 5A, top, “GABA/gly blockers”), whereas in the absence of EAAT5 the late response of the RBC was enlarged instead (Fig. 5A, top, “TBOA”). In either case, the downstream AII EPSC response was substantially increased (Fig. 5A, bottom). In the absence of conventional feedback, the AII EPSC immediately peaked at the saturation level of the synapse before falling into the more normal range. Without EAAT5 feedback, the delayed AII EPSC had a 20% smaller peak than the case without conventional blockers. This was probably due to the greater vesicle depletion that occurred in the absence of EAAT5 inhibition, slightly blunting the amplitude of the delayed peak AII EPSC. Nevertheless, the EPSC was still 2.60-fold larger than the peak amplitude of the control AII, and a new sustained component appeared, which lasted ~100 ms longer than the phasic EPSC of the control AII.
Fig. 5.
In silico model of the rod bipolar cell (RBC)-AII amacrine synapse. Readily-releasable pool of 7 vesicles. A: a virtual RBC was stimulated with an excitatory conductance in the presence or absence of each category of inhibitory conductance recorded from real cells. Top, the rod bipolar voltage response. In the absence of conventional feedback inhibition (“GABA/gly blockers”), the absence of excitatory amino acid transporter 5 (EAAT5) inhibition [dl-threo-β-benzyloxyaspartic acid (“TBOA”)], or the absence of any inhibition (“no inhibition”), the cell depolarizes excessively in response to a fixed excitatory conductance. In the presence of both forms of inhibition (“control”), the cell elicits a moderate voltage response. Bottom: the excitatory postsynaptic current (EPSC) of the downstream AII amacrine cell. In the presence of both forms of inhibition (“control”), the AII receives a moderate unsaturated transient current without a sustained phase. In the absence of conventional feedback inhibition (“GABA/gly blockers”), a rapid peak response becomes immediately saturated. In the absence of EAAT5 inhibition (“TBOA”), the peak response is also too large, and is followed by an abnormal plateau phase. B: the same virtual RBC, except that inhibition has been scaled up in amplitude. Each trace maintains the same total inhibitory conductance as the control, but the kinetics are purely one form of inhibition or the other. Control data shown in light gray. Top, the rod bipolar voltage response. When presynaptic feedback is mediated entirely by conventional receptors (“GABA/gly”), peak excitation of the RBC is shifted slightly later. When mediated entirely by EAAT5 (“EAAT5”), excitation is shifted earlier. In both cases the voltage reaches a slightly higher peak. Bottom: the AII amacrine cell EPSC. The timing of the peak AII excitation matches the peak voltage for each condition, but in both cases the amplitude of excitation is substantially increased from baseline. C: same as in B, but with each form of inhibitory conductance scaled up an additional 50%. At this level, conventional inhibition alone (GABA/gly) has finally been scaled up enough to dampen the peak of the EPSC, resulting in a delayed but unsaturated positive signal. EAAT5 alone is still inadequate to regulate the initial onset of the signal.
This suggested to us that the dual roles of these inhibitory systems are related to their differing kinetics. However, since removing either pathway results in an exaggerated response, we wanted to see whether two different forms of inhibition are truly necessary. To that end, we again ran the model with only one type of inhibition or the other, but this time we linearly scaled the amplitude of each type of inhibitory conductance to match the total inhibitory conductance of the normal control (Fig. 5B). Specifically, in the case of EAAT5, the already substantial inhibitory conductance was multiplied by a factor of only 1.39 of normal, while in the case of GABA, the relatively smaller inhibitory conductance needed to be scaled up 3.59-fold. This reflected a theoretical scenario in which the RBC has access to only one type of inhibition but is capable of increasing the inhibition it expresses to compensate for that loss of the other type of inhibition. In these hypothetical cells, the kinetics of excitation at the RBC were shifted modestly, but in both cases the AII EPSC was substantially larger than the control. In the cell with only conventional inhibition (GABA/gly), the peak AII EPSC was delayed but still substantially exceeded the level of a normal cell. In the cell with only EAAT-mediated inhibition, EAAT5 alone was not sufficient to prevent the EPSC from saturating. Thus, even in the presence of adequate total amounts of feedback inhibition, neither conventional inhibition nor EAAT5 inhibition alone would adequately dampen signaling passing through the rod bipolar-AII amacrine synapse.
Would increasing the individual component inhibitory feedback inputs even more prevent AII EPSC saturation? To see if our model cells could prevent response saturation even with additional compensation, we scaled up the level of inhibition even further, so that the total amount of either EAAT5 or conventional inhibition equaled 150% of the normal total inhibitory conductance onto the cell (Fig. 5C). Conventional GABA and glycine inhibition was finally able to dampen the AII EPSC, but by this point conventional inhibition had now been multiplied to around 5.38 times its normal levels. Reaching 150% of total inhibitory conductance with EAAT5 alone meant an ~2.08-fold increase in EAAT-mediated inhibition relative to normal, and even at that level, the slow kinetics of EAAT5 meant that the cell was still unable to prevent a rapid saturation of the AII EPSC. Because the onset of EAAT5-mediated inhibition is delayed relative to the peak of the excitatory input of the cell, we found that if EAAT5 was the only inhibitory conductance it would have to be multiplied to around 13-fold of the total normal inhibitory conductance (~18 times EAAT5 normal level) to allow the same peak EPSC amplitude as normal.
Because the normal kinetics of the RBC-AII synapse are known to be largely controlled by the status of the readily releasable pool (RRP) (Oesch and Diamond 2011), we wanted to ensure that our findings were not an artifact of the value of RRP we had chosen for the default model (7 vesicles). To that end, we reran the model with an RRP of 20 vesicles (Supplemental Fig. S1; https://doi.org/10.6084/m9.figshare.11974395).
While this change unsurprisingly did have an effect on the shape of the AII EPSC under control conditions compared with a smaller value for RRP, our overall findings from isolating and amplifying EAAT5 or conventional inhibition were unchanged.
Our modeling results demonstrate that conventional GABA and glycine receptor-mediated inhibition would need to be significantly increased to compensate for the loss of the EAAT5 channel. Likewise, the cell would also require greatly increased levels of EAAT5-mediated inhibition to substitute for the loss of conventional GABA- and glycine-mediated inhibition. Together, the models demonstrate that conventional inhibition and EAAT5 work synergistically to balance activation of the synapse far more efficiently than either could individually.
DISCUSSION
Gain modulation in sensory-system neurons is important for transmitting information on stimulus changes despite a wide range of stimulus intensities. The glutamate-activated inhibition of EAAT5 makes it a natural candidate for gain modulation. This study characterizes the relative contribution of EAAT5-mediated inhibition onto RBCs in response to physiologically relevant stimuli. It also compares the roles of EAAT5-mediated feedback and lateral, spillover inhibition. While conventional inhibitors mediate nearly all lateral inhibition onto RBCs, by contrast, EAAT5 primarily mediates RBC feedback inhibition and has a substantial impact on postsynaptic excitation of the downstream AII amacrine cell. Overall, these findings greatly increase our understanding of the function of EAAT5-associated chloride channels in RBCs.
When studying lateral networks in slice preparations rather than wholemount retinas, there is always a concern that lateral connections may be severed during dissection. Although our experiments here were performed in slice preparations, in our hands we have consistently found that 400-µm slices allow for relatively easy access to retinal bipolar cells while preserving a sufficient number of network connections. Cook et al. (1998) showed that lateral inhibition was larger in 400-µm-thick slices versus 200-µm-thick slices. In this paper, our observation of substantial conventional lateral inhibition demonstrates that our slices have maintained a significant amount of lateral inhibition.
In the past, extensive EAAT-mediated spillover transmission in RBCs has been reported (Ichinose and Lukasiewicz 2012; Veruki et al. 2006). These findings depended on either excessive direct stimulation of the neighboring cell or network-wide blockade by conventional inhibitors, which, as we demonstrate here, leads to nonphysiologic enhancement of EAAT5 signaling through disinhibition of bipolar cells throughout the network. Ichinose and Lukasiewicz (2012) demonstrated that EAAT5-mediated inhibition onto RBCs is spatially limited, consistent with the understanding that for glutamate to be released near the RBC synapse necessitates synaptic activity from either the RBC in question or a neighboring RBC close enough to lead to local, lateral spillover inhibition, which would preclude any form of long-distance lateral inhibition. Our results here are consistent with this understanding and further expand these findings by demonstrating, for the first time, that under physiological stimulus conditions and in the absence of any exogenous network blockade of GABA or glycine, EAAT5 does not play a significant role in lateral inhibition.
Nonetheless, we have demonstrated an important role for EAAT5 in RBCs: feedback inhibition. It is well characterized that conventional feedback onto RBCs is mediated by A17 amacrine cells, which form a reciprocal GABAergic synapse with them (Dong and Hare 2003; Grimes et al. 2010, 2015). Up to this point, however, study of total feedback inhibition onto RBCs has not been reported. While the existence of EAAT5-mediated autoinhibition has been previously reported, we are the first to simultaneously compare and quantify the relative contributions of conventional versus EAAT5-mediated feedback inhibition at the RBC. Our findings demonstrate that the relatively overlooked EAAT5 pathway is actually the more prominent source of feedback inhibition in the dark-adapted RBC.
Why was not the role of EAAT5 observed before at low light intensities? Earlier work suggested that EAAT5 was activated at only bright intensities, but the spontaneous EAAT5-mediated events of Veruki et al. (2006) suggested that EAAT5 should be activated at low light intensities as well. In the time since the earlier light stimulus experiments were performed, the experimental methodology has changed. Altered experimental conditions include the wait time between cellular break-in and beginning the experiment, presence or absence of glutamine in the extracellular media, the inclusion of pyruvate and lactate in the perfusion solution, the delay between application of flashes, the thickness of the slice preparations, duration of the light stimuli, temperature of the prep, use of dorsal versus ventral retina, and color of the presented light. All of these changes enhance the magnitude of the light responses. Ultimately, to assure ourselves of the validity of our findings, we assayed more than 10 times as many cells as the matching previous experiments, and our new findings presented here are also more consistent with the work of Veruki et al. (2006).
Finally, our results propose an answer to the question: why do rod bipolar cells require two different forms of feedback inhibition? EAAT-mediated inhibition onto RBCs was already known to have slower kinetics than conventional inhibition (Eggers and Lukasiewicz 2006b; Ichinose and Lukasiewicz 2012). Since then, it has been possible to speculate that the need for two different feedback systems was based around the need for both fast and slow components. However, designing an in vivo experiment to actually provide evidence for this kind of “why?” hypothesis is challenging. We have instead leveraged the strengths of computer modeling to investigate this question. In this paper we have shown that the differing kinetics of conventional inhibitory transmitters and EAAT5-mediated inhibition are functionally relevant to the cell’s ability to exert control over the complex temporal properties of the RBC-AII synapse. Our work demonstrates that EAAT5-mediated inhibition acts synergistically with conventional A17-mediated inhibition. A rod bipolar cell with access only to the faster onset, less sustained conventional inhibition is highly inefficient at inhibiting the slower component of the RBC synapse without completely suppressing the normal rapid onset of the signal. In contrast, a rod bipolar cell with access only to slower EAAT5-mediated feedback cannot prevent the rising phase of the signal from rapidly saturating. Together, a balance between the two forms of inhibition allow the cell to define a sophisticated temporal inhibitory feedback far more efficiently than either pathway alone.
We speculate that at very low light intensities the delayed feedback inhibition from EAAT5 may act to enhance the relatively slow single-photon signal in the rod pathway. At low light intensities (~10−3–10−1 photons·µm−2·s−1), the RBC is tuned to transmit single photon signals from rods. Others have shown that the transient timing of the RBC vesicle release function is the correct duration to pass a single photon signal (Oesch and Diamond 2011; Singer and Diamond 2003). EAAT5 feedback may be just delayed enough to allow the single-photon transient to pass and then inhibit the RBC to counter long-term baseline depolarization. Thus, by regulating RBC baseline depolarization slowly, EAAT5 may prevent depletion of the readily-releasable pool and maintain the right level of depolarization to allow single-photon signals to be transmitted. At medium backgrounds (~10−1–102 photons·µm−2·s−1), EAAT5 may instead be acting to allow the contrast encoding described by Oesch and Diamond (2011). At brighter backgrounds than that, RBCs are highly depolarized, and their Ca2+ channels are likely to be inactivated (Jarsky et al. 2011; Grimes et al. 2014). Future work using a combination of patch-clamp experiments and computer modeling would be well suited to determine whether this is indeed the case.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.W.B. and P.D.L. conceived and designed research; G.W.B. and J.D. performed experiments; G.W.B. and J.D. analyzed data; G.W.B., J.D., R.G.S., and P.D.L. interpreted results of experiments; G.W.B. prepared figures; G.W.B. and P.D.L. drafted manuscript; G.W.B., J.D., R.G.S., and P.D.L. edited and revised manuscript; G.W.B., J.D., R.G.S., and P.D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by National Eye Institute Grant EY-022070 (to R.G.S.), EY-023766 (to R.G.S.), EY-08922 (to P.D.L.), EY-013360 (to G.W.B.), and EY-02687 and Research to Prevent Blindness (Dept. of Ophthalmology, Washington University). We thank Drs. Ichinose, Kerschensteiner, and Rajagopal for helpful discussion and comments on this manuscript.
REFERENCES
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA 94: 4155–4160, 1997. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20: 351–384, 2001. doi: 10.1016/S1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Chávez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci 30: 2330–2339, 2010. doi: 10.1523/JNEUROSCI.5574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature 443: 705–708, 2006. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Cook PB, Lukasiewicz PD, McReynolds JS. Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. J Neurosci 18: 2301–2308, 1998. doi: 10.1523/JNEUROSCI.18-06-02301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C-J, Hare WA. Temporal modulation of scotopic visual signals by A17 amacrine cells in mammalian retina in vivo. J Neurophysiol 89: 2159–2166, 2003. doi: 10.1152/jn.01008.2002. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci 26: 3959–3970, 2006. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a. doi: 10.1113/jphysiol.2005.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b. doi: 10.1523/JNEUROSCI.2591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci 28: 95–108, 2011. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasof S, Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. J Neurosci 13: 402–411, 1993. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol 83: 1817–1829, 2000. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaught MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 375: 599–603, 1995. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Erratum: Types of bipolar cells in the mouse retina. J Comp Neurol 476: 202–203, 2004a. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004b. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Schwartz GW, Rieke F. The synaptic and circuit mechanisms underlying a change in spatial encoding in the retina. Neuron 82: 460–473, 2014. doi: 10.1016/j.neuron.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron 65: 873–885, 2010. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Tian H, Graydon CW, Hoon M, Rieke F, Diamond JS. Complex inhibitory microcircuitry regulates retinal signaling near visual threshold. J Neurophysiol 114: 341–353, 2015. doi: 10.1152/jn.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. The mode of retinal presynaptic inhibition switches with light intensity. J Neurosci 32: 4360–4371, 2012. doi: 10.1523/JNEUROSCI.5645-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Cembrowski M, Logan SM, Kath WL, Riecke H, Demb JB, Singer JH. A synaptic mechanism for retinal adaptation to luminance and contrast. J Neurosci 31: 11003–11015, 2011. doi: 10.1523/JNEUROSCI.2631-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature 377: 734–737, 1995. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci 14: 1555–1561, 2011. doi: 10.1038/nn.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltedal L, Veruki ML, Hartveit E. Passive membrane properties and electrotonic signal processing in retinal rod bipolar cells. J Physiol 587: 829–849, 2009. doi: 10.1113/jphysiol.2008.165415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli V, Strettoi E. Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol 476: 254–266, 2004. doi: 10.1002/cne.20207. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL, Penfold P. Are neuronal transporters relevant in retinal glutamate homeostasis? Neurochem Int 37: 191–198, 2000. doi: 10.1016/S0197-0186(00)00022-X. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, Eggers ED, Purgert R, Lukasiewicz PD. Nonlinear interactions between excitatory and inhibitory retinal synapses control visual output. J Neurosci 31: 15102–15112, 2011. doi: 10.1523/JNEUROSCI.1801-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider N, Cordeiro S, Machtens J-P, Braams S, Rauen T, Fahlke C. Functional properties of the retinal glutamate transporters GLT-1c and EAAT5. J Biol Chem 289: 1815–1824, 2014. doi: 10.1074/jbc.M113.517177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Enroth-Cugell C. Visual adaptation and retinal gain controls. Prog Retinal Res 3: 263–346, 1984. doi: 10.1016/0278-4327(84)90011-7. [DOI] [Google Scholar]
- Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci 22: 497–504, 1999. doi: 10.1016/S0166-2236(99)01458-7. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Shimamoto K, Yasuda-Kamatani Y, Seal RP, Yumoto N, Nakajima T, Amara SG. Effects of threo-β-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5). J Neurochem 79: 297–302, 2001. doi: 10.1046/j.1471-4159.2001.00588.x. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci 23: 10923–10933, 2003. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG. NeuronC: a computational language for investigating functional architecture of neural circuits. J Neurosci Methods 43: 83–108, 1992. doi: 10.1016/0165-0270(92)90019-A. [DOI] [PubMed] [Google Scholar]
- Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. J Neurophysiol 93: 1476–1485, 2005. doi: 10.1152/jn.00597.2004. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat Neurosci 9: 1388–1396, 2006. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Wersinger E, Schwab Y, Sahel J-A, Rendon A, Pow DV, Picaud S, Roux MJ. The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. J Physiol 577: 221–234, 2006. doi: 10.1113/jphysiol.2006.118281. [DOI] [PMC free article] [PubMed] [Google Scholar]