Abstract
Four of the five types of mammalian mechanosensors are composed of nerve endings and accessory cells. In Caenorhabditis elegans we showed that glia support the function of nose touch neurons via the activity of glial Na+ and K+ channels. We show here that a third regulator of Na+ and K+, the Na+-K+-ATPase, is needed in glia of nose touch neurons for touch. Importantly, we show that two Na+-K+-ATPase genes are needed for the function rather than structural integrity and that their ion transport activity is crucial for touch. Finally, when glial Na+-K+-ATPase genes are knocked out, touch can be restored by activation of a third Na+-K+-ATPase. Taken together, these data show the requirement in glia of touch neurons of the function of the Na+-K+-ATPase. These data underscore the importance of the homeostasis of Na+ and K+, most likely in the space surrounding touch neurons, in touch sensation, a function that might be conserved across species.
NEW & NOTEWORTHY Increasing evidence supports that accessory cells in mechanosensors regulate neuronal output; however, the glial molecular mechanisms that control this regulation are not fully understood. We show here in Caenorhabditis elegans that specific glial Na+-K+-ATPase genes are needed for nose touch-avoidance behavior. Our data support the requirement of these Na+-K+-ATPases for homeostasis of Na+ and K+ in nose touch receptors. Our data add to our understanding of glial regulation of mechanosensors.
Keywords: C. elegans, glia, mechanosensation, Na+-K+-ATPase, touch
INTRODUCTION
The study of touch sensation in mammals has historically focused on the study of the response of sensory nerve endings to pressure. However, mammalian touch receptors are composed of nerve endings and accessory cells, including epithelial-derived cells in Merkel receptors and lamellar cells of Schwann origin in Pacinian and Meissner's corpuscles. In the last two decades, researchers have started to appreciate the contribution of accessory cells of mechanosensors to touch (Woo et al. 2015). For example, Maricich and colleagues showed that in a mouse model where Merkel cells do not develop the typical neurophysiological responses that are normally mediated by Merkel cell-neurite complexes are absent (Maricich et al. 2009). Moreover, Maksimovic and colleagues, using optogenetics, showed that Merkel cells are both necessary and sufficient to evoke firing of the αβ-low-threshold mechanosensors of the Merkel cell-neurite complex (Maksimovic et al. 2014). In addition, two other independent studies reported that Merkel cells are intrinsically mechanosensitive via activity of the mechanically gated channel Piezo2 (Ikeda et al. 2014; Woo et al. 2014). Less is known about the contribution of lamellar cells of Schwann origin to the function of Pacinian and Meissner's corpuscles, but Pawson and colleagues, using a combination of immunochemical and electrophysiological techniques, showed that GABA released by the lamellar cells regulates the output of the Pacinian mechanosensors during the static portion of sustained pressure (Pawson et al. 2009). Despite appreciation of the contribution of accessory cells of mechanosensors to touch sensation, we are still far from having a clear picture of their function, especially in the context of a living behaving organism.
The study of the cellular and molecular basis of touch sensation in the model organism Caenorhabditis elegans has helped to shed new light on this sensory mechanism (Bianchi 2007). In C. elegans, 18 of the 30 postulated mechanosensory neurons (Goodman 2006) are associated with glial sheath and socket cells (Altun and Hall 2010). We and others have shown that sheath and socket glia are needed for the function of the sensory neurons they ensheath, including the function of nose touch sensing neurons (Bacaj et al. 2008; Han et al. 2013; Procko et al. 2011; Singhvi et al. 2016; Wang et al. 2008, 2012). Indeed, we have shown that glial socket cells associated with nose touch sensory neurons express epithelial Na+ channel subunits DELM-1 and DELM-2 and that these channel subunits are required for neuronal responses to touch and nose-touch-avoidance behavior (Han et al. 2013). We have also shown that mechanosensory deficits caused by knockout of delm-1 are rescued by overexpression of the worm inward rectifier K+ channel IRK-2 in socket glia. These published results suggest that homeostasis of Na+ and K+ in glia is important for the function of the associated touch receptor neurons.
The primary regulator of the homeostasis of Na+ and K+ is the Na+-K+-ATPase, which is formed by an ion-transporting α-subunit and accessory subunits β and FXYD (Geering 2001, 2006). We thus hypothesized that disruption of the function of the Na+-K+-ATPase in glia associated with mechanoreceptors may lead to touch defects. Indeed, we show here that knockdown or knockout of two α-subunits of the Na+-K+-ATPases EAT-6 and CATP-1 causes reduced nose touch sensitivity. Importantly, in eat-6 and catp-1 mutants, glia do not degenerate and nose touch neurons maintain normal morphology, supporting that these pumps are needed for function rather than structural integrity. We also show that the ion transport activity is necessary specifically in the glia for response to nose touch and that when either eat-6 or catp-1 are knocked out, nose touch sensitivity can be restored by activation in glia of a third pump, CATP-2. Our work demonstrates the absolute requirement of the function of a Na+-K+-ATPase in glia associated with touch neurons for normal responses to touch, a function that might be conserved across species.
MATERIALS AND METHODS
Contact for reagents and resource sharing.
Please contact Dr. Laura Bianchi (lbianchi@med.miami.edu) for sharing of items used in this paper, including detailed experimental protocols and C. elegans strains.
Supplemental Figs. S1–2 are available at: https://doi.org/10.6084/m9.figshare.11984388.v1.
C. elegans growth and maintenance.
All experiments were performed using healthy, young adult hermaphrodites. Animals were grown on standard nematode growth medium (NGM) seeded with Escherichia coli strain OP50 and maintained at 20°C. Wild-type animals were N2 Bristol. Imaging and behavioral assays were performed using hermaphrodite animals, with males used only for crosses. For experiments requiring animals to be grown on enriched plates, standard NGM was prepared and supplemented with 150 mM of an osmolyte [glucose or α-methyl-d-glucopyranoside (α-MDG)] before being poured into Petri dishes and seeded. Animals were grown for 24 h on enriched plates before being used for experiments.
C. elegans strains.
Nematode strains used were as follows: RB1052 trpa-1(ok999) IV, DA467 eat-6(ad467) I, EN17 catp-1(kr17) I, CX3716 lin-15B&lin-15A(n765) kyls141[osm-9::GFP5 + lin-15(+)] X, EN1279 catp-1(kr17) I; krEx251[pAF88 CAA-1(D409E); pPD115.62 Pmyo-3::GFP], EN1281 catp-1(kr17) I; krEx253[pAF90 CAA-1(R669Q); pPD115.62 Pmyo-3::GFP], BLC273 blcEx110[Pdelm-2::eat-6 RNAi; Pdelm-2::GFP], BLC276 blcEx112[Pdelm-2::catp-1 RNAi; Pdelm-2::GFP], BLC278 blcEx113[Pdelm-2::catp-2 RNAi; Pdelm-2::GFP], BLC279 blcEx114[Pdelm-2::catp-3 RNAi; Pdelm-2::GFP], BLC285 blcEx117[Pdelm-2::catp-4 RNAi; Pdelm-2::GFP], BLC339 eat-6(ad467) I; blcEx206[Pdelm2::EAT-6; Pdelm2::GFP;Punc-122::GFP], BLC288 catp-1(kr17) I; blcEx123[Pdelm-2::CATP-1 cDNA; Pdelm-2::GFP; Punc-122::GFP], BLC327 catp-1(kr17) I; kyls141[osm-9::GFP5 + lin-15(+)] X, BLC329 eat-6(ad467) I; kyls141[osm-9::GFP5 + lin-15(+)] X, BLC333 trpa-1(ok999) IV; kyls141[osm-9::GFP5 + lin-15(+)] X, RB1177 delm-1(ok1226) IV, BLC330 catp-1(kr17) I; blcEx202[Pdelm-2::eat-6 RNAi; pGEM87::GFP], BLC334 catp-1(kr17) I; blcEx53 [Pdelm-1::DELM-1; Pdelm-2::GFP], BLC335 eat-6(ad467) I; blcEx205 [Pdelm-2::catp-2 RNAi; Punc-122::GFP], BLC353 catp-1(kr17) I; blcEx207 [Pdelm-2::CAA-1(D409E); vap-1::RFP], BLC372 catp-1(kr17) I; blcEx225 [Pocr-4::catp-1; Punc-122::GFP; Pvap-1::RFP], EN1343 ccIs4251 I; sid-1 (qt2) V; krEx265[pAF92 Pdpy-7::CATP-1 dsRNA; pPD115.62 Pmyo-3::GFP; pHU4 Prab-3::GFP; 1kb+], BLC53 blcEx38[Pdelm-1::RFP; Pdelm-2::GFP], BLC377 catp-1(ky17) I; blcEx226 [Pdelm-2::GFP; Punc-122::GFP], BLC378 eat-6(ad467) I; blcEx38 [Pdelm-1::RFP; Pdelm-2::GFP], EN712 catp-1(kr17) I; krEx45[Pkr17(briggsae)::cDNAkr17(elegans); sur-5-GFP], and BCL373 blcEx224 [Pcatp-2::catp-2 genomic; Pdelm-2::GFP;Punc-122::GFP].
Molecular biology.
For RNA interference we used the method described by Esposito and colleagues (2007). Briefly, we fused the promoter of delm-2 to sense and antisense 300–500 bp exon-rich regions of the eat-6, catp-1, catp-2, catp-3, and catp-4 genes by PCR. The conditions for the PCR fusion were as described by Hobert (2002). Sense and antisense DNA fragments were verified by agarose gel electrophoresis and coinjected at the concentration of 50–100 ng/ul with unc-122::gfp (25–50 ng/ul) into the gonads of young adults.
To build the EAT-6 and CATP-1 rescue constructs, we subcloned eat-6, catp-1, and (br) catp-1(D409E) cDNA into the pPD97.77 vector containing either the promoter of delm-2 for expression in OLQ and IL socket glia or the promoter of ocr-4 for expression in OLQ neurons (Han et al. 2013; Upadhyay et al. 2016). EAT-6 and CATP-1 cDNA were cloned by PCR using gene-specific primers designed according to the corresponding predicted sequences available on WormBase. All PCR products were initially cloned into TOPO vector for sequence verification and amplification. Overexpression of DELM-1 was achieved by injecting pPD97.77 containing the delm-1 cDNA sequence under the control of delm-2 promoter (Han et al. 2013). CATP-2 overexpression was achieved by injection of the PCR product corresponding to catp-2 genomic sequence plus ~1.7-kb sequence upstream of the ATG.
Preparation of Escherichia coli.
Escherichia coli strain OP50 was spread on plates and allowed to grow overnight at room temperature to provide a food source for maintaining C. elegans. To avoid inconsistencies in bacterial thickness or health on glucose and α-methyl-d-glucopyranoside (α-MDG) supplemented plates, a thick layer of bacteria was plated and allowed to dry immediately before plating eggs.
Behavioral assays.
All nose touch behavioral assays were performed blind to genotype and plate supplementation condition. Young adult animals were placed on plates seeded with a small lawn of OP50 and allowed 30 min for recovery. An eyelash hair was gently laid on the surface of the plate in front of a forward moving animal such that the animal encountered the eyelash perpendicular to its nose. A response was recorded if the animal reversed or moved the head away upon contact with the eyelash. Ten animals per strain were tested in each experiment. Each animal was tested five times with at least 30-s intervals between each touch. Data are displayed as responses given by each animal over the course of the five touches, with means obtained by averaging the response ratios from different animals.
Fluorescence microscopy.
For imaging OLQ and IL glial cells, animals expressing green fluorescent protein (GFP) were immobilized using 20 mM sodium azide on 2% agarose pads. Fluorescent images were obtained using Evos FL Auto 2 Imaging System (Invitrogen), equipped with a ×40 objective (Olympus), and accompanying Evos FL Auto 2 software. For imaging sensory cilia, animals expressing GFP were immobilized using 10 mM muscimol on 2% agarose pads. Fluorescent images were obtained using a Zeiss 710 Duo confocal microscope equipped with a GFP filter, ×63 Water C-Apochromat objective (1.2 NA), and ORCA-Flash 4.0 V2 digital CMOS camera with HCImage image acquisition software (Hamamatsu). Images were analyzed using ImageJ. Data were analyzed and plotted using GraphPad Prism.
ATP quantification.
ATP concentration was measured using the Promega ENLITEN ATP Bioluminescence assay kit (Madison, WI) as previously described (Han et al. 2012). Synchronized eggs were seeded on NGM plates or glucose-enriched plates seeded with autoclave killed OP50. Young adults were harvested after 60 h of culture at 20°C, washed three times with M9 buffer and three times with autoclaved H2O. Following removal of the water, RAPI buffer with Protein Inhibitor Cocktail was added to the samples and worm lysis was achieved by liquid nitrogen treatment and using a homogenizer. Protein concentration was determined using the Coomassie (Bradford) Protein Assays kit following the manufacturer’s protocol (Thermo Scientific, Rockford, IL). ATP concentration was determined following the instruction of ENLITEN ATP assay kit (Promega, Madison, WI). Briefly, 50 µL of 20× diluted samples were mixed with the 50 µL of rL/L reagent in a 96-well plate. Each sample was processed in duplicate. The bioluminescence was measured by the FLUOstar Omega microplate reader (Cary, NC). The average of each duplicate was used to calculate the ATP concentration based on the standard curve built using the following concentrations of ATP: 0, 0.01, 0.05, 0.1, 0.5, 5, and 10 nM.
Microscopy for body measurements.
Animals were immobilized using 20 mM sodium azide on 2% agarose pads. Images were obtained using an Evos FL Auto 2 Imaging System (Invitrogen), equipped with a ×20 objective (Olympus) and accompanying Evos FL Auto 2 software. Images were analyzed using ImageJ. Width measurements were made right behind the pharynx, while length measurements were made from the tip of the nose to the end of the tail of each animal. Data were analyzed and plotted using GraphPad Prism.
Quantitative real-time PCR.
The following methods were carried out as previously described by Sangaletti et al. (2017). Briefly, RNA was extracted from synchronized young adults grown in NGM, 150 mM glucose, and 150 mM α-MDG supplementation using TRIzol reagent (ThermoFisher) according to manufacturer instructions with the modification that disintegration of worms was performed by five consecutive cycles of freezing in liquid N2 and thawing at 37°C. RNA was eluted in RNase-free water and optical density at 260/280 (OD260/280) between 1.8 and 2.0 was confirmed by spectroscopy. cDNA synthesis was carried out using the High-Capacity RNA-to-cDNA kit (Applied Biosystems) according to manufacturer instructions. PCR amplification was carried out in a CFX Connect Real-Time PCR Detection System (Bio-Rad) using TaqMan Universal Master Mix II (Applied Biosystems). The following FAM dye labeled probes were used: delm-1 (assay ID Ce02457448_g1; no. 4448892; exon junctions 13–14); eat-6 (assay ID Ce02483601_g1; no. 4351372; exon junctions 4–5); catp-1 (assay ID Ce02405790_m1; no. 4448892; exons 9–10, 10–11); catp-2 (assay ID Ce02474812_m1; no. 4448892; exons 1–2); catp-3 (assay ID Ce02427236_g1; no. 4448892; exons 1–2); catp-4 (assay ID Ce02441692_g1; no. 4448892; exons 1–2); and pmp-3 (assay ID Ce02485188_m1; no. 4448892; exons 3–4, 4–5). The average mRNA fold change of each target gene was calculated using the threshold cycle (Ct) applied to the 2−ΔΔCt method, as described by Livak and Schmittgen (2001) and Pfaffl (2001), and was normalized using values of the housekeeping gene pmp-3. The calibrator used to assess fold change in gene expression was either N2 or the corresponding strain [eat-6(ad467) and catp-1(kr17)] on NGM. The RNA extraction and reverse transcription was performed on three independent experiments, and each sample was used for PCR reaction in triplicates.
Quantification and statistical analysis.
Statistical analysis of data was performed using GraphPad Prism. Statistical parameters for each experiment are provided within corresponding figure legends. Unless otherwise stated, data are expressed as means ± SE, with P values obtained by ANOVA with Bonferroni correction or by t test.
RESULTS
The Na+-K+-ATPase inhibitor ouabain reduces nose touch sensitivity in C. elegans.
We have previously shown that degenerins and epithelial Na+ channel (DEG/ENaC) subunits DELM-1 and DELM-2 are expressed in C. elegans OLQ and IL socket glia and are required for mechanosensory behaviors, including nose touch mediated by OLQ sensory neurons (Han et al. 2013). We also showed that the C. elegans weak inward rectifier K+ channel IRK-2 rescues nose touch sensitivity in delm-1 mutants (Wang et al. 2008, 2012).
As Na+ and K+ gradients are maintained across the plasma membrane by the Na+-K+-ATPase, we sought to determine if glial Na+-K+-ATPases were implicated in nose touch response. To this end, we incubated wild-type worms with the Na+-K+-ATPase blocker ouabain and assayed for nose touch (Fig. 1A). We found that animals incubated in 10 mM ouabain were significantly less responsive to nose touch than animals incubated in water alone. These results suggested the possibility that pump activity might be needed either in neurons or glia (or both) of touch receptors for nose touch sensitivity.
Fig. 1.

Specific Caenorhabditis elegans Na+-K+-ATPases are needed for response to nose touch. A: Ratios of responses in wild-type (N2) animals incubated for 60 min before assay in either water (0.753 ± 0.031) or 10 mM ouabain (0.420 ± 0.048). Each animal was tested 5 consecutive times with 30 s intervals and the ratio of times each animal responded is reported. The number of animals tested in each condition was 30 for H2O and 29 for ouabain. Data are expressed as mean ± SE; ****P < 0.0001 by t test. B: ratios of responses in wild type (N2, 0.768 ± 0.010), trpa-1(ok999) (0.337 ± 0.010), eat-6 glia RNAi (Pdelm-2::eat-6 RNAi;Pdelm-2::GFP, 0.608 ± 0.018), eat-6(ad467) (0.452 ± 0.022), eat-6 glia rescue (eat-6;Pdelm-2::EAT-6, 0.693 ± 0.036), catp-1 glia RNAi (Pdelm-2::catp-1 RNAi;Pdelm-2::GFP, 0.630 ± 0.025), catp-1(kr17) (0.452 ± 0.019), catp-1 glia rescue (catp-1;Pdelm-2::CATP-1, 0.760 ± 0.041), and catp-1;eat-6 glia RNAi (catp-1;Pdelm-2::eat-6 RNAi, 0.227 ± 0.036). Number of animals assayed was 408, 410, 89, 160, 30, 130, 100, 30, and 30 respectively. Data are expressed as mean ± SE; ****P < 0.0001 by ANOVA with Bonferroni correction. Statistically significant difference when compared with wild type (N2), unless otherwise indicated. There is no statistical difference between N2 and the rescue strains.
C. elegans Na+-K+-ATPase α-subunits EAT-6 and CATP-1 are needed in OLQ and IL socket glia for nose touch sensitivity.
There are five C. elegans Na+-K+-ATPase α-subunits genes (Davis et al. 1995). To determine which of these pumps might be involved in mechanosensation by functioning specifically in glia, we used cell-specific RNAi to individually knockdown expression in OLQ and IL socket glia of each one of these subunits and assayed nose touch sensitivity (Han et al. 2013). The knockdown of α-subunits eat-6 and catp-1 significantly decreased the response to nose touch (Fig. 1B), while knockdown of α-subunits catp-2, catp-3, and catp-4 did not have an effect on this phenotype (Supplemental Fig. S1A). These results support the need for the Na+-K+-pump α-subunit genes eat-6 and catp-1 in the OLQ and IL socket glia for nose touch sensitivity.
To confirm that glial EAT-6 and CATP-1 are needed for nose touch sensitivity, we acquired the null mutants eat-6(ad467) and catp-1(kr17) and performed nose touch behavioral assays (Fig. 1B). eat-6(ad467) consists of a point mutation that causes a single amino acid substitution (L359F) near the intracellular phosphorylation site, which reduces by 80% the level of phosphorylation, thereby reducing the activity of the pump (Shima et al. 1998). catp-1(kr17) is a Mos insertion that introduces a premature stop codon, which truncates the last 3 of 10 transmembrane domains of the pump α-subunit (Ruaud and Bessereau 2007). Both mutants were significantly less responsive to nose touch than wild-type animals and the reduced nose touch phenotype was more similar to that of nose touch-insensitive trpa-1(ok999) mutants (Kindt et al. 2007). TRPA-1 is a C. elegans transient receptor potential (TRP) channel expressed in OLQ neurons (among others), which participates in mechanosensitive responses in OLQ neurons (Kindt et al. 2007). Importantly, nose touch sensitivity of eat-6 and catp-1 was reestablished by rescuing each of the corresponding genes only in the OLQ and IL socket glia using the glial-specific promoter Pdelm-2 (Han et al. 2013). Conversely, expression of CATP-1 in OLQ neurons of catp-1 mutants did not rescue nose touch insensitivity, and knockdown of catp-1 in epidermal cells, which were shown to express CATP-1 (Ruaud and Bessereau 2007) did not cause defects in nose-touch-avoidance behavior (Supplemental Fig. S1B). eat-6 rescue in neurons and RNAi in epidermis were not successful despite injection of 700+ animals each. This result suggests that these two constructs were toxic in vivo, perhaps due to overexpression, improper expression, or need of eat-6 in epidermis during development. Taken together though, these results support that eat-6 and catp-1 are needed specifically in OLQ and IL glia for nose touch responses (Fig. 1B).
To determine whether the effect of the eat-6 and catp-1 mutations on nose touch sensitivity was additive, we performed experiments on catp-1 mutants in which eat-6 had been knocked down by RNAi in OLQ and IL socket glia (Fig. 1B). This strategy was used because several attempts at building eat-6;catp-1 double mutants failed, suggesting that the combination of null mutations in both genes is lethal, probably due to the genes’ wide expression pattern (Avery 1993; Davis et al. 1995; Lakowski and Hekimi 1998; Ruaud and Bessereau 2007; Shima et al. 1998,). We found that knockdown of eat-6 by glial-specific RNAi in catp-1 mutant animals further reduced the sensitivity to nose touch compared with that of each single mutant. Together, these results suggest that these two genes are not completely redundant. We also found that, like in trpa-1 animals, both eat-6 and catp-1 mutants had faster adaption of the residual nose touch sensitivity, suggesting that overall null mutations in these Na+-K+-ATPase α-subunit genes impair animal behavior similarly to mutations in neuronal gene trpa-1 (Supplemental Fig. S1, C–F). Taken together, these results show that the Na+-K+-pump genes eat-6 and catp-1 are needed in OLQ and IL socket glia for nose touch sensitivity in C. elegans.
OLQ and IL glia are grossly normal in eat-6 and catp-1 mutant animals.
Wild-type C. elegans have four OLQ and six IL socket glial cells. We previously published that these cells are present and structurally normal in delm-1 mutant animals (Han et al. 2013). To determine if disruption in glia of the function of either EAT-6 or CATP-1 prevented the glial cells from developing or surviving, we used the glia-specific promoter Pdelm-2 to express GFP in eat-6 and catp-1 mutants such that the OLQ and IL socket glial cells could be visualized (Fig. 2, A–C). We found that knockout of either pump did not result in loss of glial cells (Fig. 2D). These data support that the nose touch insensitivity of eat-6 and catp-1 is not due to lack of OLQ and IL glia in these animals.
Fig. 2.
The OLQ and IL glia of eat-6 and catp-1 mutants are grossly normal. A: fluorescent image of wild-type (N2) young adult-expressing green fluorescent protein (GFP) under the control of the promoter of delm-2 in the OLQ and IL socket glial cells. Scale bar = 25 µm. B and C: same as in A for eat-6(ad467) (B) and catp-1(kr17) (C) mutant animals. The tip of the nose is to the left, the cell bodies of some of the OLQ (OLQsoDL and VL) and IL (IlsoDL) socket cells are labeled. D: number of OLQ and IL socket cells expressing GFP under the control of the promoter of delm-2 in wild type (N2) (8.16 ± 0.222; n = 24 animals), eat-6(ad467) (8.28 ± 0.211; n = 25 animals), and catp-1(kr17) (7.95 ± 0.245; n = 20 animals). Images were taken at room temperature and cells were counted manually. Data are expressed as individual data points and mean ± SE.
Sensory cilia in eat-6 and catp-1 mutants.
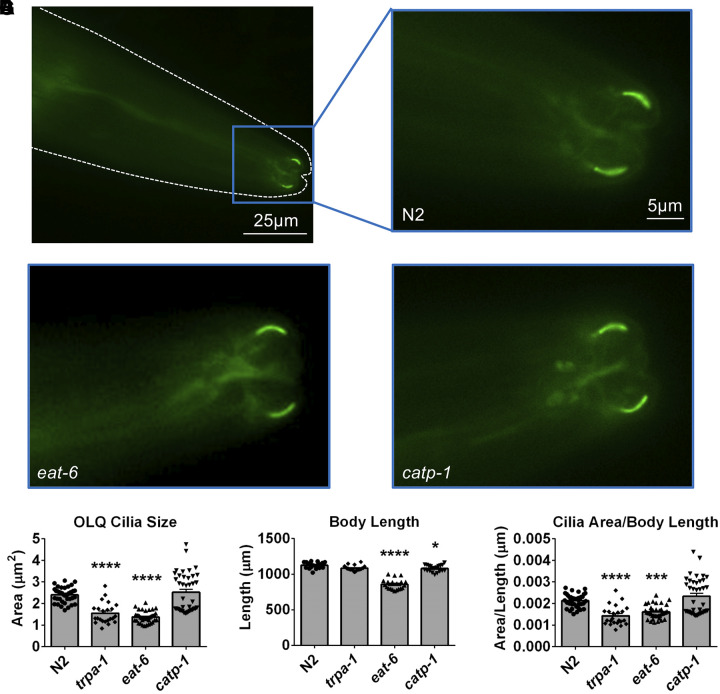
We next wondered whether mutations of these pumps caused structural changes in the OLQ neurons, and in particular the sensory endings, or cilia (Ward et al. 1975). To test this idea, we obtained a strain expressing the TRP channel OSM-9 tagged with GFP in the cilia of OLQ neurons (Colbert et al. 1997; Tobin et al. 2002). After crossing eat-6 and catp-1 with this fluorescent strain, we analyzed the shape and size of OLQ cilia (Fig. 3, A–D). We found that the OLQ cilia of eat-6 and catp-1 mutants were structurally intact. Comparison of their area showed that OLQ cilia were smaller in eat-6 but not in catp-1 mutants as compared with wild-type animals (Fig. 3E). Interestingly, nose touch-insensitive trpa-1 mutant animals also had smaller OLQ cilia area.
Fig. 3.
OLQ sensory cilia in pump mutants. A: fluorescent image of the head of a young adult wild-type (N2) worm expressing OSM-9::GFP in the dendritic endings of OLQ neurons. The tip of the nose is to the right, the dashed line is shown to outline the body of the worm. Scale bar = 25 µm. B: magnified picture of the fine structure of OLQ dendritic endings near the tip of the nose of the animal. Scale bar = 5 µm. C and D: same as in B for eat-6(ad467) and catp-1(kr17) animals. E: area in µm2 of OLQ cilia of wild type (N2, 2.396 ± 0.055; n = 39), trpa-1(ok999) (1.548 ± 0.101; n = 23), eat-6(ad467) (1.374 ± 0.042; n = 39), and catp-1(kr17) (2.522 ± 0.143; n = 39) young adult animals grown on NGM plates. ImageJ was used to measure area of cilia from fluorescence images. F: body length in µm of wild type (N2, 1124 ± 9.509; n = 20), trpa-1(ok999) (1,084 ± 7.965; n = 20), eat-6(ad467) (855.9 ± 16.150; n = 20), and catp-1(kr17) (1,080 ± 10.390; n = 20) young adult animals grown on NGM plates. G: cilia size normalized to average body length, expressed in µm, of wild type (N2, 2.133 × 10–3 ± 4.936 × 10–5; n = 39), trpa-1(ok999) (1.433 × 10–3 ± 9.382 × 10–5; n = 23), eat-6(ad467) (1.607 × 10–3 ± 4.891 × 10–5; n = 39, and catp-1(kr17) (2.336 × 10–3 ± 1.321 × 10–4; n = 39). GFP, green fluorescent protein. Data are expressed as each point representing a single measurement and means ± SE; *P < 0.05, ***P = 0.0001, ****P < 0.0001 by ANOVA with Bonferroni correction. Data were compared with measurements from wild-type (N2) animals.
eat-6 and, to a lesser degree, catp-1 mutant animals have shorter bodies than wild-type animals (Fig. 3F). After the area of the dendritic endings was normalized by the worm body length, the difference between wild-type and eat-6 or trpa-1 mutants was still significant (Fig. 3G). These results raised the possibility that the nose touch-insensitive phenotype of these mutants may result from decreased size of the OLQ nose touch neurons cilia. However, this is not a possibility for catp-1 mutants, as their OLQ cilia are similar in size to those of wild-type animals. We note that catp-1 cilia sizes showed a binomial distribution (Fig. 3, E and G). However, there was no binomial distribution in catp-1 nose touch-avoidance behavior (not shown), suggesting no correlation between cilia size and nose touch-avoidance behavior.
Causing reduction in size of the sensory cilia does not lead to nose touch insensitivity.
Because the OLQ sensory cilia were smaller in touch defective mutants trpa-1 and eat-6 (Fig. 3E), we wondered if change in cilia size could be responsible for the loss of nose touch sensitivity. We tested this idea by artificially causing cilia shrinkage and testing nose touch sensitivity in wild-type and mutants. To cause a decrease in cilia size we cultured C. elegans on plates supplemented with the nonmetabolizable sugar α-MDG. As expected, these growth conditions caused a significant shrinkage in body width (Fig. 4A) and OLQ cilia area (Fig. 4B) in all the strains. However, α-MDG did not lead to a decrease in sensitivity to nose touch in any of the C. elegans strains (Fig. 4C). These results indicate that reduced cilia area does not cause nose touch insensitivity per se and suggest that the nose touch insensitivity of eat-6 and trpa-1 mutants is attributable to other factors.
Fig. 4.
Shrinkage of sensory cilia by α-methyl-d-glucopyranoside (α-MDG) does alter nose touch sensitivity. A: ratio of width to length of animals grown on nematode growth medium (NGM) (−) and plates supplemented with 150 mM glucose and 150 mM α-MDG for wild type (N2, 0.045 ± 0.0004, 0.044 ± 0.0003 and 0.039 ± 0.0003; n = 20 for all 3 groups), trpa-1(ok999) (0.045 ± 0.0005, 0.043 ± 0.0005 and 0.035 ± 0.0004; n = 20 for all 3 groups), eat-6(ad467) (0.047 ± 0.0006, 0.045 ± 0.0005 and 0.041 ± 0.0008; n = 20 for all 3 groups), and catp-1(kr17) (0.043 ± 0.0005, 0.043 ± 0.0005 and 0.037; n = 20 for all 3 groups). Data are expressed as individual data points and means ± SE; ****P < 0.0001 by ANOVA with Bonferroni correction, comparing the growth conditions within each strain. B: cilia size normalized to average body length, expressed in µm of young adult animals grown on NGM (−) and plates supplemented with 150 mM glucose and 150 mM α-MDG for wild type (N2, 2.133 × 10–3, 2.038 × 10–3 ± 6.321 × 10–5, and 1.388 × 10–3 ± 4.007 × 10–5; n = 39 for all 3 groups), trpa-1(ok999) (1.428 × 10–3, 1.367 × 10–3 ± 9.353 × 10–5, and 0.448 × 10–3 ± 2.315 × 10–5; n = 23 for all 3 groups), eat-6(ad467) (1.606 × 10–3, 1.389 × 10–3 ± 3.235 × 10–5, and 1.072 × 10–3 ± 3.837 × 10–5; n = 39 for all 3 groups), and catp-1(kr17) (2.335 × 10–3, 2.019 × 10–3 ± 1.111 × 10–4, and 1.356 × 10–3 ± 8.277 × 10–5; n = 39, 43, and 41, respectively). Lighter grey bars indicate data presented in Fig. 3E. Data are expressed as means ± SE; ****P < 0.0001 by ANOVA with Bonferroni correction, comparing the growth conditions within each strain. C: ratio of responses to nose touch of animals grown on NGM (−) and plates supplemented with 150 mM α-MDG (+) for wild type (N2, 0.790 ± 0.034 and 0.770 ± 0.039), trpa-1(ok999) (0.190 ± 0.037 and 0.240 ± 0.037), eat-6(ad467) (0.320 ± 0.039 and 0.320 ± 0.030), and catp-1(kr17) (0.350 ± 0.032 and 0.280 ± 0.037). N was 20 each. Data are expressed as means ± SE; t test showed no statistical difference between the 2 growth conditions within each strain.
Na+-K+-ATPase ion transport function is needed for nose touch sensitivity.
We next wondered if the ion pump activity was required for response to nose touch. In the eat-6(ad467) mutant a residue near the phosphorylation site is mutated (L359F) causing reduction of the activity of the pump (Shima et al. 1998). This suggests that, at least in the case of EAT-6, the ion transport function is needed for nose touch sensitivity. However, the catp-1(kr17) mutant is a deletion leading to a truncated protein, raising the possibility that loss of other CATP-1 activities, unrelated to its ion transport function, could be needed for nose touch. To test whether CATP-1 ion transport activity is needed for its role in touch sensitivity, we obtained two strains expressing two CATP-1 mutants from Caenorhabditis briggsae (brCATP-1) expressed in the catp-1 mutant background. One of these strains has a mutation in the ATP binding site (strain EN1281, R669Q mutation) and the other a mutation in the phosphorylation site (strain EN127, D409E mutation) (Jacobsen et al. 2002; Ohtsubo et al. 1990; Pedersen and Carafoli 1987; Ruaud and Bessereau 2007) (Fig. 5A). In both of these transgenic strains, mutant (br)CATP-1 proteins are expressed but they lack ion transport function (Ruaud and Bessereau 2007). We found that both of these strains remained nose touch insensitive, just like catp-1 mutants. This was true also for a strain in which we expressed (br)CATP-1(D409E) specifically in OLQ and IL socket glia of catp-1 mutants using Pdelm-2 promoter. These results support that the ion transport function of the Na+-K+-ATPase is needed in glia for proper mechanosensation [compare with the normal nose touch sensitivity of catp-1;Pdelm-2::CATP-1 C. elegans, Fig. 1B, and with catp-1;Pcatp-1(br)::CATP-1, Fig. 5B].
Fig. 5.

Ion transport activity by the Na+-K+-ATPase is required for nose touch sensitivity. A: cartoon schematic of a Na+-K+-ATPase showing the intracellular sites for ATP-binding and phosphorylation that are mutated in CAA-1(R669Q) and CAA-1(D409E) rescue gene constructs. CAA-1 is the Caenorhabditis briggsae homolog of CATP-1, labeled in the figure as CATP-1(br). ECF and ICF, extracellular and intracellular fluid. B: ratios of responses in wild type (N2, 0.766 ± 0.021, n = 70), trpa-1(ok999) (0.220 ± 0.028; n = 40), catp-1(kr17) (0.340 ± 0.024; n = 70), catp-1(kr17);Pcatp-1(br)::CATP-1 (0.700 ± 0.053; n = 20), catp-1(kr17);Pcatp-1(br)::CATP-1(br)(R669Q) (0.330 ± 0.033; n = 20), catp-1(kr17);Pcatp-1(br)::CATP-1(br)(D409E) (0.340 ± 0.041; n = 20), and catp-1(kr17);delm-2::CATP-1(br)(D409E) (0.353 ± 0.027; n = 30). Data are expressed as means ± SE; ****P < 0.0001 by ANOVA with Bonferroni correction, comparing each strain with wild type (N2).
CATP-2 and nose touch sensitivity in pump mutants.
Our results show that the activity in OLQ and IL glia of EAT-6 and CATP-1 Na+-K+-ATPase α-subunits is needed for nose touch. C. elegans expresses three other genes with similarity to eat-6 and catp-1 (Supplemental Fig. S1A). Are Na+-K+-ATPase α-subunits interchangeable in their role in nose touch sensitivity? If so, this would further support that transport of Na+ and K+, which is the function shared by all five of these proteins, is what is needed in glia for touch sensitivity. To test this idea, we first wanted to establish using real-time PCR (RT-PCR) the basal level of expression of catp-2, catp-3, and catp-4 in eat-6 and catp-1 mutant backgrounds. This was necessary because upregulation of the expression of certain genes have been documented in mutant backgrounds across species, including C. elegans (Raj et al. 2010). We found that catp-2 mRNA levels were highly upregulated in both eat-6 (39.1 ± 12.4 times, mean ± SE, n = 3) and catp-1 (2,260 ± 487 times, mean ± SE, n = 3) mutants (Fig. 6, A and B). However, such upregulation of catp-2 mRNA does not lead to compensation, given that eat-6 and catp-1 mutants have reduced nose touch sensitivity (Fig. 1B). Furthermore, overexpression of CATP-2 (219 ± 66.9 times, mean ± SE, n = 3) per se does not cause nose touch insensitivity (Fig. 6C, 3rd column).
Fig. 6.
Rescue of nose touch sensitivity in eat-6 and catp-1 mutants by glucose and dependence on CATP-2. A and B: relative levels of eat-6, catp-1, catp-2, catp-3, catp-4, and delm-1 mRNA measured by qRT-PCR in (A) eat-6(ad467) (1.288 ± 0.065, 1.003 ± 0.054, 39.14 ± 12.47, 0.91 ± 0.114, 1.79 ± 0.515, and 19.420 ± 4.474, respectively) and catp-1(kr17) (2.221 ± 0.245, 1.697 ± 0.275, 2260 ± 487.7, 8.687 ± 3.787, 0.283 ± 0.169, and 4.743 ± 0.570, respectively) (B). Animals were grown on nematode growth medium (NGM) plates and mRNA levels reported represent the fold-change compared with those present in wild-type (N2) animals. Data are expressed as means ± SE; *P < 0.05, ****P < 0.0001 by ANOVA with Fisher’s least significant difference (LSD) test. The RNA extraction and reverse transcription was performed on 3 independent experiments, and each sample was used for PCR reaction in triplicates. C: ratio of responses to touch of animals grown on NGM (−) and plates supplemented with 150 mM glucose (+) for wild type (N2, 0.773 ± 0.016 and 0.769 ± 0.016; n = 110 and 129, respectively), Pcatp-2::CATP-2 (0.83 ± 0.033, n = 20), trpa-1(ok999) (0.186 ± 0.018 and 0.232 ± 0.017; n = 100 and 100, respectively), eat-6(ad467) (0.310 ± 0.020 and 0.705 ± 0.028; n = 40 and 40, respectively), eat-6;Pdelm-2::catp-2 RNAi (0.247 ± 0.033 and 0.347 ± 0.034; n = 30 and 30, respectively), catp-1(kr17) (0.322 ± 0.028 and 0.651 ± 0.021; n = 40 and 90, respectively), catp-1(kr17);Pcatp-1(br)::CATP-1(br)(R669Q) (0.330 ± 0.033 and 0.540 ± 0.048; n = 20 and 20, respectively), catp-1(kr17);Pcatp-1(br)::CATP-1(br)(D409E) (0.340 ± 0.041 and 0.340 ± 0.41; n = 20 and 20, respectively), catp-1(kr17);delm-2::CATP-1(br)(D409E) (0.353 ± 0.027 and 0.427 ± 0.039; n = 30 and 30, respectively), delm-1(ok1226) (0.310 ± 0.049 and 0.330 ± 0.039; n = 20 and 20, respectively), and catp-1;Pdelm-2::DELM-1 (NGM only: 0.330 ± 0.051; n = 20). Bars with dashed borders indicate data presented in Fig. 5B. Data are expressed as means ± SE; ****P < 0.0001 by ANOVA with Bonferroni correction.
To determine whether lack of compensation was due to lack of activation of CATP-2, we sought to activate this pump by increasing the concentration of glucose in cells. To do so, we cultivated worms on plates containing 150 mM glucose (Wang et al. 2018). These conditions restored the nose touch sensitivity of both eat-6 and catp-1 mutants (Fig. 6C). Interestingly, when we compared the levels of ATP in animals cultivated under standard conditions versus glucose enriched plates, we found no difference (Supplemental Fig. S2A). These data suggest that rescue by glucose is not via increase of overall concentration of ATP in cells.
To test whether rescue required the activity of catp-2, we performed nose touch in eat-6 mutant grown on glucose enriched plates in which catp-2 had been knocked down by RNAi in OLQ and IL glia. We found no rescue by glucose in these animals (Fig. 6C), suggesting requirement of catp-2. When we assessed rescue by glucose in the CATP-1 R669Q and D409E mutant strains though, we found a substantial difference. While glucose rescued nose touch avoidance in catp-1;(br)CATP-1(R669Q) mutants, it did not in catp-1;(br)CATP-1(D409E). We do not know what causes this difference as catp-2 mRNA is upregulated in the D409E mutant like it is in catp-1 mutants (Supplemental Fig. S2B). However, it is interesting to note that, contrary to CATP-1(R669Q), CATP-1(D409E) is still capable of binding ATP (Fig. 5A) (Jacobsen et al. 2002). Importantly, DELM-1 cannot rescue the nose touch-insensitive phenotype of catp-1 mutants when overexpressed in glia, supporting that overexpression of a Na+ channel cannot compensate for the lack of the Na+/K+ pump, which is responsible for both Na+ and K+ homeostasis (Fig. 6C).
To establish whether the rescuing effect of glucose on the nose touch avoidance of eat-6 and catp-1 mutants was due to effects on the size of the cilia, we quantified body size and cilia area in animals grown on glucose. We found no effect of glucose on body size (Fig. 4A) and a small effect on the size of eat-6 cilia, which are slightly shrunk in glucose (Fig. 4B). These data, together with data obtained from animals grown on α-MDG (Fig. 4, A and B), support that there is no correlation between the size of the cilia and nose touch sensitivity.
Taken together, our results support a model in which the activity of the Na+-K+-ATPase α-subunit genes eat-6, catp-1, or catp-2 is needed in OLQ and IL glia in C. elegans to maintain precise homeostasis of Na+ and K+ ions, which is essential either directly or indirectly for touch receptors’ function. Given that glia encapsulate the dendrites of nose touch neurons in C. elegans, the compartment in which the regulation of such ionic homeostasis is most likely relevant is the one between glia and neuronal dendrites. Thus this work reveals that the Na+-K+-ATPase is a regulator of the functional interaction between touch neurons and associated cells.
DISCUSSION
In this work we show that the Na+-K+-ATPase α-subunits EAT-6 and CATP-1 function in C. elegans glia associated with mechanosensory neurons OLQ and IL-1 to orchestrate responses to nose touch (Fig. 1). The data we present here support our previous findings, highlighting the crucial role of the tight regulation of the homeostasis of Na+ and K+ in glia for the function of the sensory neurons they ensheath, including mechanosensory neurons (Bacaj et al. 2008; Han et al. 2013; Wang et al. 2008, 2012).
Previous work from our laboratory demonstrated the important role of glia in ionic homeostasis for the function of associated neurons. For example, we showed that the DEG/ENaC Na+ channel ACD-1 is expressed in amphid sheath glia that surround dendrites of sensory neurons of C. elegans and contributes to acid avoidance and attraction to lysine and to isoamyl alcohol (Wang et al. 2008, 2012). We also showed that DEG/ENaC subunits DELM-1 and DELM-2 are expressed in socket glia ensheathing touch neurons and are needed for the sensitivity to nose touch (Han et al. 2013). Moreover, we showed that nose touch insensitivity of delm-1 knockout mutants is restored by overexpression in glia of worm inward rectifier K+ channel IRK-2 (Han et al. 2013). Thus this published work highlighted the importance of regulation of the concentration of Na+ and K+ by glia in sensory structures, which we further demonstrate here by showing the requirement of ion transport via glial Na+- K+-ATPase for nose touch.
In the collecting duct of the kidney, a favorable driving force for K+ excretion is established by the Na+ transport through ENaCs, a process that is made possible by the Na+-K+-ATPase (Féraille et al. 2003; Palmer 2015). Thus we speculate that in C. elegans sensory structure, glia, via function of DEG/ENaC Na+ channels, K+ channels, and the Na+-K+ pump, regulate K+ concentration in the microenvironment between glia and neurons, thus influencing neuronal output and animal behavior. Indeed, the concentration of K+ in the microenvironment around neurons is essential for setting the resting membrane potential and basal excitability of neurons, particularly those that fire action potentials. Recently, Liu and colleagues showed that at least AWA olfactory neurons in C. elegans fire all-or-none action potentials (Liu et al. 2018)). The role of Na+-K+-ATPase in the excretion of K+ into the microenvironment has also been demonstrated in the auditory system of Drosophila, in which scolopale cells, the accessory cells enwrapping neuron dendrites of the Johnston’s organ, express Na+-K+-ATPase to achieve K+ excretion into the endolymph surrounding the sensory endings, a process that is essential to hearing (Roy et al. 2013).
Both our laboratory and the Shaham laboratory have shown that glia regulate the homeostasis of other ions, in addition to Na+ and K+, in the nervous system of C. elegans. We reported that Cl− channel CLH-1 expressed in amphid glia mediates pH buffering by transport of (Grant et al. 2015), and Singhvi and colleagues showed that the dendritic shape of C. elegans thermosensory neurons AFD is controlled by the glial K+/Cl− transporter KCC-3 via regulation of local Cl− concentration (Singhvi et al. 2016). Singhvi and colleagues found that Cl− ions regulate the activity of receptor guanylyl cyclase GCY-8, which in turn modulates neuronal cGMP levels that influence dendritic shape and thermosensation (Singhvi et al. 2016). Although we found that glial Na+-K+-ATPase influences mechanosensation, we found no evidence of effects on the glial or cilia development or gross morphology, though there was some effect on cilia size, at least for EAT-6, which does not seem to correlate with nose touch insensitivity (Fig. 4).
While we cannot completely rule out submicroscopic structural changes in pump mutants, our data support that ion transport by the pump is the function that plays a key role in nose touch sensitivity. Indeed, nose touch sensitivity of catp-1 mutants is not rescued by C. briggsae mutant CATP-1 that lack pump activity (Ruaud and Bessereau 2007), but it is rescued in both eat-6 and catp-1 mutants by glucose, which leads to direct or indirect activation of CATP-2, given that the effect is lost in animals in which catp-2 is knocked down in glia. This third Na+-K+-ATPase α-subunit shares with EAT-6 and CATP-1 only 35% and 41% of identity, respectively, yet it is predicted to function as a Na+-K+-ATPase.
Interestingly, we reported here that, in contrast to aged animals (Wang et al. 2018), young adult worms grown in glucose do not show an overall increase in ATP concentration. These data suggest that glucose mediates activation of CATP-2, not via an increase of ATP concentration, but rather via another unknown factor. Given the large number of genes whose expression pattern is regulated in C. elegans by glucose (Gusarov et al. 2017; Lee et al. 2015; Wang et al. 2018), identification of this factor is beyond the scope of this publication.
Regulation of ionic homeostasis by glia is not a new concept and has been shown in other systems, including mammals. For example, astrocytes have been shown to regulate homeostasis of both Na+ and K+ (Higashi et al. 2001; Larsen et al. 2014; Olsen et al. 2015; Rose and Karus 2013; Sibille et al. 2015; Skatchkov et al. 2006) and oligodendrocytes to regulate homeostasis of Cl− (Blanz et al. 2007; Depienne et al. 2013; Hoegg-Beiler et al. 2014; Makara et al. 2003; Thiemann et al. 1992,). In particular, astrocytes have been proposed to remove excess extracellular K+ during elevated neuronal activity via the activity of inward rectifier K+ channels (Kofuji and Newman 2004). While, we cannot exclude the possibility that nose touch avoidance is regulated by other glial channels and transporters directly or indirectly dependent on Na+ and K+ homeostasis, we speculate that, at least under certain conditions, K+ could be excreted rather than taken up by glia via activity of DEG/ENaC channels, inward rectifying K+ channels, and Na+-K+-ATPase, leading to stimulation of neuronal activity as we have previously suggested (Han et al. 2013).
Taken together, results presented here underscore the fact that regulation by accessory cells including glia of the concentration of ions in touch receptors is important for touch sensation. As suggested by our previous work (Han et al. 2013; Wang et al. 2008, 2012), this function may be conserved across different sensory organs, and other regions of the nervous system of C. elegans. Given the general anatomical parallelism between nose touch receptors in C. elegans mammalian touch receptors such as the Meissner and the Pacinian corpuscles (nerve endings surrounded by accessory glia), we speculate that our findings might be applicable to mammalian touch sensation.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01-NS-070969 and R01-NS-105616A1 (to L. Bianchi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.B. conceived and designed research; C.K.J., J.F.-A., Y.W., and L.W. performed experiments; C.K.J., J.F.-A., L.W., and L.B. analyzed data; C.K.J., J.F.-A., and L.B. prepared figures; C.K.J., J.F.-A., and L.B. drafted manuscript; C.K.J., J.F.-A., L.W., and L.B. edited and revised manuscript; C.K.J., J.F.-A., L.W., and L.B. interpreted results of experiments; C.K.J., J.F.-A., L.W., and L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. K. Collins, R. Keane, K. Muller, and G. Zhai for sharing of equipment vital to collection of data presented in this paper, Nicole Encalada for technical assistance, and Dr. Jean-Louis Bessereau for the Caenorhabditis briggsae CATP-1 mutants and plasmids. We thank Dr. M. D’Amico for assistance with RT-PCR protocols and Drs. K. Collins, G. Dahl, and P. Larsson for reading of this manuscript.
REFERENCES
- Altun ZF, Hall DH. Nervous System, Neuronal Support Cells. New York: WormAtlas, 2010. [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics 133: 897–917, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science 322: 744–747, 2008. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L. Mechanotransduction: touch and feel at the molecular level as modeled in Caenorhabditis elegans. Mol Neurobiol 36: 254–271, 2007. doi: 10.1007/s12035-007-8009-5. [DOI] [PubMed] [Google Scholar]
- Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hübner CA, Jentsch TJ. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci 27: 6581–6589, 2007. doi: 10.1523/JNEUROSCI.0338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 17: 8259–8269, 1997. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MW, Somerville D, Lee RY, Lockery S, Avery L, Fambrough DM. Mutations in the Caenorhabditis elegans Na,K-ATPase α-subunit gene, eat-6, disrupt excitable cell function. J Neurosci 15: 8408–8418, 1995. doi: 10.1523/JNEUROSCI.15-12-08408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Bugiani M, Dupuits C, Galanaud D, Touitou V, Postma N, van Berkel C, Polder E, Tollard E, Darios F, Brice A, de Die-Smulders CE, Vles JS, Vanderver A, Uziel G, Yalcinkaya C, Frints SG, Kalscheuer VM, Klooster J, Kamermans M, Abbink TE, Wolf NI, Sedel F, van der Knaap MS. Brain white matter oedema due to ClC-2 chloride channel deficiency: an observational analytical study. Lancet Neurol 12: 659–668, 2013. doi: 10.1016/S1474-4422(13)70053-X. [DOI] [PubMed] [Google Scholar]
- Esposito G, Di Schiavi E, Bergamasco C, Bazzicalupo P. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395: 170–176, 2007. doi: 10.1016/j.gene.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Féraille E, Mordasini D, Gonin S, Deschênes G, Vinciguerra M, Doucet A, Vandewalle A, Summa V, Verrey F, Martin PY. Mechanism of control of Na,K-ATPase in principal cells of the mammalian collecting duct. Ann N Y Acad Sci 986: 570–578, 2003. doi: 10.1111/j.1749-6632.2003.tb07255.x. [DOI] [PubMed] [Google Scholar]
- Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr 33: 425–438, 2001. doi: 10.1023/A:1010623724749. [DOI] [PubMed] [Google Scholar]
- Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 290: F241–F250, 2006. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- Goodman MB. Mechanosensation. In: WormBook, edited by The C. elegans Research Community. 2006. doi: 10.1895/wormbook.1.62.1. [DOI] [Google Scholar]
- Grant J, Matthewman C, Bianchi L. A novel mechanism of pH buffering in C. elegans glia: bicarbonate transport via the voltage-gated ClC Cl− channel CLH-1. J Neurosci 35: 16377–16397, 2015. doi: 10.1523/JNEUROSCI.3237-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarov I, Pani B, Gautier L, Smolentseva O, Eremina S, Shamovsky I, Katkova-Zhukotskaya O, Mironov A, Nudler E. Glycogen controls Caenorhabditis elegans lifespan and resistance to oxidative stress. Nat Commun 8: 15868, 2017. doi: 10.1038/ncomms15868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Wang Y, Sangaletti R, D’Urso G, Lu Y, Shaham S, Bianchi L. Two novel DEG/ENaC channel subunits expressed in glia are needed for nose-touch sensitivity in Caenorhabditis elegans. J Neurosci 33: 936–949, 2013. doi: 10.1523/JNEUROSCI.2749-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SM, Tsuda H, Yang Y, Vibbert J, Cottee P, Lee SJ, Winek J, Haueter C, Bellen HJ, Miller MA. Secreted VAPB/ALS8 major sperm protein domains modulate mitochondrial localization and morphology via growth cone guidance receptors. Dev Cell 22: 348–362, 2012. doi: 10.1016/j.devcel.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K+ channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol 281: C922–C931, 2001. doi: 10.1152/ajpcell.2001.281.3.C922. [DOI] [PubMed] [Google Scholar]
- Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730, 2002. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- Hoegg-Beiler MB, Sirisi S, Orozco IJ, Ferrer I, Hohensee S, Auberson M, Gödde K, Vilches C, de Heredia ML, Nunes V, Estévez R, Jentsch TJ. Disrupting MLC1 and GlialCAM and ClC-2 interactions in leukodystrophy entails glial chloride channel dysfunction. Nat Commun 5: 3475, 2014. doi: 10.1038/ncomms4475. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 157: 664–675, 2014. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen MD, Pedersen PA, Jorgensen PL. Importance of Na,K-ATPase residue alpha 1-Arg544 in the segment Arg544-Asp567 for high-affinity binding of ATP, ADP, or MgATP. Biochemistry 41: 1451–1456, 2002. doi: 10.1021/bi015891h. [DOI] [PubMed] [Google Scholar]
- Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci 10: 568–577, 2007. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 129: 1045–1056, 2004. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci USA 95: 13091–13096, 1998. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J, MacAulay N. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62: 608–622, 2014. doi: 10.1002/glia.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Jeong DE, Son HG, Yamaoka Y, Kim H, Seo K, Khan AA, Roh TY, Moon DW, Lee Y, Lee SJ. SREBP and MDT-15 protect C. elegans from glucose-induced accelerated aging by preventing accumulation of saturated fat. Genes Dev 29: 2490–2503, 2015. doi: 10.1101/gad.266304.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kidd PB, Dobosiewicz M, Bargmann C. The C. elegans AWA olfactory neuron fires calcium-mediated all-or-none action potentials. Cell 175: 57–70.E17, 2018. doi: 10.1016/j.cell.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Makara JK, Rappert A, Matthias K, Steinhäuser C, Spät A, Kettenmann H. Astrocytes from mouse brain slices express ClC-2-mediated Cl- currents regulated during development and after injury. Mol Cell Neurosci 23: 521–530, 2003. doi: 10.1016/S1044-7431(03)00080-0. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509: 617–621, 2014. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science 324: 1580–1582, 2009. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M, Noguchi S, Takeda K, Morohashi M, Kawamura M. Site-directed mutagenesis of Asp-376, the catalytic phosphorylation site, and Lys-507, the putative ATP-binding site, of the α-subunit of Torpedo californicaNa+K+-ATPase. Biochim Biophys Acta 1021: 157–160, 1990. doi: 10.1016/0005-2736(90)90028-M. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J Neurosci 35: 13827–13835, 2015. doi: 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 10: 1050–1060, 2015. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson L, Prestia LT, Mahoney GK, Güçlü B, Cox PJ, Pack AK. GABAergic/glutamatergic-glial/neuronal interaction contributes to rapid adaptation in pacinian corpuscles. J Neurosci 29: 2695–2705, 2009. doi: 10.1523/JNEUROSCI.5974-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PL, Carafoli E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem Sci 12: 146–150, 1987. doi: 10.1016/0968-0004(87)90071-5. [DOI] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko C, Lu Y, Shaham S. Glia delimit shape changes of sensory neuron receptive endings in C. elegans. Development 138: 1371–1381, 2011. doi: 10.1242/dev.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Rifkin SA, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature 463: 913–918, 2010. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Karus C. Two sides of the same coin: sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia 61: 1191–1205, 2013. doi: 10.1002/glia.22492. [DOI] [PubMed] [Google Scholar]
- Roy M, Sivan-Loukianova E, Eberl DF. Cell-type-specific roles of Na+/K+ ATPase subunits in Drosophila auditory mechanosensation. Proc Natl Acad Sci USA 110: 181–186, 2013. doi: 10.1073/pnas.1208866110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruaud AF, Bessereau JL. The P-type ATPase CATP-1 is a novel regulator of C. elegans developmental timing that acts independently of its predicted pump function. Development 134: 867–879, 2007. doi: 10.1242/dev.02790. [DOI] [PubMed] [Google Scholar]
- Sangaletti R, D’Amico M, Grant J, Della-Morte D, Bianchi L. Knock-out of a mitochondrial sirtuin protects neurons from degeneration in Caenorhabditis elegans. PLoS Genet 13: e1006965, 2017. doi: 10.1371/journal.pgen.1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima Y, Tada Y, Furuki M, Hara Y, Ohta H. A missense mutation of the gene for Na+,K(+)-ATPase α-subunit causes abnormal feeding behavior in Caenorhabditis elegans. Biochem Biophys Res Commun 248: 778–782, 1998. doi: 10.1006/bbrc.1998.8981. [DOI] [PubMed] [Google Scholar]
- Sibille J, Dao Duc K, Holcman D, Rouach N. The neuroglial potassium cycle during neurotransmission: role of Kir4.1 channels. PLOS Comput Biol 11: e1004137, 2015. doi: 10.1371/journal.pcbi.1004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi A, Liu B, Friedman CJ, Fong J, Lu Y, Huang XY, Shaham S. A glial K/Cl transporter controls neuronal receptive ending shape by chloride inhibition of an rGC. Cell 165: 936–948, 2016. doi: 10.1016/j.cell.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skatchkov SN, Eaton MJ, Shuba YM, Kucheryavykh YV, Derst C, Veh RW, Wurm A, Iandiev I, Pannicke T, Bringmann A, Reichenbach A. Tandem-pore domain potassium channels are functionally expressed in retinal (Müller) glial cells. Glia 53: 266–276, 2006. doi: 10.1002/glia.20280. [DOI] [PubMed] [Google Scholar]
- Thiemann A, Gründer S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356: 57–60, 1992. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Madsen DM, Kahn-Kirby A, Peckol EL, Moulder G, Barstead R, Maricq AV, Bargmann CI. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318, 2002. doi: 10.1016/S0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Upadhyay A, Pisupati A, Jegla T, Crook M, Mickolajczyk KJ, Shorey M, Rohan LE, Billings KA, Rolls MM, Hancock WO, Hanna-Rose W. Nicotinamide is an endogenous agonist for a C. elegans TRPV OSM-9 and OCR-4 channel. Nat Commun 7: 13135, 2016. doi: 10.1038/ncomms13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Beaudoin-Chabot C, Thibault G. Glucose increases the lifespan of post-reproductive C. elegans independently of FOXO (Preprint). bioRxiv 347435, 2018. doi: 10.1101/347435. [DOI]
- Wang Y, Apicella A Jr, Lee SK, Ezcurra M, Slone RD, Goldmit M, Schafer WR, Shaham S, Driscoll M, Bianchi L. A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans. EMBO J 27: 2388–2399, 2008. doi: 10.1038/emboj.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, D’Urso G, Bianchi L. Knockout of glial channel ACD-1 exacerbates sensory deficits in a C. elegans mutant by regulating calcium levels of sensory neurons. J Neurophysiol 107: 148–158, 2012. doi: 10.1152/jn.00299.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol 160: 313–337, 1975. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lumpkin EA, Patapoutian A. Merkel cells and neurons keep in touch. Trends Cell Biol 25: 74–81, 2015. doi: 10.1016/j.tcb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509: 622–626, 2014. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]