Abstract
Coronavirus disease 2019 (COVID-19) is a pandemic infectious disease caused by the novel coronavirus called SARS-CoV-2. There is a gap in our understanding regarding the immunopathogenesis of COVID-19. However, many clinical trials are underway across the world for screening effective drugs against COVID-19. Nevertheless, currently, no proven effective therapies for this virus exists. The vaccines are deemed as a significant part of disease prevention for emerging viral diseases, since, in several cases, other therapeutic choices are limited or non‐existent, or that diseases result in such an accelerated clinical worsening that the efficacy of treatments is restricted. Therefore, effective vaccines against COVID-19 are urgently required to overcome the tremendous burden of mortality and morbidity correlated with SARS-CoV-2. In this review, we will describe the latest evidence regarding outstanding vaccine approaches and the challenges for vaccine production.
Keywords: COVID-19, SARS-CoV-2, Immunopathogenesis, Vaccines
1. Introduction
Corona virus disease 2019 (COVID-19), also known as 2019-nCoV acute respiratory disease, is caused by SARS-CoV-2 that has led the current ongoing pandemic worldwide [1]. The first SARS-CoV-2 infection was discovered in Wuhan, Hubei, China, and rapidly spread around the world by July 30, including more than 16,523,815 confirmed cases 655,112 confirmed deaths in 216 countries and territories [2], [3]. Most human coronaviruses are originated from bats [4], and importantly, a genetic similarity between the bat Betacoronavirus and SARS-CoV-2 has been recently demonstrated [4]. Although the certain transmission route to humans has partially remained unknown, according to some reports, the spike gene of SARS-CoV-2 may have originated from pangolins [4].
The SARS-CoV-2 colonizes the respiratory tract system and causes symptoms similar to those of common cold, including respiratory disorders, runny nose, dry cough, dizziness, sore throat, and body aches, accompanied by headaches and fever for several days [5], [6], [7]. In people with defective immune systems, such as immunocompromised and elderly individuals, COVID-19 symptoms can become more severe, causing pneumonia and bronchitis [8]. The case fatality rate of this disease seems to be age-dependent, with a higher percentage in the elderly, particularly men, and an overall fatality rate up to 3% [9]. There is currently no specific treatment for COVID-19; however, research is ongoing, and efforts are currently directed at repurposing licensed antivirals drugs. Still, the best way to deal with this disease is by taking proper preventive measures; thus, hopes are pinned on developing effective vaccines [10]. Although by August 2020, no vaccines have been developed for COVID-19, various organizations are attempting for vaccine development [10]. Three vaccination strategies could be considered [11], [12].
As the first strategy, researchers hope to produce a complete virus vaccine. Such vaccine aims to induce a rapid immune response in humans against SARS-CoV-2 [11], [12]. The second strategy is the development of a sub-unit vaccine to induce the immune system for the identification of specific viral subunits [11], [12]. In case of SARS-CoV-2, such research focuses on spike (S) proteins that facilitate binding of virus to the angiotensin-converting enzyme 2 (ACE2) [13]. The third strategy is the development of nucleic acid vaccines (DNA or RNA vaccines) [11], [12], [14]. Although experimental vaccines developed by either of these strategies should be tested for safety and efficacy, developing effective and safe vaccines is urgently needed to prevent SARS-CoV-2 infections. In this review, we will describe various strategies for developing COVID-19 vaccines based on current understandings of various coronaviruses, particularly the novel SARS-CoV-2.
2. Roles of viral S, M and N proteins
Coronaviruses (including SARS-CoV-2) are enveloped viruses and their envelopes are composed of a lipid bilayer originated from the host cell membrane when being released from the infected cells. Structurally, SARS-CoV-2 has four main structural proteins, including S glycoprotein, small envelope (E) glycoprotein, membrane (M) glycoprotein, nucleocapsid (N) protein, as well as and several accessory proteins [15]. The S glycoprotein is a transmembrane protein with a molecular weight of about 150 kDa found on the viral outer membrane [16]. S protein forms homotrimers that protrudes the viral surface and facilitates binding of viral envelope to host cells by interacting with ACE2 expressed on the lower respiratory tract cells [16]. This glycoprotein is cleaved by the host cell furin-like protease into 2 subunits, namely S1 and S2 [16]. S1 component is responsible for cellular tropism with the receptor-binding domain while S2 mediates viral fusion to host cells [16]. The N protein is the structural component of coronaviruses bound to the viral nucleic acid [16]. As this protein is bound to RNA, it is involved in processes related to viral genome, viral replication cycle, and the host cell responses to viral infections [16]. The N protein is also strongly phosphorylated and is suggested to be involved in structural changes, thereby enhancing affinity for viral RNA [16]. The most abundant structural protein is the M glycoprotein; it spans the membrane bilayer three times, leaving a short NH2-terminal domain outside the virus and an extended COOH terminus (cytoplasmic domain) inside the virion [17], [18]. The S protein as a type I M glycoprotein constitutes the peplomers. The primary target of neutralizing antibodies is the S protein [17], [18]. Within the envelope, molecular interactions between proteins probably determine the coronaviral membrane formation and composition [17], [18]. The M protein plays a predominant role in the intracellular formation of viral particles without requiring the S protein. In the presence of tunicamycin, which inhibits protein glycosylation, coronavirus continues proliferation and produces spikeless, noninfectious virions that contain the M proteins but lack the S proteins [17], [18]. N proteins of many coronaviruses are highly immunogenic and are expressed abundantly during infection [19]. High levels of immunoglobulin G antibodies against the N protein have been detected in sera of SARS patients. The N protein in a vaccine setting induces SARS-specific T-cell proliferation and cytotoxic activity [20].
3. Immune reactions and pathogenesis of COVID-19
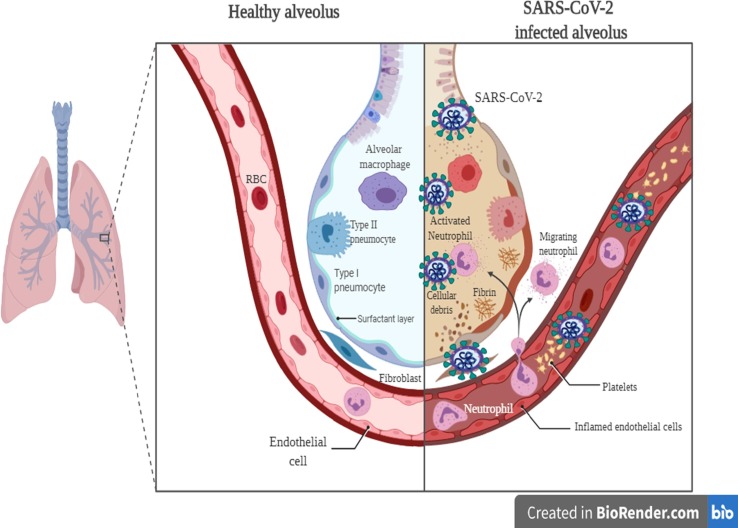
Findings from COVID-19 have shown that in severe cases, a cytokine storm has been observed that causes the acute respiratory distress syndrome [21], [22], [23]. Other reports on severe COVID-19 cases have indicated a high rate of pro-inflammatory cytokines and immune cell subsets [23], [24]. During COVID-19 infection, various immune cells are synergistically involved in antiviral reactions [23]. Elevated leukocytes, neutrophils, as well as the neutrophil-lymphocyte ratio, have been documented in severe COVID-19 cases [23]. Lymphopenia is indicative of impaired immune reactions in most SARS-CoV-2-infected cases [25], [26] (Fig. 1 ). Hence, it seems that leukocytes and neutrophils along with lymphocytes can prompt the cytokine storm in SARS-CoV-2-infected individuals [23]. The levels of T lymphocyte subsets with crucial roles in the equilibrium of immune reactions vary based due to different viral pathogenesis mechanisms [23]. It has been previously found that the number of T lymphocytes decrease in SARS-CoV infected individuals [23], [27]. Findings have noted that COVID-19 infection could lead to immune dysregulation by affecting T lymphocytes. The significant reduction in the number of T lymphocyte has been demonstrated in COVID-19, which is pronounced in severe cases [23]. The numbers of T helper lymphocytes, cytotoxic suppressor T lymphocytes, and regulatory T lymphocytes in patients with SARS-CoV are below the normal ranges. It has been found that SARS-CoV-2 causes an immune dysregulation by inducing aberrant cytokine activity and alterating the rate of lymphocyte subsets, thereby leading to cytokine storm and tissue injury. Excessive inflammatory responses during a cytokine storm lead to severe disorders and facilitate the prognosis of SARS-CoV-2 infection. Severe cases of COVID-19 infections are found to have lower lymphocyte levels and higher inflammatory cytokine rates [21], [24], [28]. Overall, in late stages of COVID-19 infection, cytokine storm is the leading cause of disease progression and death (26). The elevated levels of IL-1β, IFN-γ, IL-10, IL-2, TNF-α, and IL-4 have led to the induction of granulocyte-colony stimulating factor (GCS-F), IFNγ-induced protein-10 (IP-10), macrophage chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1α (MIP-1α) [28].
Fig. 1.
The SARS-CoV-2 immunopathogenesis in the lung.
4. Coronavirus vaccines
Previous works on vaccine development for Coronaviridae have aimed at SARS and MERS [29]; nevertheless, these vaccines have been evaluated in non-human models. By 2020, no approved vaccine has been reported to be safe and protective in humans; however, the identification and development of novel vaccines and medicines to tackle with SARS-CoV is a priority for public health agencies around the world. In this section, we review the latest strategies for evaluating candidate vaccines for SARS and MERS, and most importantly, we provide an overview of SARS-CoV-2 candidate vaccines.
5. Candidate vaccines for severe acute respiratory syndrome
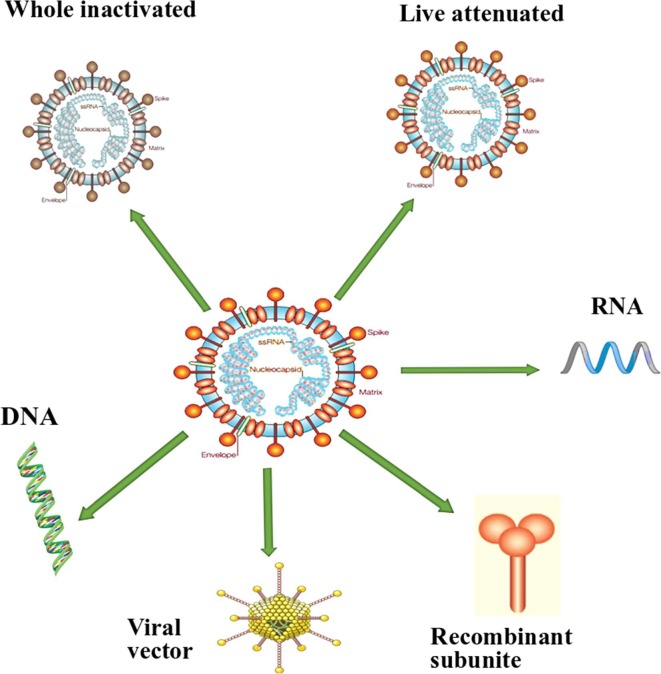
Several approaches have been considered for the development of SARS vaccines, such as inactivated virus vaccines, live-attenuated SARS vaccines, recombinant vector vaccines, recombinant SARS proteins, and DNA vaccines [30], [31], [32], [33] (Fig. 2 ). The inactivated whole vaccine is an attractive candidate as it is easily produced and presents an antigenic moiety similar to what the host immune cells meet during viral infections [30]. Besides, this vaccine presents many proteins on its surface for recognition by the immune system [30]. Many researchers have developed an inactivated whole SARS vaccine and found that it stimulates neutralizing antibodies [34], [35], [36], [37]. Stadler et al. [38] found that inactivated whole vaccine prevented pulmonary SARS replication in mouse model. Another study showed that in the absence or presence of alum adjuvant, the inactivated whole vaccine induced protection against SARS infection by stimulating neutralizing antibodies and decreasing viral loads in the respiratory system [39]. Additionally, formaldehyde-inactivated SARS vaccines were administered to rhesus monkeys and the results showed their immunogenicity and safety [30]. Interestingly, inactivated SARS (a.k.a SARS CoV1) vaccines have been used in humans and stimulated neutralizing antibodies. These vaccines were found to be effective and safe [40]. Overall, findings indicated that the inactivated whole vaccine is safe, induces neutralizing antibodies for SARS, and stimulates T lymphocytes.
Fig. 2.
The current approaches considered for SARS-CoV-2.
Recombinant virus vaccine consists of live replicating viruses with the ability of inducing efficient immune reactions mediated by T and B lymphocytes as it can directly infects antigen-presenting cells (APCs) [41]. This vaccine produces target proteins within the host cell that can be processed by antigen-processing machinery to be expressed by MHC I and subsequently be presented to CD8+ T cells [30]. Some viruses have been shown to express the SARS protein for triggering potent cellular immunity as well as neutralizing antibodies [30].
Viral vector vaccines combine many of the positive aspects of DNA vaccines with those of live attenuated vaccines [42]. Similar to DNA vaccines, viral vector vaccines transfer DNA into the host cell for the generation of antigenic proteins with the ability of provoking a variety of immune responses, including antibodies, T helper cells (CD4+ T cells), and cytotoxic T lymphocytes (CTLs) [42]. Unlike DNA vaccines, viral vector vaccines have the potential to actively attack host cells and replicate within them, much like a live attenuated vaccine, further stimulating the immune system similar to an adjuvant [42]. Therefore, the viral vector vaccine generally consists of a live-attenuated virus that is genetically engineered to transfer DNA coding for protein antigens from a pathogenic organism. Currently, viral vectors expressing pathogenic proteins are being developed as vaccines against viral pathogens [42]. For some diseases, viral vectors are being used in combination with another strategy called the heterologous prime-boost approach [42]. One vaccine is given as a priming step, followed by an alternative vaccine as a booster [42]. The heterologous prime-boost strategy aims to provide a more robust immune response [42].
Adenovirus vaccine lacks pathogenicity when administered through oral and nasal routes and promotes mucosal immunity [43]. The limitation of the adenovirus vaccine is its restricted host range leading to difficulties in in vivo settings [41], [44]. Protection against SARS by adenovirus-vectored vaccines was primarily evaluated in mice. It triggered high rates of anti-N protein interferon-gamma as well as neutralizing antibodies, and reduced viral titers [39]. Importantly, the intranasal administration triggered immunoglobulin A, which could effectively block viral replication in both the nose and the lungs [30]. These data show that the intranasal administration of the N and S recombinant adenovirus vaccines can trigger protective host mucosal immunity. Also, adenoviruses that express SARS proteins have been tested in rhesus macaques and a ferret model. The results showed the immunogenicity of these vaccines as the decreased severity of pneumonia and viral titer were reported [45].
Subunit vaccines are safe, easily provided, and often less protective due to the presentation of produced proteins by MHC II and less ability of inducing CTLs [30]. The S protein can be employed for the SARS subunit vaccine development as it induces the protective immunity [46], [47]. This protein is responsible for binding host receptor ACE2; hence, it is a suitable target for vaccine development [48], [49]. Additionally, it has been found that the N protein can represent another target for vaccine development [50], [51] as it has been documented to stimulate the host cell immunity via CD8+ T lymphocytes [30].
DNA vaccines encoding viral proteins can stimulate cellular and humoral immune reactions. Protein antigens are expressed and presented by MHC I, thereby triggering CD8+ T lymphocytes [30]. Some DNA vaccines have been developed for SARS based on the S, N, and M proteins [30], [52], [53], [54]. A DNA-S protein vaccine triggered neutralizing antibodies as well as T lymphocyte responses and subsequently decreased SARS replication [55]. Also, a DNA vaccine based on multiple-epitope strategy triggered the generation of antibodies for the S and M proteins, which could prevent SARS-CoV infection [54]. Reportedly, the efficacy of DNA vaccines in most clinical studies have not been very promising; thus, numerous approaches have been used to enhance their efficacy [30]. For example, it has been suggested that a DNA vaccine in conjunction with inactivated viral proteins as well as vectors can improve immune reactions, in particular, specific T helper 1/T helper 2 responses [54], [56], [57]. Overall, these work on SARS-CoV vaccine suggest strategies that could work for SARS-CoV2.
Finally, attenuated viral vaccines are the most prominent and effective vaccines as they possess immune-activating moieties. These vaccines usually very effective, and a single dose is often enough to induce long-lasting immunity [30]. The problem with attenuated vaccines is that mutations can cause virulence, as found in poliovirus [30]. Virulence has not been indicated following SARS-attenuated vaccine administration; however, several mutations have been reported [30]. The protective efficacy and immunogenicity of a live-attenuated vaccine with recombinant SARS lacking the E gene showed that hamsters immunized with this vaccine had a high rate of neutralizing antibodies that prevented SARS replication and respiratory symptoms [58]. Therefore, the deletion of E gene can be considered as a primary step in the development of a live-attenuated SARS vaccine. Additionally, it has been found that the deletion of nsp-1 gene in murine hepatitis virus can be used for the development of a highly efficacious attenuated vaccine, suggesting a promising approach for the development of an attenuated vaccine for SARS-CoV-1 [59].
6. Candidate vaccines for middle east respiratory syndrome (MERS)
For MERS prevention, several candidate vaccines are under development, including subunit vaccines, DNA vaccines, peptide vaccines, vector vaccines, and live-attenuated vaccines [60]. It has been found that the intramuscular injection of MERS-DNA vaccine expressing the S protein stimulated S-specific neutralizing antibody and T lymphocyte responses that led to IFN-γ, TNF-α, and IL-2 induction in rhesus macaques [61]. Protective efficacy was found in rhesus macaques with decreased viral titer and the absence of pneumonia. This finding led to the first phase I clinical trial of a MERS DNA-vaccine developed by some companies [60], [61]. In an in vivo study, Wang et al. [62] demonstrated that the combination of S DNA vaccine subunit protein in alum triggered neutralizing antibodies against MERS. The S DNA vaccine induced greater humoral reactions in mice [62]. Immunization with S DNA protein in primates also decreased pulmonary infiltrates and consolidation [62].
Commonly, subunit vaccines are safe and tolerated, and induce specific CD4+ T lymphocytes [63], [64]. In SARS, the receptor-binding domain, S1 subunit, has been identified as the primary target for neutralizing antibodies [65], [66], [67]. Additionally, in MERS, the S protein, as the receptor-binding domain, has been applied to evaluate its efficacy and its ability to induce neutralizing antibodies in rhesus macaques, rabbits, and mice [68], [69], [70], [71]. It has been shown that the modified receptor-binding domain of the S protein from MERS could trigger potent cellular and humoral reactions [64], [72]. Also, Coleman et al. [73], [74] showed that recombinant S nanoparticles combined with M adjuvant stimulated neutralizing antibodies and decreased viral loads in murine lungs.
However, more investigations are required to evaluate the safety, immunogenicity and the efficacy of nanoparticles in human clinical trials for further developments. Reportedly, vector-based MERS vaccines that express the S protein can induce a robust neutralizing antibody response and reduce the viral replication in the respiratory tract [75]. A chimpanzee adenovirus-based MERS vaccine prepared by the Jenner Institute entered the clinical trial phase [76]. Several Chimpanzee-adenovirus vaccines have been tested in vivo for several viral infections such as HIV (human immunodeficiency virus), Ebola, hepatitis C, rabies, as well as SARS, and the results showed their potent immunity and efficacy [60]. Additionally, immune reactions caused by MERS candidate vaccines such as Chimpanzee-adenovirus and modified vaccinia virus Ankara (Modified Vaccinia Ankara) were investigated. These vaccines induced neutralizing antibodies and immune reactions in vivo [77]. Also, a live attenuated vaccine that can be developed by the deletion of virulence genes has been found to trigger immune reactions [60]. Recently, a live-attenuated vaccine for MERS has been prepared via a replication-competent virus [78].
7. Antibody-dependent enhancement
Antibody-dependent enhancement (ADE), sometimes less accurately referred to as immune enhancement or disease enhancement, is an episode during which binding of a virus to non-neutralizing antibodies enhances its insertion into host the cells [79]. This event, which leads to both increased infectivity and virulence, has been observed in viruses such as Dengue virus, Yellow fever virus, Zika virus, HIV, and coronaviruses [80], [81], [82], [83]. The concern with ADE of coronaviruses infection initially raised from studies on feline infectious peritonitis virus (FIPV) [84]. FIPV infects myeloid-derived cells, such as macrophages, in cats [85]. As the target cell of FIPV also displays fragment crystallizable (Fc) receptors, this virus can interact with Fc receptors to enter macrophages [84]. Indeed, vaccines that provide low titers of neutralizing antibodies led to peritonitis and higher mortality rates in kittens [86]. Concerns were also raised on the possibility of ADE following SARS-CoV and MERS-CoV infections [87].
ADE begins when antibody-bound virus attaches activating Fc receptors to start Fc receptor-mediated endocytosis or phagocytosis [84]. This process promotes virus entry into Fc receptor-expressing monocytes, macrophages, and dendritic cells [84]. However, binding active Fc receptors alone is inadequate for ADE [84]. This is because activated Fc receptors trigger signaling molecules that provoke IFN-stimulated gene (ISG) expression [17]. ISGs have potent antiviral activities [84]. Consequently, for ADE to happen, viruses need to develop ways to suppress such antiviral responses in target cells [84]. For example, ADE of dengue virus infection is also reliant on the binding of dengue virus to the leukocyte immunoglobulin-like receptor B1 (LILRB1) [88]. Signaling from LILRB1 represses the pathway involved in ISG expression to generate an intracellular environment advantageous for viral replication [88], [89], [90]. Moreover, we have lately described that in addition to binding LILRB1, DENV has also developed other ways to fundamentally alter the host cell response throughout antibody-mediated infection to support viral replication [91]. Consequently, viruses that employ ADE must (1) target Fc receptor-expressing cells for infection, and (2) evolve mechanisms to overcome antiviral responses in myeloid-derived cells [92]. For viruses to evolve such abilities, Fc receptor-expressing cells must be their primary targets so that positive selection can take place [84]. However, currently, SARS-CoV-2 has so far been discovered to infect ACE2-expressing epithelial cells [93]. Further studies will be demanded to define the potential of SARS-CoV-2 in infecting myeloid-derived cells and the role of ADE of SARS-CoV-2 in viral pathogenesis [84]. It is critical to define which vaccines and adjuvants can evoke protective antibody responses to SARS-CoV-2 [94]. Previous investigations have revealed that the immunization of mice with inactivated whole SARS- CoV, the immunization of rhesus macaques with MVA- encoded S protein and the immunization of mice with DNA vaccine encoding full- length S protein could induce ADE or eosinophil- mediated immunopathology to some degree, probably owing to low quality and quantity of antibody production [95], [96]. It has been noted that it is essential to consider ADE to develop countermeasures toward the SARS-CoV-2. Data from previous coronaviruses research strongly suggest that ADE may play a role in viral pathology. If this is the case with SARS-CoV-2, then careful design and testing of vaccines or alternative prophylaxic approaches will be needed to prevent ADE.
8. SARS-CoV-2 targets for the development of the vaccine
Similar to SARS-CoV and MERS-CoV, the recent SARS-CoV-2 belongs to the Betacoronavirus genus [97]. The genome size of the virus is about 30 kilobases, which, similar to other coronaviruses, encodes several structural and non-structural proteins [98]. The structural proteins include the S, E, M, and N proteins [98]. Elementary studies have recommended that SARS-CoV-2 is very similar to SARS-CoV based on the full-length genome phylogenetic analysis, and the putatively similar cell entry mechanisms and human cell receptors [97], [98]. Due to this apparent similarity among the two viruses, recent research that has presented an understanding of protective immune responses toward SARS may be furthered to facilitate SARS-CoV-2 vaccine development.
Several reports related to SARS have recommended a protective role of both humoral and cell-mediated immune responses. In case of the former, antibody responses produced against the S protein are protective against infection in mouse models [55], [99]. Many studies have indicated that antibodies generated against the N protein of SARS were particularly widespread in SARS-infected patients [100], [101]. While being active, the antibody response was affirmed to be short-lived in recovering SARS patients [102]. In contrast, T cell-associated responses have been shown to induce long-term immunity, even up to 11 years post-infection, and have therefore drawn attention as a promising vaccine against SARS-CoV [102], [103]. SARS structural proteins are the most immunogenic in the convalescent SARS patients compared to the non-structural proteins as they significantly induce T cell responses [104]. Furthermore, T cell responses against the S and N proteins have been reported to be the most dominant and long-lasting immune reaction [105].
The SARS-CoV S protein is made of two subunits; the S1 subunit comprises a receptor-binding domain that interacts with the host cell receptor ACE2 and the S2 subunit mediates fusion between the viral and host cell membranes [106]. The S protein has critical roles in the induction of neutralizing-antibodies and T-cell responses, as well as protective immunity throughout SARS infection [106]. Particular antibodies toward SARS-CoV (IgG and IgM) were detectable about two weeks post-infection, reached a peak at 60 days post-infection, and remained at high levels 180 days post-infection [107]. High titers of neutralizing antibodies and SARS-specific cytotoxic T lymphocyte responses were detected in patients who had recovered from SARS. The severity of the responses correlated with the disease outcome [108], [109], [110].
Neutralizing antibodies and T-cell immune responses can directly target several SARS-CoV-2 proteins, but mainly the S protein. This suggests that S protein-induced specific immune responses is vital in the fight against SARS-CoV-2 infection [106]. SARS-CoV-2 S protein has also a crucial role in overcoming the species-dependent barriers. The adaptive evolution of S protein can contribute to the animal-to-human transmission route of SARS-CoV-2 [106]. As the S protein of SARS-CoV-2 is implicated in receptor recognition and virus attachment and entry, it represents one of the most critical components for the construction of SARS-CoV-2 vaccines and therapeutics. Genomic analyses indicated that SARS-CoV-2 shares genomic relationships with SARS-CoV in the receptor-binding motif that directly interacts with the human receptor ACE2 [111]. For both of these coronaviruses, the S protein is crucial for viral transmission and infection and defines the tropism of the virus to host cell entry [111]. SARS-CoV-2 binds the ACE2 receptor similar to MERS-S that binds the cellular receptor dipeptidyl peptidase 4 (DPP4) via the receptor-binding domain (RBD) in the N-terminal surface sub-unit (S1). It then employs its C-terminal transmembrane subunit (S2) to fuse with the host cell membrane [111]. Due to this vital functional feature and established antigenicity, the S protein is a principal target for vaccine development.
9. Ongoing vaccines for severe acute respiratory syndrome coronavirus 2
Vaccines for acute viral infections are developed such that they can recapitulate immune reactions towards natural infections [112]. Basic knowledge is now absent for COVID-19, including whether equilibrium and the type of cells that respond to the virus differ corresponding to the course and its severity. This understanding can help us choose the vaccine that is most likely to stimulate the immune systems against SARS-CoV-2. Due to the fast spread of SAR-CoV-2 infections in various countries, many companies have attempted to develop an effective SAR-CoV-2 vaccine.
Vaccination for prophylaxis stimulates a sufficient amount of neutralizing antibodies and memory cytotoxic T cells specifically aimed at viruses present in the lung to stop viral replication [113]. To prevent viral replication, co-presence and co-activation of APCs, T cells, and B cells are needed in lymph nodes [113]. Considering the speed and level of respiratory immunopathology caused by SARS-CoV-2, vaccines should optimally induce the formation of neutralizing antibodies and local cellular immunity to hinder infection progression [113]. Although such immunity by a vaccine is achievable in the young, it may still be more challenging in the elderly as low efficacy of seasonal influenza vaccines has been observed in this population [113]. By understanding the path adopted for the development of SARS and MERS vaccines, many researchers have started to find a strategy to develop the SAR-CoV-2 vaccine following the outbreak. Bellow, we will explain the currently promising approaches for COVID-19 vaccine development.
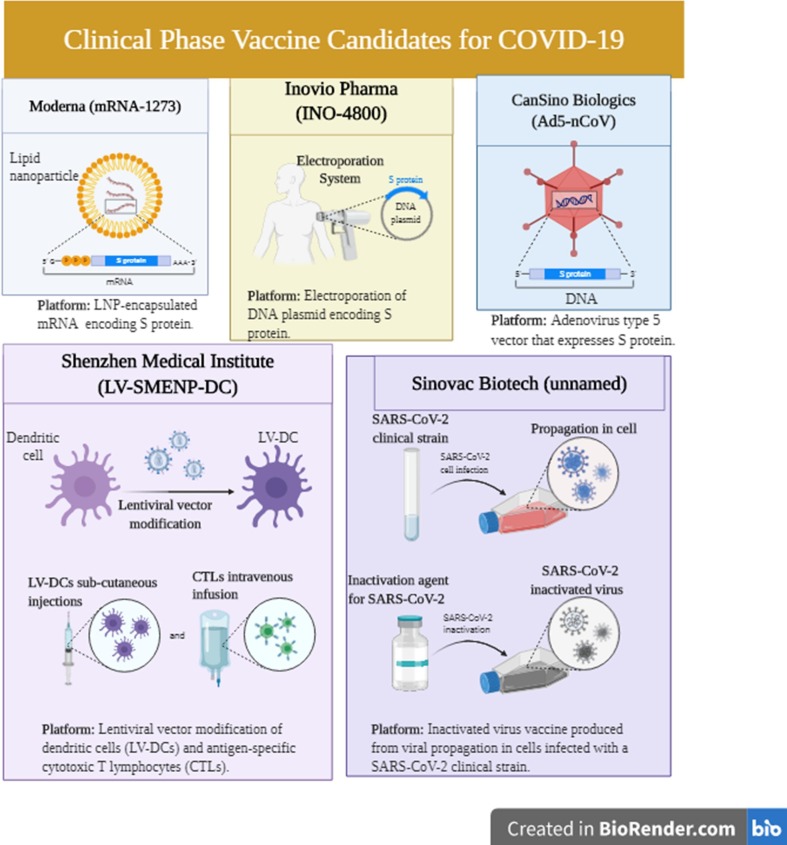
As of July 2020, 115 vaccine candidates have been announced for fighting COVID-19, among which 78 are being actively investigated, and 37 have been disregarded [114]. Among the 78 confirmed active projects, 73 are at the preclinical stages. Candidate vaccine that were shown to be the most promising including mRNA-1273 (developed by Moderna), Ad5-nCoV (developed by Can Sino Biologicals), and INO-4800 (developed by Inovio) have reached the clinical trial stage [114]. Information about specific SARS- CoV-2 antigen(s) employed for vaccine development is publicly limited. Publicly announced vaccines are known to induce neutralizing antibodies aimed at the S protein, thereby hindering virus uptake by the ACE2 receptor [114].
Nevertheless, the genomics of the virus is unknown, and the interrelationship between various forms and variants of the S protein employed in candidate vaccines is unclear. Research by Shenzhen Geno- Immune Medical Institute has shown the potency of LV- SMENP- DC and pathogen-specific APCs for developing SARS vaccines. Several other companies that are working on vaccine development are planning to initiate clinical trials (1 0 2). One of these companies is Cambridge, Massachusetts-based Moderna [115], which is on the front line of developing a COVID-19 vaccine, mRNA-1273 that is currently undergoing clinical trials [115]. This mRNA vaccine codes for a prefusion-stabilized form of the S protein [115]. This vaccine somehow resembles gene therapy and when administered, it leads to protein production by human cells, thereby triggering immune responses that prepare the body against viral infections [115].
Ad5-nCoV is considered as the first coronavirus vaccine that entered the clinical trial phase in China [116]. This candidate vaccine is built according to the Can Sino BIO’s adenovirus-based viral vector vaccine platform [116] and is genetically engineered such that the replication-defective adenovirus type 5 (Ad5) expresses the SARS-CoV-2 S protein [116]. Animal studies in preclinical stages suggested that Ad5-nCoV can stimulate an efficient immune reaction [116]. These studies also showed the safety of these vaccines [116]. The new Ad5-vectored COVID-19 vaccine is the primary vaccine that has been tested in a clinical trial [117]. This vaccine uses a weakened form of adenovirus to deliver genetic substances coding for the SARS-CoV-2 S protein [116]. The S proteins are produced and delivered to the lymph nodes, where the immune responses generate neutralizing antibodies against the S protein [116]. Feng-Cai Zhu et al. [117] showed that the Ad5-vectored COVID-19 vaccine could induce immunogenicity 28 days following vaccination. Humoral immunity towards SARS-CoV-2 was at its optimal levels 28 days following vaccination in healthy individuals. Rapid and specific T-cell responses were also observed following 14 days of vaccination [117]. Their results indicated that the Ad5-vectored COVID-19 vaccine requires more investigation. Notably, a phase II clinical placebo-controlled double-blinded trial has been started to assess the recombinant vaccine for COVID-19 (Ad5 vector) (CTII-nCoV) [118]. This trial addresses the healthy adults aged over 18 years who met all inclusion criteria [118] and assesses the safety and immunogenicity of Ad5-nCoV [118]. Five hundred individuals will be included, 250 subjects receiving the middle-dose vaccine, 125 subjects receiving the low-dose vaccine, and a group of placebo [118]. Immunogenic properties of the vaccine will be assessed on days 0, 14, 28, and 180 following vaccine administration [118].
In phase III clinical trials in 6 countries, reducing health care workers absenteeism in COVID-19 pandemic through BCG vaccine (BCG-CORONA) will start in a placebo-controlled adaptive multi-center randomized controlled trial. Subjects will be categorized into two groups, one of which receives the BCG vaccine via intracutaneous administration, and the other receives the placebo in a 1:1 ratio [119]. BCG vaccine was initially developed against tuberculosis, but several studies have indicated its potency against various infectious diseases [119] (Fig. 3 ). A promising effect has been indicated against viral pathogens, including yellow fever, herpes simplex virus, respiratory syncytial virus, and human papillomavirus [119]. Due to the ability of BCG in reducing the occurrence of respiratory tract infections in children, it is hypothesized that it induces immunity towards COVID-19 infection [119].
Fig. 3.
The current ongoing clinical trial for candidate SARS-CoV-2 vaccines.
Researchers at the University of Oxford have started to study subjects in a phase 2/3 clinical trial of Astra Zeneca-partnered COVID-19 vaccine AZD1222 (the recombinant adenovirus vaccine) (aka ChAdOx1 nCoV-19) [120]. The next step of the program, which includes a 1,000-subject phase I trial, is designed to enroll 10,260 subjects in the U.K. to obtain promising results for confirming the initial shipments to customers at the end of the summer (1 0 8). Some other companies include the International Vaccine Institute (IVI), INOVIO, and KNIH partnered with CEPI that are conducting the phase I/II clinical trial of INOVIO’s COVID-19 DNA vaccine (DNA plasmid delivered by electroporation) in South Korea [121].
Kim et al. [122] described the usage of microneedle arrays (MNAs) for delivering MERS-CoV vaccines and indicated their immunogenicity in preclinical studies. According to the results, the MNA that delivered MERS-S1 subunit vaccines prolonged antigen-specific antibody induction [122]. Notably, these vaccines induced efficient antigen-specific antibodies two weeks following immunization [122]. Therefore, they can be promising for fighting against coronavirus infections [122] Efforts to develop MNA-MERS-S1 subunit vaccines have enabled researchers to rapidly develop MNA-SARS-CoV-2 subunit vaccines capable of provoking efficient virus-specific antibody responses [122]. Collectively, their results demonstrate the clinical development of MNA-delivered recombinant protein subunit vaccines against COVID-19. In a non-clinical research by Safoni, this candidate SARS vaccine demonstrated immunogenicity and provided relative protection in animal models [123]. As a current licensed vaccine has been based on this platform, clinical research can be conducted almost quickly. Currently, several clinical and preclinical vaccines are being studied around the globe, and a complete list is depicted in Table 1 .
Table 1.
Candidate vaccines in various clinical evaluations towards COVID-19.
| Candidate vaccine | Type | Status | Description | References |
|---|---|---|---|---|
| Ad5-nCoV (NCT04313127) | Non-replicating viral vector | Phase II Clinical trial |
Ad5-nCoV is a genetically engineered vaccine that uses the replication-defective adenovirus type 5 as the vector to display S protein(s) of SARS-CoV-2. It is designed to block the COVID-19. | [142] |
| ChAdOx1 nCoV-19 (AZD1222) (NCT04324606) | Non-replicating viral vector | Ongoing to Phases II clinical trial | hAdOx1 nCoV-19 is generated from a virus (ChAdOx1), a weakened form of a chimpanzee’s adenovirus that has been genetically modified so that it cannot grow in humans. A genetic element has been incorporated into the ChAdOx1 construct to produce S proteins of the SARS-CoV-2. This protein is commonly located outside of the SARS-CoV-2, and has a vital role in SARS-CoV-2 pathogenesis. | [143] |
| mRNA-1273 (NCT04283461) | RNA vaccine | Phase I clinical trial | mRNA-1273 is an mRNA vaccine against SARS-CoV-2 coding for a prefusion-stabilized form of the S protein. Consistent with the antibody data, mRNA-1273 vaccination elicited neutralizing antibodies in participants. | [144] |
| INO-4800 (NCT04336410) | DNA Vaccine | Phase I clinical trial | INO-4800 has induced robust neutralizing antibodies and T cell immune responses in preclinical models. Participants experienced one ID injection of 1.0 mg INO-4800. The engineered construct, INO-4800, results in S protein expression. | [145] |
| LV-SMENP-DC vaccine (NCT04276896) | Modified APC | Phase II clinical trial | Based on the genomic sequence of the novel coronavirus, conserved and essential structural and protease protein domains have been chosen to engineer lentiviral SMENP minigenes to express COVID-19 antigens. -SMENP-DC vaccine is made by the modification of DC with lentivirus vectors displaying COVID-19 minigene SMENP and immune-modulatory genes. CTLs are stimulated by LV-DC, which presents COVID-19 specific antigens. | [146] |
| COVID-19/aAPC vaccine (NCT04299724) | Engineered lentiviral minigenes (Modified APC) | Phase I clinical trial | Based on the genomic sequence of SARS-CoV-2, conserved and necessary structural and protease protein domains are selected to engineer lentiviral minigenes for SARS-CoV-2 antigen expression. The COVID-19/aAPC vaccine is prepared by lentiviral modifications, including immune-modulatory genes and the viral minigenes to the artificial antigen-presenting cells (aAPCs). | [119] |
| Bacille Calmette-Guerin (NCT04328441) | Other | Phase III/IV clinical trial | The Bacille Calmette-Guerin is a live attenuated vaccine comprised of the causative agent of bovine tuberculosis (Mycobacterium bovis). Amazingly, BCG vaccination seems to not only protect humans against severe childhood tuberculosis but has non-specific protective effects against other respiratory tract infections both in vitro and in vivo. Thus, this vaccine is being repurposed to see whether it can diminish morbidity and mortality correlated with SARS-CoV-2 infection. | [120] |
| AV-COVID-19 (NCT04386252) | Modified APC | Phase I/II clinical trial | The AV-COVID-19 vaccine is obtained from autologous dendritic cells (DC) loaded with antigens from the SARS-CoV-2 to prevent COVID-19. To produce this vaccine, monocytes of a healthy individual will be isolated. These monocytes will be differentiated into DCs using IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) followed by incubation with SARS-CoV-2 antigens to create AV-COVID-19. This vaccine will be administered subcutaneously with or without extra GM-CSF. | [147] |
| SARS-CoV-2 rS (NCT04368988) | Protein subunit | Phase I clinical trial | The SARS-CoV-2 rS vaccine is an intramuscularly administered nanoparticle vaccine. The nanoparticles are designed by infecting Sf9 insect cells with baculoviruses vectors that express the SARS-CoV-2 S protein. The proteins are correctly folded, undergo a series of programmed modifications, and are eventually transferred to the cell surface. Correctly folded and modified S proteins are then extracted from the cell surface and purified to preserve their three-dimensional structure and biological function, ultimately serving as the immunogenic molecules in the vaccine. The vaccine is also being tested with Matrix M adjuvant. This saponin-based adjuvant acts in part by stimulating the entry of antigen-presenting cells into the injection site and intensifying antigen presentation in the local lymph nodes. | [148] |
| bacTRL-Spike (NCT04334980) | DNA-based | Phase I clinical trial | bacTRL-Spike-1 is an oral vaccine containing live Bifidobacterium longum, which has been modified to deliver plasmids containing synthetic DNA coding for S proteins of SARS-CoV-2 to human cells. These S proteins can then induce immune responses. | [149] |
| V-SARS (NCT04380532) | Inactivated virus | Phase I/II clinical trial | The V-SARS vaccine is made from the heat-inactivated plasma of donors with COVID-19. The idea behind this vaccine is that individuals with COVID-19 will have to circulate SARS-CoV-2. Thus, heat-inactivation of their plasma will efficiently deliver an inactived virus to healthy individuals, inducing an immune response against this pathogen. This vaccine will be administered orally in pill form. | [150] |
| BNT162 (a1, b1, b2, c2) (EudraCT 2020001038-36) | RNA-based vaccine | Phase I/II clinical trial | The BNT162 vaccine trial is comprised of four (a1, b1, b2, and c2) prophylactic SARS-CoV-2 RNA vaccines against COVID-19. Two candidates are the modified nucleoside mRNA (modRNA), one is uridine-containing mRNA (uRNA), and the other candidate is the self-amplifying mRNA (saRNA). These first two vaccines are the spike sequences from SARS-CoV-2, and the other two are only the receptor-binding domain (RBD) sequences. | [151] |
| SARS-CoV-2 inactivated vaccine (Unnamed Candidate) (ChiCTR2000031809) | Inactivated virus | Phase I/II clinical trial | Inactive viral vaccines are generated by multiplying viruses in cell culture (such as in Vero cells) followed by inactivation with a chemical reagent (such as beta-propiolactone). Upon vaccination, this allows the body to induce diverse immune responses against various viral antigens while having no threat of actually being infective. | [152] |
| Measles-Mumps-Rubella Vaccine (NCT04357028) | Other | Phase III clinical trial | The measles-mumps-rubella (MMR) vaccine is composed of live-attenuated viruses. This vaccination is a safe and effective way to prevent these infections, especially in young children. Interestingly, young children do not seem to be overly susceptible to COVID-19. One hypothesis is that antibodies against measles (due to the MMR vaccine) may be cross-reactive with SARS-CoV-2. Thus, vaccination with the MMR vaccine may be protective against COVID-19. | [153] |
| DPX-COVID-19 | protein subunit, lipid-based delivery | Preclinical Phase | DPX is the company’s established lipid-based delivery platform with no aqueous elements in the final formulation. The DPX platform can be formulated with peptide antigens. Its unique “no release” mechanism of action allows antigen-presenting cells (APCs) to be attracted to the injection site, facilitating a robust and sustained immune response within the lymph nodes. This patented technology leverages a novel mechanism of action that allows the in vivo programming of immune cells, which is directed at producing potent new synthetic therapeutics. IMV’s lead candidate, DPX-Survivac, is a T cell-activating immunotherapy combined with survivin. IMV is currently evaluating DPX-Survivac in advanced ovarian cancer, as well as in combination therapy in various clinical studies with Merck’s Keytruda®. IMV is also developing a DPX-based vaccine to combat COVID-19. | [154] |
| LUNAR-COV19 | RNA, mRNA | Preclinical Phase | Low dosage, single-shot, self-replicating mRNA vaccine lacks viral elements or co-adjuvants. The vaccine is based on STARR™ (Self-Transcribing And Replicating RNA), which combines self-replicating RNA with LUNAR® (Lipid-enabled and Unlocked Nucleomonomer Agent modified RNA) lipid-mediated delivery system into a single solution to generate proteins inside the human body. | [154] |
| CureVac | RNA-based vaccine | Preclinical Phase | CureVac proclaimed a positive pre-clinical outcome at a low dose for its lead vaccine candidate against SARS-CoV-2. Its leading vaccine candidate for coronavirus has generated a high level of virus-neutralizing titers after 2 µg dose of vaccination in pre-clinical investigations. CureVac has demanded started the clinical trial Phase 1/2a in June 2020. | [154] |
| PittCoVacc | Protein subunit | Preclinical Phase | PittCoVacc vaccine is an elementary protein vaccine made from a small fragment of one of the viral proteins. It uses a process similar to that in seasonal flu shots. They also leveraged a new method called a microneedle array to deliver the drug for increasing the potency of the vaccine. | [154] |
| AdCOVID | Non-replicating viral vector | Preclinical Phase | The COVID-19 vaccine, named AdCOVID, is a single-dose vaccine candidate that is delivered by an intranasal spray and is designed to activate several components of the immune system including humoral (antibodies), cellular (T-cell) and mucosal immunity. | [154] |
| CoroFlu | Inactivated virus | Preclinical Phase | An intranasal vaccine for coronavirus, ‘CoroFlu’ is under progress. CoroFlu is built on the backbone of FluGen’s flu vaccine candidate known as M2SR. The research group will insert gene sequences from SARS-CoV-2 into M2SR so that the new vaccine will induce immunity against this virus. CoroFlu will also display the influenza virus hemagglutinin protein, which is the dominant influenza virus antigen, so that immune responses will be induced against both coronavirus and influenza (H2N2 seasonal flu strain). | [154] |
10. Adjuvants
Vaccine candidates toward SARS-CoV have been examined in many investigations and include inactivated whole virus vaccine, recombinant S protein preparations, and several viral vectors containing genes coding for SARS-CoV proteins [124], [125], [126]. Many of such vaccine candidates efficiently induced the production of neutralizing antibodies. Such antibodies target the S protein of the SARS-CoV-2, subsequently blocking the binding of viruses to their cellular receptor and inhibiting cell entry. However, there are a few fundamental limitations in vaccine development [127]. One critical issue in vaccine design is to guarantee the efficacy of vaccine while reducing the possible risks correlated with it [127]. The inactivated virus vaccine may not evoke a sufficient immune response and may require multiple booster doses [127].
Similarly, new-generation vaccines that consist of recombinant viral proteins require immunostimulatory molecules [128], [129]. Besides, as with other RNA viruses, these coronaviruses frequently experience recombination; hence, a live attenuated vaccine may reverse to a virulent form and may pose a severe threat to human lives [127]. Ideally, a vaccine against highly pathogenic viruses, including coronaviruses, should generate a protective antibody response to minimize antigen doses quickly and with no adverse reactions [127]. Most of the above issues may be solved by employing a suitable adjuvant in vaccine preparations, which will help elicit a robust immune response, while possibly decreasing the antigen load and the need for multiple doses of vaccine. Thus, selecting an efficacious adjuvant becomes crucial for the development of an adjuvanted vaccine against COVID-19.
Recent data on SARS-CoV-2 vaccine investigations in non-human primates (NHPs) appears promising. An inactivated vaccine (PiCoVacc) adjuvanted with alum, elicited S and RBD protein-specific neutralizing antibodies and protected the macaques from SARS-CoV-2 challenge [127]. Importantly, this study did not show ADE in immunized animals [130]. When administered to macaques, The recombinant S1 protein of the virus fused with Fc and adjuvanted with saponin microemulsion induced potent anti-S1 neutralizing antibodies [131]. The Oxford University's vaccine candidate, ChAdOx1 nCoV-19 encoding the S protein of SARS-CoV-2, protected rhesus monkeys from developing pneumonia [127]. This vaccine significantly diminished the viral loads in bronchoalveolar lavage fluid and respiratory tract tissue without inducing disease progression in vaccinated monkeys [132]. Further, a recent phase I clinical trial with Ad5-nCoV expressing S protein vaccine reported promising results [127]. This vaccine was safe, well-tolerated, and induced both humoral and cellular immunity [133]. Supportive results have also been received from a recent phase I clinical trial for the MERS-CoV vaccine [127]. An MVA-MERS-S (MVA: modified vaccinia virus Ankara) DNA vaccine was safe, well-tolerated, and produced humoral and cell-mediated immune responses in 87% of the participants after receiving the second dose [134]. The MVA vector technology may be repurposed to develop a COVID-19 vaccine by combining SARS-CoV-2 S protein [127]. Moreover, to facilitate SARS-CoV-2 vaccine development, many leading vaccine companies are collaborating to share their previously approved molecular compounds [127]. To date, some vaccine candidates have progressed to either phase I or II clinical trials. Among these, Ad5-nCoV (CanSino Biologics, Inc) is progressing immediately and has reached the phase II clinical trial [127]. Many of these programs are applying the established adjuvant system with their COVID-19 vaccine candidates. An adjuvant based on TLR9 agonists (CpG 1018), which has been developed by Dynavax Technologies Corp., USA, is being used in a recombinant S protein (S-Trimer) vaccine candidate against COVID-19 announced by Clover Biopharmaceutics, China [127]. The CpG 1018 adjuvant has been used in an FDA-approved hepatitis B vaccine (HEPLISAV-B®) [127]. GlaxoSmithKline has also teamed up with many firms such as Xiamen Innovax Biotech and Sanofi, to make an adjuvant technology available to its collaborators for their vaccine candidates [127]. Both Sanofi and Xiamen Innovax Biotech used GSK's AS03 adjuvant (squalene-based) in their recombinant protein vaccine candidates [127]. Similarly, CSL (Commonwealth Serum Laboratories, Australia) and Seqirus (Germany), which employ a novel molecular-clam technology in their COVID-19 vaccine candidate, have tied up to use the MF59 adjuvant [127]. Novavax, (USA), on the other hand, is using its proprietary Matrix-M™ adjuvant for a vaccine candidate (NVX-CoV2373), consisting of nanoparticles carrying SARS-CoV-2 S protein antigens [127]. Another Biopharma company, Soligenix, Inc., has collaborated with BTG. Speciality Pharmaceutics Soligenix is using a novel vaccine adjuvant (CoVaccine HT) from BTG., with its COVID-19 vaccine candidate [127]. CoVaccine HT is an oil-in-water emulsion consisting of sucrose fatty acid sulfate easter (SFASE) and squalene, which has been reported to induce both humoral and cell-mediated immunity [135], [136]. iBio has signed up an agreement with Infectious Disease Research Institute (IDRI, Seattle, US) to utilize their novel adjuvants such as GLA (Glucopyranosyl Lipid Adjuvant), a synthetic analogue of the MPL, for SARS-CoV-2 VLP vaccine development [127], [137].
The experience and knowledge generated from the past vaccine studies with different adjuvants against similar coronaviruses may expedite the development of an adjuvanted vaccine against COVID-19. Inclusion of adjuvant may significantly cut down the amount of antigen in a vaccine, especially when vaccine candidates employ the recombinant S/RBD protein. This could address an overwhelming demand for a vaccine during a pandemic in a short time.
11. Vaccine production
Vaccine development for human application can take years, mainly when novel technologies that have not been widely examined for safety or scaled up for mass production are used. As no coronavirus vaccine is on the market and no large-scale production capability is present, we will need to develop new processes and capacities that can be slow and time-consuming for the first time. The Coalition for Epidemic Preparedness Innovation (CEPI) has granted funds to many highly innovative professionals in the field, and many of them will likely succeed in ultimately producing a SARS-CoV-2 vaccine [12]. However, none of these companies and organizations have an established pipeline to progress such a vaccine to late-stage clinical trials that allow licensure by regulatory agencies and they cannot currently produce the number of doses needed.
An mRNA-based vaccine, which presents target antigens in vivo following injection of mRNA encapsulated in lipid nanoparticles was co-developed by Moderna and the Vaccine Research Center at the National Institutes of Health and a phase I clinical trial has lately started [12]. Curevac is working on a similar vaccine but is still in the preclinical phase [12]. New approaches in preclinical steps comprise recombinant-protein-based vaccines (focused on the S protein, e.g., ExpresS2ion, iBio, Novavax, Baylor College of Medicine, University of Queensland, and Sichuan Clover Biopharmaceuticals), viral-vector-based vaccines (focused on the S protein, e.g., Vaxart, Geovax, University of Oxford, and Cansino Biologics), DNA vaccines (focused on the S protein, e.g., Inovio and Applied DNA. Sciences), live-attenuated vaccines (Codagenix with the Serum Institute of India, etc.), and inactivated virus vaccines [12]. These platforms have several advantages and disadvantages, and it is not plausible to predict which approach will be faster or more prosperous. Johnson & Johnson (J&J) and Sanofi recently joined efforts to develop SARS-CoV-2 vaccines [12]. However, J&J uses an experimental adenovirus vector platform that has not yet resulted in a licensed vaccine [12]. Using a process similar to the means employed for their approved Flublok recombinant influenza virus vaccine [138], Sanofi's vaccine is also months, if not years, from being ready for use.
There are currently no licensed human coronavirus vaccines. Also, many applied technologies are new and need to be entirely examined for safety. Vaccine's target, the S protein, has been identified, and vaccine candidates are being generated. This is usually supported by two critical steps that are typically needed before bringing a vaccine into clinical trials. First, the vaccine is examined in suitable animal models to understand whether it is protective. However, using animal models for SARS-CoV-2 infection might be challenging. The virus does not grow in wild-type mice and only provokes a mild disease in transgenic animals representing human ACE2 [139]. Other potential animal models include ferrets and NHPs, for which pathogenicity studies are ongoing [12]. Even in the absence of an animal model, it is possible to assess the vaccine as serum from vaccinated animals can be examined through in-vitro neutralization assays [12]. Post-challenge safety data should also be obtained in these circumstances for vaccine development such as the ones collected for SARS-CoV-1 and MERS-CoV vaccines [12]. Moreover, vaccines need to be investigated for toxicity in animals such as rabbits [12]. This examination, which has to be performed in compliant with GLP (Good Laboratory Practice), typically takes 3–6 months to be completed [12]. For some vaccine platforms, safety testing elements might be skipped if there is already adequate data for similar vaccines made with same procedures.
Vaccines for humans are constructed in accordance with current Good Manufacturing Practice (cGMP) to ensure consistent their quality and safety [12]. This demands state-of-the-art facilities, trained personnel, proper documentation, and raw materials according to cGMP [12]. These methods have to be planned or corrected to fit SARS-CoV-2 vaccines. For many vaccine candidates in the preclinical phase, such methods do not yet exist and must be improved from scratch. Once sufficient preclinical data are available and initial quantities of the vaccine have been produced in accordance with cGMP, clinical trials might be launched [12]. Typically, clinical vaccine development begins with small phase I trials to evaluate the safety of vaccine candidates in humans [12]. These are followed by phase II (formulation and doses are established to prove initial efficacy) and phase III trials. The efficacy and safety of a vaccine need to be exhibited in a larger cohort [12]. However, in an unusual situation like the current one, this plan might be compromised, and an accelerated approval process might be developed. If efficacy is shown, a vaccine might be licensed by regulatory agencies. Another critical point is that the capacity to produce sufficient amounts of cGMP-quality vaccine needs to be available. For vaccines based on existing platforms, such as inactivated or live attenuated vaccines, this can be relatively easy to achieve, because existing infrastructure can be used. For vaccines based on novel technologies, e.g., mRNA, reaching this capacity typically takes time. Although it would be advantageous even if a restricted number of doses were available to protect healthcare operators and the most vulnerable populations, the goal should be to make vaccines accessible to the global population. Even for influenza virus vaccines, for which many production facilities exist in high-income countries, as well as low- and middle-income countries, the demand in the case of a pandemic would by far surpass the production capacity [12]. Finally, it takes time to distribute vaccines and administer them. To vaccinate a large proportion of the population would likely take weeks [12]. Given that the community is currently naive to SARS-CoV-2, it is highly likely that more than one dose of the vaccine will be necessary [12]. Prime-boost vaccination regimens are typically employed in such cases, and the two vaccinations are regularly spaced 3–4 weeks apart [12]. Protective immunity will likely be achieved only 1–2 weeks following the second vaccination [12]. This, therefore, adds another 1–2 months to the timeline [12]. Even if alternatives for many of the steps discussed earlier can be found, it is unlikely that a vaccine would be accessible earlier than 6 months after the initiation of clinical trials [12]. Realistically, SARS-CoV-2 vaccines will not be available for another 12–18 months [12].
12. The challenges and limitations of SARS-CoV-2 vaccine development
An interesting aspect of the COVID-19 vaccine development is the variety of technology platforms currently being under investigation, including vaccines based on nucleic acid (DNA and RNA), virus-like particles, peptides, viral vectors, recombinant proteins, live-attenuated vaccines and inactivated vaccines [114]. Currently, most vaccines are not based on these platforms. On the other hand, next-generation approaches can be helpful in rapid vaccine development [114]. Some of these vaccine platforms may possibly be suitable for the old population, children, women during pregnancy or lactation, or immune-compromised patients [114]. For some of the platforms mentioned above, adjuvants can be used for improving immunogenic properties and lowering the required dose of administration, thereby increasing the number of individuals who can receive vaccination without compromising immunity.
Vaccine development for human infections can take several years, especially when applying new technologies for improving safety or increasing the production scale. No coronavirus vaccines or no large-scale production are currently present and initial works can be laborious and time-consuming. However, CEPI has granted funds to some of the most innovative research companies in the field, many of which will most probably succeed in making a SARS-CoV-2 vaccine [12]. Nevertheless, none of these centers have developed an FDA-approved COVID-19 vaccine to late-stage clinical trials to be manufactured in large scales. An mRNA-based vaccine that can accurately express the target antigen after the administration of lipid nanoparticle-encapsulated mRNA has been developed by Moderna [12]. Although it seems that the RNA vaccine technology by Moderna, Pfizer, and BioNTech has progressed rapidly, there are some risks regarding this platform, and the efficacy of the vaccine has not yet been approved. Primary data indicate the safety of these vaccines; however, unwanted immune responses may be observed. Furthermore, there is a risk of worsened condition of the disease for all available vaccines where the immune reactions may be unexpected [12]. As expected, all currently investigated vaccines may not be practical in the future as they may not show proper safety or immunogenicity. Moreover, large-scale manufacturing poses a great hurdle. Following the development of a potent and efficient vaccine against COVID-19, immediate large-scale production is required. As of June 30, there are higher than 16, 523, 815 verified cases of COVID-19 worldwide, but another 8 billion people are at risk that can benefit from a vaccine. Although Moderna’s vaccine seems to be efficient and safe up to now, no company has prior experience of RNA vaccine production, let alone in the scale necessary. This company states that it can manufacture millions of doses of the vaccine per month at a factory already established for a different vaccine and are trying to attract other partners. In case a vaccine becomes available in the coming year, first candidates of vaccine administration would be doctors, nurses, paramedics, infants, toddlers, and pregnant women. Other people in priority of receiving the vaccine include high-risk patients and people ≥65 years of age.
Additionally, there are several challenges for development of a vaccine for coronaviruses as these viruses infect the upper respiratory tract, where our immune system is not strong [140] as the upper respiratory tract is considered as an external part of the body. Discovering an approach for neutralizing the virus “outside” of the body is extremely hard and since the epithelial cells get infected, strong immune responses are not activated. Apparently, vaccine production against viruses while not overly inducing the immune reactions is difficult. In case a vaccine induces the immune reaction while not affecting the target cells, subsequent results could be worse than the initial viral infection.
Generally, possible limitations for vaccine development for other viruses must be taken into consideration for COVID-19 vaccine including a: adverse side effects, b: limitation in usage for the immunocompromised patients, c: reversion to a virulent vaccine in case of using live-attenuated vaccines, d: the need for several booster administrations, and e: the shorter period of protection [141].
13. Conclusion
A better understanding regarding the pathogenesis, transmission, and immune reactions against SARS-CoV-2 in animals and humans is still required. Uncertain detection of the virus within the target population(s), the variability of the S protein, and the absence of standardized assays and/or proper animal models are the main reasons for the current gaps in our knowledge. Nonetheless, several vaccine candidates have been developed and reached the clinical trials and other vaccine candidates are soon to be developed. The effective SARS-COV-2 vaccine will induce antibodies with the ability of preventing viral proteins or inducing T cells to destroy infected cells. Currently, clinical trials are ongoing to evaluate the efficacy and potency of different drugs. These trials will let us identify the potential therapies for SARS-CoV-2 infection. For preventing the COVID-19 pandemic, rapid development of a vaccine is required. Despite the lower speed of vaccine development compared to the spread of viral infection, ongoing research is promising. Further understanding of the life cycle and pathogenesis of SARS-CoV-2 will facilitate vaccine and drug development to prevent and treat COVID-19 in the future.
Funding
This work did not receive any particular grant from funding agencies in the commercial and public sectors.
Declaration of Competing Interest
Nothing declared.
Acknowledgments
This work was supported by the Hamadan University of Medical Sciences, Hamadan, Iran.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106928.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.T.W.H. Organization, Coronavirus disease (COVID-19) pandemic, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 4.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z., Cui J., Lu J. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parvin F., Islam S., Urmy Z., Ahmed S. The symptoms, contagious process, prevention and post treatment of COVID-19. Europ. J. Physiotherapy Rehabilit. Stud. 2020 [Google Scholar]
- 6.M.A. Ozma, P. Maroufi, E. Khodadadi, Ş. Köse, I. Esposito, K. Ganbarov, S. Dao, S. Esposito, T. Dal, E. Zeinalzadeh10, Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (Covid-19) during the outbreak period, Inf. Med 28 (2020). [PubMed]
- 7.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulamir A.S., Hafidh R.R. The possible immunological pathways for the variable immunopathogenesis of COVID–19 infections among healthy adults, elderly and children. Electron. J. General Med. 2020;17(4) [Google Scholar]
- 9.Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; 2020. Features, evaluation and treatment coronavirus (COVID-19), Statpearls [internet] [PubMed] [Google Scholar]
- 11.U.S. Pharmacist, Three Strategies Emerging to Develop a COVID-19 Vaccine, 2020. https://www.uspharmacist.com/article/three-strategies-emerging-to-develop-a-covid19-vaccine.
- 12.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020 doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pourjafar M., Samadi P., Khoshinani H.M., Saidijam M. Are mimotope vaccines a good alternative to monoclonal antibodies? Immunotherapy. 2019;11(9):795–800. doi: 10.2217/imt-2018-0213. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020 doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astuti I., Ysrafil Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Haan C.A., Kuo L., Masters P.S., Vennema H., Rottier P.J. Coronavirus particle assembly: primary structure requirements of the membrane protein. J. Virol. 1998;72(8):6838–6850. doi: 10.1128/jvi.72.8.6838-6850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Y. Cong, M. Ulasli, H. Schepers, M. Mauthe, P. V’kovski, F. Kriegenburg, V. Thiel, C.A. de Haan, F. Reggiori, Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle, J. Virol. 94(4) (2020). [DOI] [PMC free article] [PubMed]
- 20.Leung D.T.M., Chi Hang T.F., Chun Hung M., Sheung Chan P.K., Cheung J.L.K., Niu H., Tam J.S.L., Lim P.L. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid. J. Infect. Dis. 2004;190(2):379–386. doi: 10.1086/422040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Liu H., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia, Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chinese J. Tuberculosis Respiratory Dis. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 23.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs, Turk. J. Med. Sci. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020 [Google Scholar]
- 25.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T., Qiu Z., Zhang L., Han Y., He W., Liu Z., Ma X., Fan H., Lu W., Xie J. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 2004;189(4):648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong J., Tang J., Ye C., Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho H., Excler J.-L., Kim J.H., Yoon I.-K. Development of Middle East respiratory syndrome coronavirus vaccines–advances and challenges. Human Vacc. Immunother. 2018;14(2):304–313. doi: 10.1080/21645515.2017.1389362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev Vaccines. 2009;8(7):887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(6):567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes K.V. SARS coronavirus: a new challenge for prevention and therapy. J. Clin. Investig. 2003;111(11):1605–1609. doi: 10.1172/JCI18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navas-Martín S., Weiss S.R. Coronavirus replication and pathogenesis: implications for the recent outbreak of severe acute respiratory syndrome (SARS), and the challenge for vaccine development. J. Neurovirol. 2004;10(2):75–85. doi: 10.1080/13550280490280292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong S., Wang Y.-F., Zhang M.-Y., Liu X.-J., Zhang C.-H., Liu S.-S., Qian C.-W., Li J.-X., Lu J.-H., Wan Z.-Y. Immunogenicity of SARS inactivated vaccine in BALB/c mice. Immunol. Lett. 2004;95(2):139–143. doi: 10.1016/j.imlet.2004.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y., Zhou Y., Siddiqui P., Jiang S. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem. Biophys. Res. Commun. 2004;325(2):445–452. doi: 10.1016/j.bbrc.2004.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takasuka N., Fujii H., Takahashi Y., Kasai M., Morikawa S., Itamura S., Ishii K., Sakaguchi M., Ohnishi K., Ohshima M. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int. Immunol. 2004;16(10):1423–1430. doi: 10.1093/intimm/dxh143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu D., Zheng B., Yao X., Guan Y., Yuan Z.-H., Zhong N.-S., Lu L.-W., Xie J.-P., Wen Y.-M. Intranasal immunization with inactivated SARS-CoV (SARS-associated coronavirus) induced local and serum antibodies in mice. Vaccine. 2005;23(7):924–931. doi: 10.1016/j.vaccine.2004.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadler K., Roberts A., Becker S., Vogel L., Eickmann M., Kolesnikova L., Klenk H.-D., Murphy B., Rappuoli R., Abrignani S. SARS vaccine protective in mice. Emerg. Infect. Dis. 2005;11(8):1312. doi: 10.3201/eid1108.041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.See R.H., Zakhartchouk A.N., Petric M., Lawrence D.J., Mok C.P., Hogan R.J., Rowe T., Zitzow L.A., Karunakaran K.P., Hitt M.M. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J. Gen. Virol. 2006;87(3):641–650. doi: 10.1099/vir.0.81579-0. [DOI] [PubMed] [Google Scholar]
- 40.Lin J., Zhang J.-S., Su N., Xu J.-G., Wang N., Chen J.-T., Chen X., Liu Y.-X., Gao H., Jia Y.-P. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antiviral Therapy. 2007;12(7):1107. [PubMed] [Google Scholar]
- 41.Rocha C.D., Caetano B.C., Machado A.V., Bruña-Romero O. Recombinant viruses as tools to induce protective cellular immunity against infectious diseases. Int. Microbiol. 2004;7:83–94. [PubMed] [Google Scholar]
- 42.Draper S.J., Heeney J.L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010;8(1):62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 43.Rolph M.S., Ramshaw I.A. Recombinant viruses as vaccines and immunological tools. Curr. Opin. Immunol. 1997;9(4):517–524. doi: 10.1016/s0952-7915(97)80104-5. [DOI] [PubMed] [Google Scholar]
- 44.Imler J.-L. Adenovirus vectors as recombinant viral vaccines. Vaccine. 1995;13(13):1143–1151. doi: 10.1016/0264-410x(95)00032-v. [DOI] [PubMed] [Google Scholar]
- 45.Kobinger G.P., Figueredo J.M., Rowe T., Zhi Y., Gao G., Sanmiguel J.C., Bell P., Wivel N.A., Zitzow L.A., Flieder D.B. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine. 2007;25(28):5220–5231. doi: 10.1016/j.vaccine.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enjuanes L., Smerdou C., Castilla J., Antón I.M., Torres J.M., Sola I., Golvano J., Sánchez J.M., Pintado B. Springer; 1995. Development of protection against coronavirus induced diseases, Corona-and Related Viruses; pp. 197–211. [DOI] [PubMed] [Google Scholar]
- 47.Navas-Martin S., Weiss S.R. SARS: lessons learned from other coronaviruses. Viral Immunol. 2003;16(4):461–474. doi: 10.1089/088282403771926292. [DOI] [PubMed] [Google Scholar]
- 48.See R.H., Roper R.L., Brunham R.C., Finlay B.B. Rapid response research-SARS coronavirus vaccines and application of processes to other emerging infectious diseases. Curr. Immunol. Rev. 2005;1(2):185–200. [Google Scholar]
- 49.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsen C.W. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 1993;36(1–2):1–37. doi: 10.1016/0378-1135(93)90126-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antón I.M., González S., Bullido M.J., Corsín M., Risco C., Langeveld J.P., Enjuanes L. Cooperation between transmissible gastroenteritis coronavirus (TGEV) structural proteins in the in vitro induction of virus-specific antibodies. Virus Res. 1996;46(1–2):111–124. doi: 10.1016/S0168-1702(96)01390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu M.-S., Pan Y., Chen H.-Q., Shen Y., Wang X.-C., Sun Y.-J., Tao K.-H. Induction of SARS-nucleoprotein-specific immune response by use of DNA vaccine. Immunol. Lett. 2004;92(3):237–243. doi: 10.1016/j.imlet.2004.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim T.W., Lee J.H., Hung C.-F., Peng S., Roden R., Wang M.-C., Viscidi R., Tsai Y.-C., He L., Chen P.-J. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004;78(9):4638–4645. doi: 10.1128/JVI.78.9.4638-4645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X., Xu W., Tong D., Ni J., Gao H., Wang Y., Chu Y., Li P., Yang X., Xiong S. A chimeric multi-epitope DNA vaccine elicited specific antibody response against severe acute respiratory syndrome-associated coronavirus which attenuated the virulence of SARS-CoV in vitro. Immunol. Lett. 2008;119(1–2):71–77. doi: 10.1016/j.imlet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z.-Y., Kong W.-P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zakhartchouk A.N., Liu Q., Petric M., Babiuk L.A. Augmentation of immune responses to SARS coronavirus by a combination of DNA and whole killed virus vaccines. Vaccine. 2005;23(35):4385–4391. doi: 10.1016/j.vaccine.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma C., Yao K., Zhou F., Zhu M. Comparative immunization in BALB/c mice with recombinant replication-defective adenovirus vector and DNA plasmid expressing a SARS-CoV nucleocapsid protein gene. Cell Mol. Immunol. 2006;3(6):459–465. [PubMed] [Google Scholar]
- 58.Lamirande E.W., DeDiego M.L., Roberts A., Jackson J.P., Alvarez E., Sheahan T., Shieh W.-J., Zaki S.R., Baric R., Enjuanes L. A live attenuated severe acute respiratory syndrome coronavirus is immunogenic and efficacious in golden Syrian hamsters. J. Virol. 2008;82(15):7721–7724. doi: 10.1128/JVI.00304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Züst R., Cervantes-Barragán L., Kuri T., Blakqori G., Weber F., Ludewig B., Thiel V. Coronavirus non-structural protein 1 is a major pathogenicity factor: implications for the rational design of coronavirus vaccines. PLoS Pathog. 2007;3(8) doi: 10.1371/journal.ppat.0030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho H., Excler J.-L., Kim J.H., Yoon I.-K. Development of Middle East Respiratory Syndrome Coronavirus vaccines - advances and challenges. Human Vacc. Immunother. 2018;14(2):304–313. doi: 10.1080/21645515.2017.1389362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muthumani K., Falzarano D., Reuschel E.L., Tingey C., Flingai S., Villarreal D.O., Wise M., Patel A., Izmirly A., Aljuaid A. A synthetic consensus anti–spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 2015;7(301) doi: 10.1126/scitranslmed.aac7462. 301ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L., Shi W., Joyce M.G., Modjarrad K., Zhang Y., Leung K., Lees C.R., Zhou T., Yassine H.M., Kanekiyo M. Evaluation of candidate vaccine approaches for MERS-CoV. Nat. Commun. 2015;6(1):1–11. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lan J., Deng Y., Chen H., Lu G., Wang W., Guo X., Lu Z., Gao G.F., Tan W. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang N., Channappanavar R., Ma C., Wang L., Tang J., Garron T., Tao X., Tasneem S., Lu L., Tseng C.-T.K. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell. Mol. Immunol. 2016;13(2):180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du L., Kou Z., Ma C., Tao X., Wang L., Zhao G., Chen Y., Yu F., Tseng C.-T.K., Zhou Y. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu W.J., Zhao M., Liu K., Xu K., Wong G., Tan W., Gao G.F. T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan Y., Cao D., Zhang Y., Ma J., Qi J., Wang Q., Lu G., Wu Y., Yan J., Shi Y. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lan J., Yao Y., Deng Y., Chen H., Lu G., Wang W., Bao L., Deng W., Wei Q., Gao G.F. Recombinant receptor binding domain protein induces partial protective immunity in rhesus macaques against Middle East respiratory syndrome coronavirus challenge. EBioMedicine. 2015;2(10):1438–1446. doi: 10.1016/j.ebiom.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C., Zheng X., Gai W., Zhao Y., Wang H., Wang H., Feng N., Chi H., Qiu B., Li N. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget. 2017;8(8):12686. doi: 10.18632/oncotarget.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma C., Wang L., Tao X., Zhang N., Yang Y., Tseng C.-T.K., Li F., Zhou Y., Jiang S., Du L. Searching for an ideal vaccine candidate among different MERS coronavirus receptor-binding fragments—the importance of immunofocusing in subunit vaccine design. Vaccine. 2014;32(46):6170–6176. doi: 10.1016/j.vaccine.2014.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tai W., Zhao G., Sun S., Guo Y., Wang Y., Tao X., Tseng C.-T.K., Li F., Jiang S., Lanying D. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology. 2016;499:375–382. doi: 10.1016/j.virol.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang J., Zhang N., Tao X., Zhao G., Guo Y., Tseng C.-T.K., Jiang S., Du L., Zhou Y. Optimization of antigen dose for a receptor-binding domain-based subunit vaccine against MERS coronavirus. Human Vacc. Immunother. 2015;11(5):1244–1250. doi: 10.1080/21645515.2015.1021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C., Glenn G.M., Smith G.E., Frieman M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman C.M., Venkataraman T., Liu Y.V., Glenn G.M., Smith G.E., Flyer D.C., Frieman M.B. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017;35(12):1586–1589. doi: 10.1016/j.vaccine.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volz A., Kupke A., Song F., Jany S., Fux R., Shams-Eldin H., Schmidt J., Becker C., Eickmann M., Becker S. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2015;89(16):8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warimwe G., Gesharisha J., Carr B., Otieno S., Otingah K., Wright D., Charleston B., Okoth E., Elena L., Lorenzo G. Chimpanzee adenovirus vaccine provides multispecies protection against Rift Valley fever. Sci. Rep. 2016;6:20617. doi: 10.1038/srep20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A., Grehan K., Temperton N., Lambe T., Warimwe G. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35(30):3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Almazán F., DeDiego M.L., Sola I., Zuñiga S., Nieto-Torres J.L., Marquez-Jurado S., Andrés G., Enjuanes L. Engineering a replication-competent, propagation-defective Middle East respiratory syndrome coronavirus as a vaccine candidate. MBio. 2013;4(5):e00650–e713. doi: 10.1128/mBio.00650-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tirado S.M.C., Yoon K.-J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 80.Khandia R., Munjal A., Dhama K., Karthik K., Tiwari R., Malik Y.S., Singh R.K., Chaicumpa W. Modulation of Dengue/Zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in Zika virus infection. Front. Immunol. 2018;9:597. doi: 10.3389/fimmu.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gould E.A., Buckley A. Antibody-dependent enhancement of yellow fever and Japanese encephalitis virus neurovirulence. J. General Virol. 1989;70(Pt 6):1605–1608. doi: 10.1099/0022-1317-70-6-1605. [DOI] [PubMed] [Google Scholar]
- 82.Martín-Acebes M.A., Saiz J.-C., Jiménez de Oya N. Antibody-dependent enhancement and Zika: real threat or phantom menace? Front. Cell Infect. Microbiol. 2018;8:44. doi: 10.3389/fcimb.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., Zhou Y., Du L., Li F. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020;94(5):e02015–e2019. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Alwis R., Chen S., Gan E.S., Ooi E.E. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. 2020;55:102768. doi: 10.1016/j.ebiom.2020.102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tekes G., Thiel H.-J. Feline coronaviruses: pathogenesis of feline infectious peritonitis, Advances in virus research. Elsevier. 2016:193–218. doi: 10.1016/bs.aivir.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vennema H., De Groot R., Harbour D., Dalderup M., Gruffydd-Jones T., Horzinek M., Spaan W. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J. Virol. 1990;64(3):1407–1409. doi: 10.1128/jvi.64.3.1407-1409.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yip M., Leung H., Li P., Cheung C., Dutry I., Li D., Daeron M., Bruzzone R., Peiris J.S., Jaume M. Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med J. 2016;22(3 Suppl 4):25–31. [PubMed] [Google Scholar]
- 88.Chan K.R., Ong E.Z., Tan H.C., Zhang S.L.-X., Zhang Q., Tang K.F., Kaliaperumal N., Lim A.P.C., Hibberd M.L., Chan S.H. Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue. Proc. Natl. Acad. Sci. 2014;111(7):2722–2727. doi: 10.1073/pnas.1317454111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nimmerjahn F., Lux A. LILR-B1 blocks activating FcγR signaling to allow antibody dependent enhancement of dengue virus infection. Proc. Natl. Acad. Sci. 2014;111(7):2404–2405. doi: 10.1073/pnas.1324286111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ong E., Zhang S., Tan H., Gan E., Chan K., Ooi E. Dengue virus compartmentalization during antibody-enhanced infection. Sci. Rep. 2017;7:40923. doi: 10.1038/srep40923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan C.Y., Low J.Z.H., Gan E.S., Ong E.Z., Zhang S.L.-X., Tan H.C., Chai X., Ghosh S., Ooi E.E., Chan K.R. Antibody-Dependent dengue virus entry modulates cell intrinsic responses for enhanced infection. Msphere. 2019;4(5):e00528–e619. doi: 10.1128/mSphere.00528-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilder-Smith A., Ooi E.-E., Horstick O., Wills B. Dengue. The Lancet. 2019;393(10169):350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020:1–3. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Z.-Y., Werner H.C., Kong W.-P., Leung K., Traggiai E., Lanzavecchia A., Nabel G.J. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. 2005;102(3):797–801. doi: 10.1073/pnas.0409065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Viruses. 2020;12(3):254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deming D., Sheahan T., Heise M., Yount B., Davis N., Sims A., Suthar M., Harkema J., Whitmore A., Pickles R. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3(12) doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ying L., Xu S., Yang R.F., Li Y.X., Ji Y.Y., He Y.Y., De Shi M., Wei L., Shi T.L., Jin W. Identification of an epitope of SARS-coronavirus nucleocapsid protein. Cell Res. 2003;13(3):141–145. doi: 10.1038/sj.cr.7290158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.J. Wang, J. Wen, J. Li, J. Yin, Q. Zhu, H. Wang, Y. Yang, E.d. Qin, B. You, W. Li, Assessment of immunoreactive synthetic peptides from the structural proteins of severe acute respiratory syndrome coronavirus, Clinical Chemistry 49(12) (2003) 1989-1996. [DOI] [PMC free article] [PubMed]
- 102.Tang F., Quan Y., Xin Z.-T., Wrammert J., Ma M.-J., Lv H., Wang T.-B., Yang H., Richardus J.H., Liu W. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J. Immunol. 2011;186(12):7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 103.Ng O.-W., Chia A., Tan A.T., Jadi R.S., Leong H.N., Bertoletti A., Tan Y.-J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34(17):2008–2014. doi: 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.C.K.-f. Li, H. Wu, H. Yan, S. Ma, L. Wang, M. Zhang, X. Tang, N.J. Temperton, R.A. Weiss, J.M. Brenchley, T cell responses to whole SARS coronavirus in humans, J. Immunol. 181(8) (2008) 5490-5500. [DOI] [PMC free article] [PubMed]
- 105.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88(19):11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mo H.-Y., Xu J., Ren X.-L., Zeng G.-Q., Tan Y.-X., Chen R.-C., Chan-Yeung M., Zhong N.-S. Evaluation by indirect immunofluorescent assay and enzyme linked immunosorbent assay of the dynamic changes of serum antibody responses against severe acute respiratory syndrome coronavirus. Chin. Med. J. 2005;118(6):446–450. [PubMed] [Google Scholar]
- 108.Xu X., Gao X.-M. Immunological responses against SARS-coronavirus infection in humans. Cell Mol. Immunol. 2004;1(2):119–122. [PubMed] [Google Scholar]
- 109.Zhong X., Yang H., Guo Z.-F., Sin W.-Y.F., Chen W., Xu J., Fu L., Wu J., Mak C.-K.G., Cheng C.-S.S. B-cell responses in patients who have recovered from severe acute respiratory syndrome target a dominant site in the S2 domain of the surface spike glycoprotein. J. Virol. 2005;79(6):3401–3408. doi: 10.1128/JVI.79.6.3401-3408.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li T., Xie J., He Y., Fan H., Baril L., Qiu Z., Han Y., Xu W., Zhang W., You H. Long-term persistence of robust antibody and cytotoxic T cell responses in recovered patients infected with SARS coronavirus. PLoS ONE. 2006;1(1) doi: 10.1371/journal.pone.0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L., Korkmaz E., Falo L.D., Jr., Gambotto A. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Graham B.S. Advances in antiviral vaccine development. Immunol. Rev. 2013;255(1):230–242. doi: 10.1111/imr.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pawelec G., Weng N.-P. Can an effective SARS-CoV-2 vaccine be developed for the older population? Immun. Ageing. 2020;17:8. doi: 10.1186/s12979-020-00180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le T.T., Andreadakis Z., Kumar A., Roman R.G., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 115.BioSpace, The Hopes and Challenges of a COVID-19 Vaccine 2020. https://www.biospace.com/article/the-covid-19-vaccine-challenge-timelines-and-innovation/.
- 116.P. Vaccinations, Ad5-nCoV COVID-19 Vaccine, 2020. https://www.precisionvaccinations.com/vaccines/ad5-ncov-covid-19-vaccine.
- 117.F.-C. Zhu, Y.-H. Li, X.-H. Guan, L.-H. Hou, W.-J. Wang, J.-X. Li, S.-P. Wu, B.-S. Wang, Z. Wang, L. Wang, S.-Y. Jia, H.-D. Jiang, L. Wang, T. Jiang, Y. Hu, J.-B. Gou, S.-B. Xu, J.-J. Xu, X.-W. Wang, W. Wang, W. Chen, Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial, The Lancet. [DOI] [PMC free article] [PubMed]
- 118.clinicaltrials, A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) (CTII-nCoV), 2020. https://clinicaltrials.gov/ct2/show/NCT04341389.
- 119.clinicaltrials.gov, Safety and Immunity of Covid-19 aAPC Vaccine, 2020. https://clinicaltrials.gov/ct2/show/NCT04299724.
- 120.clinicaltrials.gov, Reducing Health Care Workers Absenteeism in Covid-19 Pandemic Through BCG Vaccine (BCG-CORONA), 2020. https://clinicaltrials.gov/ct2/show/NCT04328441.
- 121.T.I.V.I. (IVI), IVI, INOVIO, and KNIH to partner with CEPI in a Phase I/II clinical trial of INOVIO’s COVID-19 DNA vaccine in South Korea, 2020. https://www.ivi.int/ivi-inovio-and-knih-to-partner-with-cepi-in-a-phase-i-ii-clinical-trial-of-inovios-covid-19-dna-vaccine-in-south-korea/.
- 122.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanofi, Sanofi joins forces with U.S. Department of Health and Human Services to advance a novel coronavirus vaccine, 2020. https://www.sanofi.com/en/media-room/press-releases/2020/2020-02-18-16-00-00.
- 124.Roberts A., Lamirande E.W., Vogel L., Jackson J.P., Paddock C.D., Guarner J., Zaki S.R., Sheahan T., Baric R., Subbarao K. Animal models and vaccines for SARS-CoV infection. Virus Res. 2008;133(1):20–32. doi: 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du L., Tai W., Zhou Y., Jiang S. Vaccines for the prevention against the threat of MERS-CoV. Expert review of vaccines. 2016;15(9):1123–1134. doi: 10.1586/14760584.2016.1167603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang S., He Y., Liu S. SARS vaccine development. Emerg. Infect. Dis. 2005;11(7):1016. doi: 10.3201/eid1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int. Immunopharmacol. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonam S.R., Partidos C.D., Halmuthur S.K.M., Muller S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017;38(9):771–793. doi: 10.1016/j.tips.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 129.Aguilar J., Rodriguez E. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 130.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., Gao H., Ge X., Kan B., Hu Y., Liu J., Cai F., Jiang D., Yin Y., Qin C., Li J., Gong X., Lou X., Shi W., Wu D., Zhang H., Zhu L., Deng W., Li Y., Lu J., Li C., Wang X., Yin W., Zhang Y., Qin C. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.W. Ren, H. Sun, G.F. Gao, J. Chen, S. Sun, R. Zhao, G. Gao, Y. Hu, G. Zhao, Y. Chen, X. Jin, F. Fang, J. Chen, Q. Wang, S. Gong, W. Gao, Y. Sun, J. Su, A. He, X. Cheng, M. Li, C. Xia, M. Li, L. Sun, Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralizing responses in nonhuman primates, bioRxiv (2020) 2020.04.21.052209. [DOI] [PMC free article] [PubMed]
- 132.N. van Doremalen, T. Lambe, A. Spencer, S. Belij-Rammerstorfer, J.N. Purushotham, J.R. Port, V. Avanzato, T. Bushmaker, A. Flaxman, M. Ulaszewska, ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques, bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 133.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X., Wu S.-P., Wang B.-S., Wang Z., Wang L. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koch T., Dahlke C., Fathi A., Kupke A., Krähling V., Okba N.M., Halwe S., Rohde C., Eickmann M., Volz A. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lakhan N., Stevens N.E., Diener K.R., Hayball J.D. CoVaccine HT™ adjuvant is superior to Freund's adjuvants in eliciting antibodies against the endogenous alarmin HMGB1. J. Immunol. Methods. 2016;439:37–43. doi: 10.1016/j.jim.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 136.Bodewes R., Kreijtz J., van Amerongen G., Geelhoed-Mieras M., Verburgh R., Heldens J., Bedwell J., van den Brand J., Kuiken T., van Baalen C. A single immunization with CoVaccine HT-adjuvanted H5N1 influenza virus vaccine induces protective cellular and humoral immune responses in ferrets. J. Virol. 2010;84(16):7943–7952. doi: 10.1128/JVI.00549-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coler R.N., Bertholet S., Moutaftsi M., Guderian J.A., Windish H.P., Baldwin S.L., Laughlin E.M., Duthie M.S., Fox C.B., Carter D. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou Z., Post P., Chubet R., Holtz K., McPherson C., Petric M., Cox M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24(17):3624–3631. doi: 10.1016/j.vaccine.2006.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.L. Bao, W. Deng, B. Huang, H. Gao, L. Ren, Q. Wei, P. Yu, Y. Xu, J. Liu, F. Qi, The pathogenicity of 2019 novel coronavirus in hACE2 transgenic mice. bioRxiv 2020, preprint 10.1101/2020.02. 07.939389.
- 140.Gillim-Ross L., Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 2006;19(4):614–636. doi: 10.1128/CMR.00005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bowick G.C., McAuley A.J. Vaccine and adjuvant design for emerging viruses: mutations, deletions, segments and signaling. Bioengineered Bugs. 2011;2(3):129–135. doi: 10.4161/bbug.2.3.15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.clinicaltrials.gov, Phase I Clinical Trial of a COVID-19 Vaccine in 18-60 Healthy Adults (CTCOVID-19), 2020. https://clinicaltrials.gov/ct2/show/NCT04313127.
- 143.clinicaltrials.gov, A Study of a Candidate COVID-19 Vaccine (COV001), 2020. https://clinicaltrials.gov/ct2/show/NCT04324606.
- 144.clinicaltrials.gov, Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19), 2020. https://clinicaltrials.gov/ct2/show/NCT04283461.
- 145.clinicaltrials.gov, Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers, 2020. https://clinicaltrials.gov/ct2/show/NCT04336410.
- 146.clinicaltrials.gov, Immunity and Safety of Covid-19 Synthetic Minigene Vaccine, 2020. clinicaltrials.gov.
- 147.clinicaltrials.gov, Phase Ib-II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Frontline Healthcare Workers and First Responders, 2020. https://clinicaltrials.gov/ct2/show/NCT04386252.
- 148.clinicaltrials.gov, Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant, 2020. https://clinicaltrials.gov/ct2/show/NCT04368988.
- 149.clinicaltrials.gov, Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19, 2020. https://clinicaltrials.gov/ct2/show/NCT04334980.
- 150.clinicaltrials.gov, Tableted COVID-19 Therapeutic Vaccine (COVID-19), 2020. https://clinicaltrials.gov/ct2/show/NCT04380532.
- 151.clinicaltrialsregister.eu, Full Title: A Multi-site Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-2019 Using Different Dosing Regimens, 2020. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001038-36.
- 152.C.C.T.R. (ChiCTR), A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells), 2020. http://www.chictr.org.cn/showprojen.aspx?proj=52227.
- 153.clinicaltrials.gov, Measles Vaccine in HCW (MV-COVID19), 2020. https://clinicaltrials.gov/ct2/show/NCT04357028.
- 154.covid-vaccine-tracker, COVID-19 Vaccine & Therapeutics Tracker, 2020. https://biorender.com/covid-vaccine-tracker/details/v-0CV3/bacille-calmette-guerin-bcg.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.