Abstract
We conducted a study of combined treatment with docetaxel, bevacizumab, and everolimus in patients with chemotherapy-naive metastatic castrate-resistant prostate cancer. Although we establish a safe dose for coadministration of these 3 agents and our early results suggested encouraging levels of anti-cancer efficacy, our final results do not support further exploration of this treatment regimen for castrate-resistant prostate cancer.
Background
Previous data suggests that co-targeting mammalian target of rapamycin and angiogenic pathways may potentiate effects of cytotoxic chemotherapy. We studied combining mammalian target of rapamycin and vascular endothelial growth factor inhibition with docetaxel in castrate-resistant prostate cancer (CRPC).
Methods
Eligible patients had progressive, metastatic, chemotherapy-naive CRPC. Docetaxel and bevacizumab were given intravenously day 1 with everolimus orally daily on a 21-day cycle across 3 dose levels (75:15:2.5, 75:15:5, and 65:15:5; docetaxel mg/m2, mg/kg bevacizumab, and mg everolimus, respectively). Maintenance therapy with bevacizumab/everolimus without docetaxel was allowed after ≥ 6 cycles.
Results
Forty-three subjects were treated across all dose levels. Maximal tolerated doses for the combined therapies observed in the phase 1B portion of the trial were: docetaxel 75 mg/m2, bevacizumab 15 mg/kg, and everolimus 2.5 mg. Maximal prostate-specific antigen decline ≥ 30% and ≥ 50% was achieved in 33 (79%) and 31 (74%) of patients, respectively. Best response by modified Response Evaluation Criteria In Solid Tumors criteria in 25 subjects with measurable disease at baseline included complete or partial response in 20 (80%) patients. The median progression-free and overall survival were 8.9 months (95% confidence interval, 7.4–10.6 months) and 21.9 months (95% confidence interval, 18.4–30.3 months), respectively. Hematologic toxicities were the most common treatment-related grade≥ 3 adverse events including: febrile neutropenia (12; 28%), lymphopenia (12; 28%), leukocytes (10; 23%), neutrophils (9; 21%), and hemoglobin (2; 5%). Nonhematologic grade ≥ 3 adverse events included: hypertension (8; 19%), fatigue (3; 7%), pneumonia (3; 7%), and mucositis (4; 5%). There was 1 treatment-related death owing to neutropenic fever and pneumonia in a patient treated at dose level 3 despite dose modifications and prophylactic growth factor support.
Conclusions
Docetaxel, bevacizumab, and everolimus can be safely administered in CRPC and demonstrate a significant level of anticancer activity, meeting the predetermined response criteria. However, any potential benefit of combined therapy must be balanced against increased risk for toxicities. Our results do not support the hypothesis that this combination of agents improves upon the results obtained with docetaxel monotherapy in an unselected population of chemotherapy-naive patients with CRPC.
Keywords: Anti-angiogenic therapy, Combination chemotherapy, Clinical trial, mTOR inhibitor
Background
The phosphatase and tensin homolog (PTEN)/PI3 kinase/ mammalian target of rapamycin (mTOR) axis is a potential pharmacologic target in castrate resistant prostate cancer (CRPC). The PI3 kinase controls cellular response to growth factors, stress, and survival. Deregulation of the PI3 kinase axis is common in prostate cancer, often initiated by loss of function of negative regulators such as PTEN.1 The mTOR is a key downstream effector in the PI3 kinase pathway. Everolimus (formerly RAD001) is an orally available rapamycin analog that inhibits mTOR and is now approved by the United States Food and Drug Administration for certain subpopulations of patients with breast, renal, and neuro-endocrine cancers. Preclinical data shows that mTOR inhibitors are active in a number of different cancer models, particularly when alterations in the PTEN/PI3 kinase axis are present.2,3 Everolimus has been shown to potentiate chemotherapy-induced apoptosis in laboratory-based studies.4 Phase I studies have demonstrated the safety of everolimus in patients with solid tumors, including prostate cancer.5,6 Phase II studies of mTOR inhibitors (everolimus, temsirolimus, ridaforolimus) administered as single agents in CRPC demonstrated generally low activity in CRPC.7–10 Other studies explored safety and potential efficacy of adding everolimus or temsirolimus to docetaxel11–13 or bicalutamide14,15 without evidence of definitive benefit. The combination of an mTOR inhibitor with chemotherapy or hormone therapy for CRPC has not been studied in randomized, phase III trials.
Angiogenesis is another pathway that is relevant to the growth and metastatic progression of CRPC. Vascular endothelial growth factor (VEGF) is a key circulating factor secreted by cancer cells and tissues, which contributes to neovascularization and tumor formation.16 Plasma and urine VEGF levels are elevated in CRPC and have been shown to be independent predictors of survival.17,18 Bevacizumab (Avastin) is a humanized antibody-neutralizing VEGF that is approved by the United States Food and Drug Administration for the treatment of certain forms of lung, colorectal, renal, gynecologic, and central nervous system cancer. Noncomparative studies suggested bevacizumab would enhance docetaxel efficacy in CRPC.19–21 However, a randomized phase III trial investigating adding bevacizumab to docetaxel for CRPC failed to demonstrate improved overall survival (hazard ratio, 0.91; 95% confidence interval [CI], 0.78–1.05) despite improvement in progression-free survival (9.9 months vs. 7.5 months; P < .001) and objective responses (49.4% vs. 35.5%; P = .001).22 Toxicity was also a concern in this trial, with a higher rate of treatment-related death in the bevacizumab arm (4%) compared with the standard treatment arm (1.2%).
Although some previous studies support the activation and therapeutic potential of targeting both mTOR and VEGF in metastatic prostate cancer, pair-wise targeting of either pathway along with cytotoxic chemotherapy in CRPC has generally not been successful. We hypothesized that co-targeting both of these pathways would be feasible and more effective than standard docetaxel in patients with metastatic CRPC. Here, we describe the safety and efficacy from the phase IB/II clinical trial of docetaxel plus bevacizumab and everolimus.
Methods
Study Population
Eligible patients were required to have with histologically proven metastatic adenocarcinoma of the prostate progressing in the presence of castrate levels of circulating testosterone. Progressive disease was defined by any one of the following criteria: objective disease progression according to Response Evaluation Criteria In Solid Tumors (RECIST) 1.0 criteria23; appearance of 1 or more new lesions on bone scan; or a rise in PSA ≥ 5 ng/mL on at least 2 occasions≥ 1 week apart. To be included, patients were required to be ≥ 18 years of age with Eastern Cooperative Oncology Group performance status≤ 2, and with adequate organ and hematologic function. Major exclusion criteria included: prior treatment with cytotoxic chemotherapy for metastatic cancer; prior use of antangiogenic agents; prior use of an mTOR inhibitor; known brain metastases (brain imaging was not required); congestive heart failure (New York Heart Association Class II or above); uncontrolled hypertension; bleeding diathesis; history of arterial or venous thromboembolic events; unstable angina or recent myocardial infarction (prior 12 months); proteinuria; peripheral neuropathy ≥ grade 2.
The protocol was approved by the Institutional Review Boards at the Cedars Sinai Medical Center and the University of Southern California and registered with the US National Clinical Trials Registry (NCT00574769). Written in formed consent was documented from all patients prior to the performance of any study-related procedure.
Study Design and Methods
The study was designed as a phase IB safety and dose-finding trial followed by a phase II dose-expansion trial to explore efficacy for the combination treatment. Combined treatment with docetaxel, everolimus, and bevacizumab was continued for up to 12 cycles followed by a "maintenance" phase of everolimus and bevacizumab without docetaxel continuing for up to 12 additional cycles. Transition to the maintenance phase was allowed after cycle 6 for patients who achieved a maximal clinical response or unacceptable toxicity related to docetaxel.
The primary objective of the phase IB portion of the trial was to establish a maximal tolerated dose of docetaxel/everolimus/bevacizumab. Adverse events were coded using National Cancer Institute Common Toxicity Criteria v.3 criteria. Dose-limiting toxicities (DLTs) were defined as grade ≥ 4 anemia, grade ≥ 4 neutropenia (including neutropenic fever), grade 3 thrombocytopenia, or other ≥ grade 3 nonhematologic toxicity. Sequential enrollment into dosing cohorts preceded as follows: (1) if 1 of 3 subjects in a cohort experienced a DLT, then that cohort was expanded with an additional 3 subjects; (2) if 2 or more patients in any cohort experience a DLT, than the previous dose level will be defined as the maximal tolerated dose.
The primary objective of the phase II portion of the trial was to evaluate the clinical efficacy of this combination in terms as best overall clinical response and progression-free survival in patients with CRPC. Clinical efficacy was determined using predetermined criteria for the composite endpoints of disease stabilization, measurable tumor regression, and/or biochemical responses (prostate-specific antigen [PSA] declines). A clinically meaningful rate of response using this composite endpoint was defined as 35% as indicated by the lower bound of a 2-sided 90% CI. An actual response rate of 50% would be required to achieve this lower bound with 30 patients. Progression-free survival was defined according to Prostate Cancer Working Group 2 criteria (summarized as a confirmed rise in PSA ≥ 25% and ≥ 2 ng/mL above the nadir) as the main consensus criteria for progression in use while the trial was active. Progression in soft tissue or bone lesions was defined according to a modified version of RECIST criteria incorporating changes in PSA with the caveat that confirmatory scans were not always obtained prior to start of next therapy.23
Drug Treatment
For the phase IB, dose-finding portion of the trial, patients were enrolled sequentially into treatment cohorts of 3 to 6 subjects (Table 1). The bevacizumab dose remained constant at 15 mg/kg day 1 administered prior to docetaxel across all dose levels. The initial protocol included 2 dose levels for docetaxel/everolimus summarized as: level 1: 75 mg/m2/2.5 mg and level 2: 75 mg/m2/5 mg. After DLTs were observed in 2 of 2 subjects at level 2, the protocol was amended to incorporate a third dose level with a ~15% dose reduction in docetaxel from 75 mg/m2 to 65 mg/m2 while maintaining the same everolimus dose intensity (5 mg daily). Docetaxel was administered by vein on day 1, and everolimus was administered continuously on a 21-day cycle starting day 2 of cycle 1. Pre-medications included dexamethasone 8 mg, which was administered orally 12 hours and 6 hours prior to docetaxel, along with other standard anti-emetic and supportive measures. Prophylactic myeloid growth factors were not allowed during cycle 1, but could be started for following cycles based on the clinical judgment of the treating physician. In general, dose modifications or interruptions were required for grade ≥ 3 toxicities. Concurrent anti-cancer treatment was not allowed except for a gonadotropin-releasing hormone agonist/antagonist and/or a bone-stabilizing agent (bisphosphonate, denosumab).
Table 1.
Enrollment by Dose Levels
| Dose Level | Docetaxel, mg/m2 | Everolimus, mg | Bevacizumab, mg/kg | Enrollment |
|---|---|---|---|---|
| 1 | 75 | 2.5 | 15 | n = 37a |
| 2 | 75 | 5 | 15 | n = 2 |
| 3 | 65 | 5 | 15 | n = 4 |
Includes 6 subjects enrolled In Phase 1B and 31 subjects enrolled In Phase 2.
Results
Patient Characteristics
A total of 43 subjects were enrolled in the study across all cohorts, including 12 patients enrolled in the phase IB portion between November 2007 and June 2008 and 31 subjects in the phase II cohort from November 2008 to July 2014 (Table 1). An interruption in enrollment occurred between June 2009 and March 2010 owing to the transfer of the primary investigator between institutions. Baseline characteristics for the patients according to dosing cohorts are summarized in Table 1. Across all subjects, median (range) for demographic variables are summarized as: age, 65 (50–79) years; PSA, 76.6 (0–1847) ng/mL; alkaline phosphatase, 114 (37–768) U/L; and hemoglobin 12.5 (0.0–15.7) g/dL. Metastatic sites included: bone (38; 88%), lymph nodes (19; 44%), and viscera in (8; 19%) patients. Given the long period of enrollment, newer, highly-specific androgen receptor signaling inhibitors were not available during the initial period of trial activation; thus no patients received these agents during the phase IB portion of the trial. More recently, many patients enrolled on the trial did receive prior treatment with highly-specific androgen receptor signaling inhibitors such as abiraterone, orteronel, or enzalutamide obtained either through a clinical trial or as standard care as summarized in Table 1.
Defining Optimal Phase II Dose
Twelve patients were treated in the dose-finding cohorts (Table 2). One subject experienced 2 DLTs at the level 1 dose (docetaxel 75 mg/m2, everolimus 2.5 mg) recorded as grade 3 neutropenic fever and pneumonia. Therefore, this cohort was expanded to include another 3 subjects with no subsequent DLTs observed. Two subjects were then enrolled at the level 2 dose (docetaxel 75 mg/m2, everolimus 5 mg). Two DLTs were observed in the first 2 subjects (both grade 3 neutropenic fevers); therefore, this cohort was closed to subsequent enrollment. The protocol was then amended to include a level 3 cohort preserving the everolimus dose at 5 mg and lowering docetaxel dose to 65 mg/m2. DLTs were observed in 3 of 4 patients enrolled at this dose including neutropenic fever, grade 3 and grade 5, and pulmonary embolism, grade 3. A patient in cohort 3 died from neutropenic fever and sepsis at cycle 3 despite dose reductions and growth factor support instituted after experiencing a neutropenic fever during cycle 1. Therefore, dose level 1 was declared the maximal tolerated dose to be used in the dose expansion study.
Table 2.
Demographic and Clinical Characteristics
| Median (Range) | |||
|---|---|---|---|
| Phase 1B (n = 12) | Phase II (n = 31) | Total (n = 43) | |
| Age, y | 69 (58–77) | 64 (50–79) | 65 (50–79) |
| PSA, ng/dL | 115 (4–1335) | 69 (0–1847) | 76.6 (0–1847) |
| Alkaline phosphatase, U/mL | 108 (37–763) | 127 (45–768) | 114 (37–768) |
| Hgb, g/dL | 12.4 (10.3–13.7) | 12.5 (9.0–15.7) | 12.5 (9.0–15.7) |
| Metastatic sites, N (%) | |||
| Bone | 11 (92) | 27 (87) | 38 (88) |
| Viscera | 2(17) | 6 (19) | 8(19) |
| Lymph node | 5(42) | 14 (45) | 19 (44) |
| Prior treatment with highly-specific androgen inhibitor, N (%) | |||
| Abiraterone | 0/12 (0) | 11/31 (39) | 11/43 (26) |
| Enzalutamide | 0/12 (0) | 2/31 (6) | 2/43 (5) |
| Orteronel | 0/12 (0) | 3/31 (10) | 3/43 (7) |
Abbreviations: Hgb = hemoglobin; PSA = prostate-specific antigen.
Study Treatment Delivered
A total of 150 cycles of treatment were delivered over the entire course of the study, divided as 116 cycles with docetaxel and 44 as maintenance. On a per patient basis, the median cycles of docetaxel delivered per patient was 9 (range, 3–12). Considering only patients treated at the recommended phase II dose (level 1), maintenance therapy without docetaxel was given to 22 of 37 subjects for a median of 3 (range, 1–10) cycles.
Clinical Response
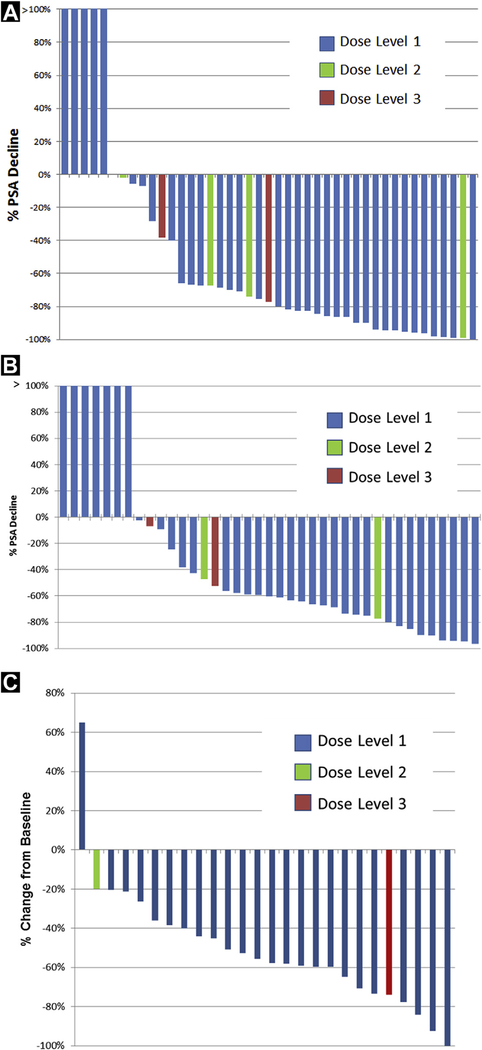
Clinical response according to dose levels is summarized in Figure 1 and Table 3. PSA response was assessable in 41 subjects with baseline PSA ≥ 2 ng/mL according to Prostate Cancer Working Group 2 guidelines.24 Maximal PSA decline ≥ 30% and ≥ 50% was achieved in 32 (78%) and 30 (73%) patients, respectively. At the 12-week landmark, PSA decline ≥ 30% and ≥ 50% were noted in in 27 (71%) and 24 (63%) patients, respectively. Response was evaluable according to RECIST criteria in 26 subjects.23 Investigator assessed maximal responses are summarized as: 1 (4%) complete response with confirmed resolution of retroperitoneal lymphadenopathy; partial response in 19 (76%) subjects, stable disease in 4 (16%) subjects, and progressive disease in 1 (4%) subject.
Figure 1.
Clinical Response Represented Per Patient. Maximal (A) and Change at 12 Weeks (B) Compared With Baseline Percent PSA Changes on Treatment. (C) Best Response for Target Lesion Measurements From Baseline According to Response Evaluation Criteria in Solid Tumors Criteria23
Table 3.
Clinical Response by Dose Levels
| PSA Change From Baselinea | Level 1 (n = 33) N (%) | Level 2 (n = 2) N (%) | Level 3 (n = 4) N (%) | Total (n = 39) N (%) |
| Maximum decline | ||||
| ≥30% | 25 (81) | 2 (100) | 3(75) | 30 (77) |
| ≥50% | 24 (77) | 1 (50) | 3(75) | 38 (72) |
| Decline at 12 wk | ||||
| ≥30% | 22 (67) | 2 (100) | 2(50) | 26 (67) |
| ≥50% | 21 (64) | 1 (25) | 1 (25) | 23 (59) |
| RECIST | Level 1 (n = 22) N (%) | Level 2 (n = 2) N (%) | Level 3 (n = 1) N (%) | Total (n = 25) N (%) |
| Stable disease | 4(18) | 1 (50) | 5(20) | |
| Partial response | 18 (82) | 1 (100) | 19 (76) | |
| Complete response | 1 (5) | 1 (4) |
Abbreviations: PSA = prostate-specific antigen; RECIST = Response Evaluation Criteria In Solid Tumors.
Baseline PSA ≥ 2 ng/mL.
The primary endpoint of the phase II portion of the trial was a composite endpoint incorporating declines in PSA and measurable disease defined as ≥ 30% decline in PSA, stable disease or better by imaging criteria, or both. Thus, a positive composite endpoint was achieved in 34 (92%) of 37 subjects treated at the recommended (level 1) dose schedule.
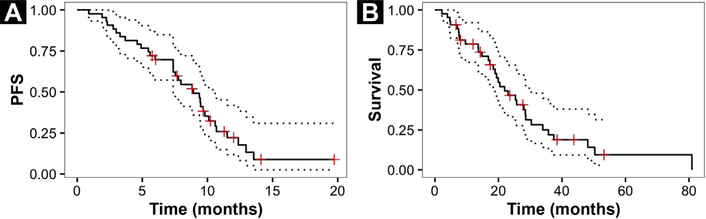
The median progression-free and overall survivals were 8.9 months (95% CI, 7.4–10.6 months) and 21.9 months (95% CI, 18.4–30.3 months), respectively (Figure 2).
Figure 2.
Kaplan-Meier Analysis With 95% Confidence Intervals for Progression-free (A) and Overall Survival (B)
Toxicity
Common toxicities are summarized in Table 4 and a detailed list of toxicities is provided in the Supplementary Table 1. Hematologic toxicities were the most common treatment-related grade ≥ 3 adverse events including: febrile neutropenia (12; 28%), lymphopenia (12; 28%), leukocytes (10; 23%), neutrophils (9; 21%), and hemoglobin (2; 5%). Nonhematologic grade ≥ 3 adverse events included: hypertension (8; 19%), fatigue (3; 7%), pneumonia (3; 7%), and mucositis (4; 5%). There was 1 treatment-related death owing to neutropenic fever and pneumonia in a patient treated at dose level 3 despite dose modifications and prophylactic growth factor support.
Table 4.
Common and Important Toxicities by CTC Grade and Body System
| Body System | CTC Name | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|
| Blood/bone marrow | Hemoglobin | 14 | 16 | 2 | 0 | 0 |
| Leukocytes | 6 | 5 | 6 | 4 | 0 | |
| Lymphopenia | 10 | 4 | 12 | 0 | 0 | |
| Constitutional symptoms | Fatigue | 47 | 17 | 3 | 0 | 0 |
| Cardiac general | Hypertension | 2 | 2 | 8 | 0 | 0 |
| Gastrointestinal | Anorexia | 34 | 5 | 1 | 0 | 0 |
| Diarrhea | 20 | 4 | 1 | 0 | 0 | |
| Mucositis | 46 | 15 | 2 | 0 | 0 | |
| Nausea | 31 | 4 | 0 | 0 | 0 | |
| Hemorrhage/bleeding | Hemorrhage (all sites) | 18 | 1 | 1 | 0 | 0 |
| Infection | Febrile neutropenia | 0 | 0 | 11 | 2 | 1 |
| Pneumonia | 0 | 0 | 3 | 0 | 1 | |
| Pulmonary embolism | 0 | 0 | 1 | 0 | 0 | |
| Metabolic/laboratory | Hyperglycemia | 9 | 4 | 0 | 0 | 0 |
| Hypertriglyceridemia | 15 | 2 | 0 | 0 | 0 | |
| Proteinuria | 3 | 10 | 0 | 0 | 0 | |
| Neurology | Neuropathy-sensory | 29 | 1 | 0 | 0 | 0 |
| Pulmonary/upper respiratory | Pneumonitis/pulmonary infiltrates | 1 | 0 | 2 | 0 | 0 |
| Vascular | Embolism | 0 | 1 | 0 | 0 | 0 |
| Thrombosis | 0 | 2 | 1 | 0 | 0 |
Abbreviations: CTC = common toxicity criteria; G = grade.
Discussion
The primary goal for the initial phase of this trial was to define a safe dose for the combination of docetaxel with everolimus and bevacizumab. Daily prednisone was not included owing to concern for overlapping immunosuppressive effects with everolimus. When used as monotherapy for breast, lung, renal, and brain cancer, the commonly used dose ranges for these agents are docetaxel 75 to 100 mg/m2 every 21 days, everolimus 10 mg daily, and bevacizumab 5 to 15 mg/kg every 14 to 21 days. Previously, several studies have reported increased risk for myelosuppression when everolimus is used with docetaxel. Moulder et al reported significant hematologic toxicity in a phase I study investigating dose ranges of docetaxel 40 to 75 mg/m2 day 1 and everolimus 20 to 50 mg day 1 and day 8 on a 21-day cycle.25 Similarly, Ramalingam et al defined docetaxel 60 mg/m2 every 21 days and everolimus 5 mg days 1 to 19 as the maximum tolerated dose in patients with none—small-cell lung cancer owing to hematologic toxicities (especially neutropenia) seen at higher dose levels.26 Courtney et al reported the results of a dose-escalation study in CRPC with docetaxel every 21 days/everolimus daily given in 3 dose ranges: 60 mg/m2/5 mg, 60 mg/m2/10 mg, and 70 mg/mm2/5 mg.12 Significant myelosuppression was observed at the highest dose level, leading these investigators to define the middle dose level (docetaxel 60 mg/m2, everolimus 10 mg) as the maximum tolerated dose. In the present study, we found that we could maintain the standard dose intensity for docetaxel (75 mg/m2) without myeloid growth factors only when used with an everolimus dose significantly less than the standard dose used as monotherapy (2.5 mg vs. 10 mg as monotherapy). A limitation of the current study is the lack of pharmacodynamics measurements as it is uncertain if mTOR was effectively targeted in target tissue at this dose level.
Many previous studies have shown much less potential for additive hematologic toxicity for bevacizumab used with taxanes.27 Most of the other toxicities encountered in this trial were consistent with the known safety profiles for each of these agents when used alone (Table 4). Mild (grade 1–2) fatigue, alopecia, nausea, and peripheral neuropathy were observed at rates consistent with docetaxel/prednisone monotherapy.22,28,29 Mucositis and hypertriglyceridemia and hypertension were observed at frequencies similar to everolimus monotherapy.30 Bevacizumab has been associated with less frequent, but more serious, adverse events in monotherapy trials, including bleeding, thrombosis, and visceral perforation.31 In this trial, only isolated instances of these more serious adverse events were observed. We conclude that docetaxel 75 mg/m2 day 1/everolimus 5 mg daily/bevacizumab 10 mg/kg day 1 on a 21-day cycle represents a safe combination regimen. However, as the incidence of neutropenic fever is ~25% on a per-subject basis, future trials should utilize prophylactic myeloid growth factor support according to current guidelines.32
The overall goal of this trial was to explore the efficacy of a combined targeting approach incorporating both VEGF- and mTOR-directed agents with docetaxel as initial chemotherapy in metastatic CRPC. Results from the phase II portion of the trial met the predefined endpoint with the observation of clinical response evidenced by a 50% decline in PSA at 12 weeks and partial response or better by RECIST criteria in 73% and 80% of subjects, respectively. However, a limitation of the nonrandomized trial design mandates that these results are interpreted in the context of other reports covering the efficacy of these and similar agents for metastatic CRPC. Docetaxel was established as first-line cytotoxic chemotherapeutic agent to treat CRPC in 2004 based on compelling results of the TAX-327 and Southwest Oncology Group (SWOG) 99–16 phase III trials demonstrating that docetaxel/prednisone-based treatment increased overall survival of CRPC patients compared with mitoxantrone/prednisone.29,33 Of note, SWOG 99–16 included estramustine combined with docetaxel/ prednisone based on preliminary data indicating synergy. However, estramustine is now rarely used owing to a lack of compelling data supporting an additive benefit with docetaxel and an increased incidence of adverse events.34 Thus, docetaxel 75 mg/m2 every 3 weeks with prednisone 5 mg twice a day is the current standard first-line chemotherapy treatment for metastatic CRPC.35
Over the last decade, many phase III clinical trials have unsuccessfully sought to add to the efficacy of docetaxel/prednisone for CRPC based primarily on preclinical observations and preliminary clinical trials for efficacy. The list of agents tested in phase III trials with the goal of improving on outcomes observed with docetaxel alone includes a broad range of agents with different purported mechanisms of actions, including a prostate cancer cellular vaccine (GVAX36); high dose vitamin D (calitriol37); endothelin receptor antagonists (atrasentan,38 zibotentan39); a Src-tyrosine kinase inhibitor (dasatinib40); a bone-targeted radiopharmaceutical (Strontium-8941); a clusterin-directed antisense oligonucleotide (custirsen42); a combined immuno-modulating/anti-angiogenic agent (lenolidomide43); and anti-angiogenic agents targeting VEGF (aflibercept,44 bevacizumab22). Of particular relevance to the current report are trials that involve either an anti-angiogenic or an mTOR inhibitor in addition to docetaxel as initial chemotherapy for CRPC (Table 5). The results in the current trial are most similar to those reported for the docetaxel/bevacizumab arm in Cancer and Leukemia Group B (CALGB) 90401. Specifically, the rate of PSA decline ≥ 50% (69.5% vs. 73%) and median overall survival (22.6 vs. 24 months) are very similar for patients treated with docetaxel/ bevacizumab versus docetaxel/bevacizumab/everolimus, respectively. This suggests that the triplet combination would not likely improve upon the results of CALGB 90401 in randomized testing.
Table 5.
Selected Results of Phase II/III Trials for Patient Cohorts Treated With Docetaxel-based Therapies
| ≥50% Maximal Reduction in PSA | Partial/Complete Objective Response Rate | Overall Survival, mo | ||
|---|---|---|---|---|
| Docetaxel monotherapy | ||||
| TAX 32745 | N = 335 | 131/291 = 45% | 17/141 = 12% | 18.9 (95% CI, 17.0–21.2) |
| SWOG 99–1633 | N = 386 | 155/309 = 50% | 17/103 = 17% | 17.5 |
| Docetaxel/anti-angiogenic agent | ||||
| Docetaxel/thalidomide/bevacizumab20 | N = 60 | 52/58 = 89.6% | 64% (21/33) | 28.2 |
| Docetaxel/bevacizumab/lenolidomide46 | N = 63 | 55/61 = 90% | 86% (24/29) | 24.6 |
| Docetaxel/lenolidomide, (MAINSAIL)43 | N = 525 | 59% | 22% (118/533) | 17.7 (95% CI, 14.8–18.8) |
| Aflibercept44 | N = 612 | 68.6 (64.7–72.4) | 38.4% (124/323) | 22.1 (95% CI, 20.3–24.1) |
| Docetaxel/estramustine/bevacizumab21 | N = 77 | 75% | 59% (23/39) | 24 |
| Docetaxel/bevacizumab (CALGB 90401)15 | N = 524 | 69.5% | 49.4% | 22.6 (95% CI, 21.1–24.5) |
| Present report | ||||
| Docetaxel/bevacizumab/everolimus | N = 43 | 73% | 55% | 21.9 (95% CI, 18.4–30.3) |
Abbreviations: CI = confidence interval; PSA = prostate-specific antigen.
At least 2 broad theories can be proposed to explain the discordance between the positive effects observed with combination therapy in laboratory studies with the disappointing effects of combination therapy observed in patients. First, a reductionist explanation would propose that, although the fundamental observations supporting the use of combination therapies are sound, more data and models are needed to better predict clinical effects in individual patients. For example, as clinical data supports pathologic activation of the PTEN/PI3 kinase/mTOR pathway in most cases of advanced prostate cancer, then continued efforts should be directed at devising new ways to target this pathway. The use of mTOR inhibitors in some PTEN-deficient cell lines leads to upregultion of pro-growth pathways.47 Thus, PI3 kinase itself upstream from mTOR, with or without simultaneously targeting of the androgen receptor pathway, would seem like a valid therapeutic strategy. It is notable that more recent reports utilizing direct PI3 kinase pathway inhibitors with or without androgen pathway inhibitors (abiraterone or enzalutamide) have generally not shown activity in unselected population of patients with CRPC,48–50 whereas an ongoing trial is seeking to select patients with PTEN alterations diagnosed in metastatic tissue as a possible predictive marker associated with response (clinicaltrials.gov: NCT02215096). In short, the reductionist explanation would suggest that a more complete molecular characterization of cancer and patient would allow for a more precise prediction of how individual patients would respond to any particular therapy.
Alternatively, a counter-explanation for the discordance between laboratory and clinical observations holds that cancer is a complex adaptive system. Complexity science posits that the growth and progression of cancer is an emergent behavior reflecting intricate connections and interactions of a large number of subsystems. Thus, any attempt to predict cancer growth or response to any one, or combination, of therapies based on simple preclinical models is destined to fail. Complexity theory holds that cancer is a system with emergent behavior reflecting an interwoven series of systems that may not always respond to the same stimulus in a predictable way.
Conclusions
In conclusion, our study was successful at defining safe doses for combination therapy with docetaxel, everolimus, and bevacizumab. Although the primary endpoint for efficacy was met, we conclude that the results of the current study do not support moving forward with docetaxel/bevacizumab/everolimus in unselected patients with CRPC.
Supplementary Material
Clinical Practice Points.
This study shows that everolimus 2.5 mg orally daily/bevacizumab 15 intravenously mg/kg every 21 days/docetaxel 75 mg/m2 intravenously every 21 days is safe and well-tolerated in CRPC.
Combined treatment with everolimus, bevacizumab, and docetaxel demonstrates significant clinical activity in chemotherapy-naive CRPC based on intermediate endpoints.
However, longer-term endpoints, including progression-free and overall survival, appear to be similar to treatment with docetaxel given as a single agent.
When interpreted in the context of other docetaxel-based combination therapy trials, the results of this trial do not support pursuing this combination further in CRPC.
Acknowledgments
The authors thank Daniel Ruderman, PhD, for data analysis, and the nurses and research staff at the USC Westside Cancer Center and the USC Norris Comprehensive Cancer Center who contributed to this trial.
Footnotes
Disclosure
Research support was provided by Novartis Pharmaceuticals Corp; study medications were provided by Novartis and Genentech/Roche. This work was partially supported by National Cancer Institute Cancer Center Shared Grant award P30CA014089. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. None of the funding influenced the design, analysis, or conclusions of the study. The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental table accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.clgc.2017.07.003.
References
- 1.Visakorpi T. The molecular genetics of prostate cancer. Urology 2003; 62(5 Suppl 1):3–10. [DOI] [PubMed] [Google Scholar]
- 2.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A 2001; 98:10314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res 2002; 62:5027–34. [PubMed] [Google Scholar]
- 4.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 2005; 120:747–59. [DOI] [PubMed] [Google Scholar]
- 5.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008; 26:1603–10. [DOI] [PubMed] [Google Scholar]
- 6.Lerut E, Roskams T, Goossens E, et al. Molecular pharmacodynamic (MPD) evaluation of dose and schedule of RAD001 (everolimus) in patients with operable prostate carcinoma (PC). Proc Am Soc Clin Oncol 2005; 23 (abstract 3071). [Google Scholar]
- 7.Templeton AJ, Dutoit V, Cathomas R, et al. , Swiss Group for Clinical Cancer Research (SAKK). Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). Eur Urol 2013; 64:150–8. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong AJ, Shen T, Halabi S, et al. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clin Genitourin Cancer 2013; 11: 397–406. [DOI] [PubMed] [Google Scholar]
- 9.Kruczek K, Ratterman M, Tolzien K, Sulo S, Lestingi TM, Nabhan C. A phase II study evaluating the toxicity and efficacy of single-agent temsirolimus in chemotherapy-naive castration-resistant prostate cancer. Br J Cancer 2013; 109: 1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amato RJ, Wilding G, Bubley G, Loewy J, Haluska F, Gross ME. Safety and preliminary efficacy analysis of the mTOR inhibitor ridaforolimus in patients with taxane-treated, castration-resistant prostate cancer. Clin Genitourin Cancer 2012; 10:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross RW, Manola J, Oh WK, et al. Phase I trial of RAD001 (R) and docetaxel (D) in castration resistant prostate cancer (CRPC) with FDG-PET assessment of RAD001 activity. J Clin Oncol 2008; 26 (abstract 5069). [Google Scholar]
- 12.Courtney KD, Manola JB, Elfiky AA, et al. A phase I study of everolimus and docetaxel in patients with castration-resistant prostate cancer. Clin Genitourin Cancer 2015; 13:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emmenegger U, Booth CM, Berry S, et al. Temsirolimus maintenance therapy after docetaxel induction in castration-resistant prostate cancer. Oncologist 2015; 20:1351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakabayashi M, Werner L, Courtney KD, et al. Phase II trial of RAD001 and bicalutamide for castration-resistant prostate cancer. BJU Int 2012; 110:1729–35. [DOI] [PubMed] [Google Scholar]
- 15.Chow H, Ghosh PM, deVere White R, et al. A phase 2 clinical trial of everolimus plus bicalutamide for castration-resistant prostate cancer. Cancer 2016; 122:1897–904.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 2006; 12:5018–22. [DOI] [PubMed] [Google Scholar]
- 17.Bok RA, Halabi S, Fei DT, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a Cancer and Leukemia Group B study. Cancer Res 2001; 61:2533–6. [PubMed] [Google Scholar]
- 18.George DJ, Halabi S, Shepard TF, et al. , Cancer and Leukemia Group B. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res 2001; 7:1932–6. [PubMed] [Google Scholar]
- 19.Huang X, Ning YM, Gulley JL, et al. Phase II trial of bevacizumab (A), lenalidomide (R), docetaxel (D), and prednisone (P) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2011; 29(suppl 7) (abstract 138). [Google Scholar]
- 20.Ning YM, Gulley JL, Arlen PM, et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:2070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picus J, Halabi S, Kelly WK, et al. , Cancer and Leukemia Group B. A phase 2 study of estramustine, docetaxel, and bevacizumab in men with castrate-resistant prostate cancer: results from Cancer and Leukemia Group B Study 90006. Cancer 2011; 117:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol 2012; 30:1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205–16. [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Halabi S, Tannock I, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26:1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulder S, Gladish G, Ensor J, et al. A phase 1 study of weekly everolimus (RAD001) in combination with docetaxel in patients with metastatic breast cancer. Cancer 2012; 118:2378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramalingam SS, Harvey RD, Saba N, et al. Phase 1 and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel for recurrent/refractory nonsmall cell lung cancer. Cancer 2010; 116: 3903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan A, Miles DW, Pivot X. Bevacizumab in combination with taxanes for the first-line treatment of metastatic breast cancer. Ann Oncol 2010; 21:2305–15. [DOI] [PubMed] [Google Scholar]
- 28.Petrylak DP, Tangen C, Hussain M, et al. SWOG 99–16: Randomized phase III trial of docetaxel (D)/estramustine (E) versus mitoxantrone(M)/prednisone(p) in men with androgen-independent prostate cancer (AIPCA). J Clin Oncol 2004; 23 (abstract 3). [Google Scholar]
- 29.Eisenberger MA, De Wit R, Berry W, et al. A multicenter phase III comparison of docetaxel (D) + prednisone (P) and mitoxantrone (MTZ) + P in patients with hormone-refractory prostate cancer (HRPC). J Clin Oncol 2004; 23 (abstract 4). [Google Scholar]
- 30.Aapro M, Andre F, Blackwell K, et al. Adverse event management in patients with advanced cancer receiving oral everolimus: focus on breast cancer. Ann Oncol 2014; 25:763–73. [DOI] [PubMed] [Google Scholar]
- 31.Shord SS, Bressler LR, Tierney LA, Cuellar S, George A. Understanding and managing the possible adverse effects associated with bevacizumab. Am J Health Syst Pharm 2009; 66:999–1013. [DOI] [PubMed] [Google Scholar]
- 32.Smith TJ, Bohlke K, Lyman GH, et al. , American Society of Clinical Oncology. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015; 33:3199–212. [DOI] [PubMed] [Google Scholar]
- 33.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351:1513–20. [DOI] [PubMed] [Google Scholar]
- 34.Fizazi K, Le Maitre A, Hudes G, et al. , Meta-analysis of Estramustine in Prostate Cancer (MECaP) Trialists’ Collaborative Group. Addition of estramustine to chemotherapy and survival of patients with castration-refractory prostate cancer: a meta-analysis of individual patient data. Lancet Oncol 2007; 8:994–1000. [DOI] [PubMed] [Google Scholar]
- 35.Basch E, Loblaw DA, Oliver TK, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer:American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol 2014; 32:3436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small E, Demkow T, Gerritsen WR, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC). Genitourinary Cancers Symposium 2009. (abstract 7). [Google Scholar]
- 37.Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol 2011; 29:2191–8. [DOI] [PubMed] [Google Scholar]
- 38.Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol 2013; 14:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fizazi K, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol 2013; 31:1740–7. [DOI] [PubMed] [Google Scholar]
- 40.Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol 2013; 14:1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Tu S-M, Pagliaro LC, et al. A prospective randomized phase III trial comparing consolidation therapy with or without strontium-89 following induction chemotherapy in androgen-independent prostate cancer. J Clin Oncol 2014; 32(suppl 4) (abstract 90). [Google Scholar]
- 42.Chi KN, Higano CS, Blumenstein BA, et al. Phase III SYNERGY trial: Docetaxel +/- custirsen and overall survival in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and poor prognosis. J Clin Oncol 2015; 33(suppl) (abstract 5009). [Google Scholar]
- 43.Petrylak DP, Vogelzang NJ, Budnik N, et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2015; 16:417–25. [DOI] [PubMed] [Google Scholar]
- 44.Tannock IF, Fizazi K, Ivanov S, et al. , VENICE Investigators. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol 2013; 14:760–8. [DOI] [PubMed] [Google Scholar]
- 45.Tannock IF, de Wit R, Berry WR, et al. , TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351:1502–12. [DOI] [PubMed] [Google Scholar]
- 46.Madan RA, Karzai FH, Ning YM, et al. Phase II trial of docetaxel, bevacizumab, lenalidomide, and prednisone in patients with metastatic castration-resistant prostate cancer. BJU Int 2016; 118:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011; 19:575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong AJ, Halabi S, Healy P, et al. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur J Cancer 2017; 81:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei XX, Hseih AC, Kim W, et al. A phase I study of abiraterone acetate combined with BEZ235, a dual PI3K/mTOR inhibitor, in metastatic castration resistant prostate cancer. Oncologist 2017; 22:503–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massard C, Chi KN, Castellano D, et al. Phase Ib dose-finding study of abiraterone acetate plus buparlisib (BKM120) or dactolisib (BEZ235) in patients with castration-resistant prostate cancer. Eur J Cancer 2017; 76:36–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.