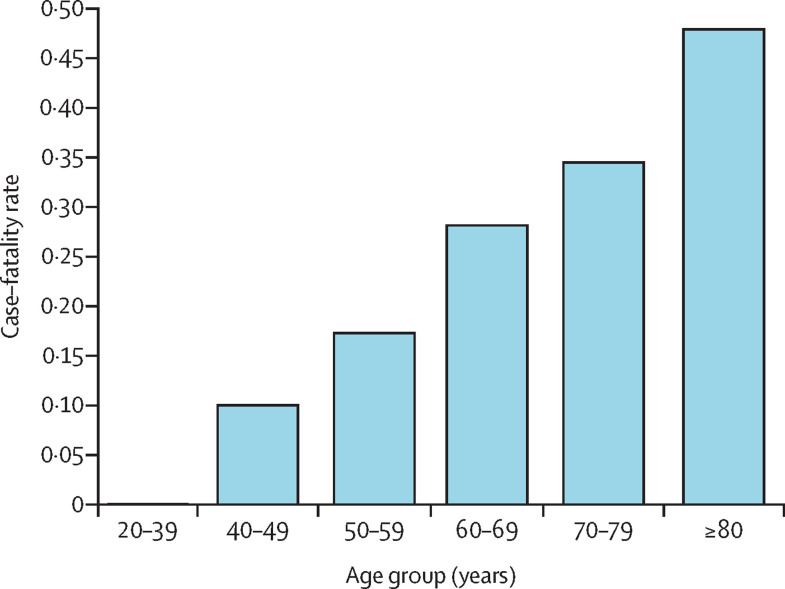Abstract
Background
Patients with cancer are purported to have poor COVID-19 outcomes. However, cancer is a heterogeneous group of diseases, encompassing a spectrum of tumour subtypes. The aim of this study was to investigate COVID-19 risk according to tumour subtype and patient demographics in patients with cancer in the UK.
Methods
We compared adult patients with cancer enrolled in the UK Coronavirus Cancer Monitoring Project (UKCCMP) cohort between March 18 and May 8, 2020, with a parallel non-COVID-19 UK cancer control population from the UK Office for National Statistics (2017 data). The primary outcome of the study was the effect of primary tumour subtype, age, and sex and on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) prevalence and the case–fatality rate during hospital admission. We analysed the effect of tumour subtype and patient demographics (age and sex) on prevalence and mortality from COVID-19 using univariable and multivariable models.
Findings
319 (30·6%) of 1044 patients in the UKCCMP cohort died, 295 (92·5%) of whom had a cause of death recorded as due to COVID-19. The all-cause case–fatality rate in patients with cancer after SARS-CoV-2 infection was significantly associated with increasing age, rising from 0·10 in patients aged 40–49 years to 0·48 in those aged 80 years and older. Patients with haematological malignancies (leukaemia, lymphoma, and myeloma) had a more severe COVID-19 trajectory compared with patients with solid organ tumours (odds ratio [OR] 1·57, 95% CI 1·15–2·15; p<0·0043). Compared with the rest of the UKCCMP cohort, patients with leukaemia showed a significantly increased case–fatality rate (2·25, 1·13–4·57; p=0·023). After correction for age and sex, patients with haematological malignancies who had recent chemotherapy had an increased risk of death during COVID-19-associated hospital admission (OR 2·09, 95% CI 1·09–4·08; p=0·028).
Interpretation
Patients with cancer with different tumour types have differing susceptibility to SARS-CoV-2 infection and COVID-19 phenotypes. We generated individualised risk tables for patients with cancer, considering age, sex, and tumour subtype. Our results could be useful to assist physicians in informed risk–benefit discussions to explain COVID-19 risk and enable an evidenced-based approach to national social isolation policies.
Funding
University of Birmingham and University of Oxford.
Introduction
The disease course of individuals who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is phenotypically diverse. Many people have only mild symptoms, and it is becoming increasingly apparent from antibody data that some others have no symptoms at all, but still actively carry and transmit the virus. However, some individuals display severe symptoms and can have an extreme phenotype, with development of respiratory failure, cytokine release syndrome, and multi-organ failure. Subgroups of patients with COVID-19 have been identified who appear to be at increased risk of morbidity and mortality, including patients of older age, male sex (vs female), and those with comorbidities, such as hypertension, chronic lung disease, diabetes, and cancer.1
Since COVID-19 began to spread across the globe in early 2020, patients with cancer were identified as a particularly susceptible subgroup of the population. Patients with cancer have been reported to be at increased risk of infection with SARS-CoV-2 and a more severe disease course, with a large proportion of people requiring high levels of intensive care, having a more rapidly evolving disease, and having an increased risk of death.2, 3, 4 However, cancer encompasses many different diseases, with a diverse array of primary tumour subtypes and stages, affecting a heterogeneous group of patients of all ages, with very different prognoses and outcomes. Therefore, labelling all patients with cancer as susceptible to COVID-19 is probably neither reasonable nor informative.
Research in context.
Evidence before this study
We searched PubMed for studies published between Dec 1, 2019, and July 1, 2020, relating to severe acute respiratory coronavirus 2 (SARS-CoV-2) infection susceptibility and the clinical course of COVID-19 in patients with cancer, using the search terms “COVID-19”, “SARS-CoV-2”, and “cancer”. The search was not limited to English language publications. Several studies described correlations between patient demographics, specifically age and sex, and increased COVID-19 morbidity and mortality. Two cancer studies identified no significant effect of chemotherapy on mortality (Lee and colleagues, 2020; Kuderer and colleagues, 2020), and this was also noted in another study (Vaugnat and colleagues, 2020). However, a smaller study identified a small increased risk of mortality with recent chemotherapy (Yang and colleagues, 2020). The effect of cancer subtype remains to be elucidated. Dai and colleagues (2020) identified that patients with haematological or lung malignancies had a poorer disease course than those with other types of cancer. The elevated risk in haematological malignancies was also noted by Yang and colleagues (2020) and for lung cancer in another study (Garassino and colleagues, 2020), but another did not identify increased mortality by cancer subtype (Kuderer and colleagues, 2020).
Added value of this study
This UK Coronavirus Cancer Monitoring Project (UKCCMP) study is a national monitoring project of patients with cancer who develop COVID-19. We compared cancer patients enrolled in the UKCCMP and a parallel UK cancer control population cohort without COVID-19 and analysed the effect of tumour features (primary subtype and stage) and patient demographics (age and sex) on the risk and trajectory of COVID-19 disease. Tumour features (primary subtype and stage) and patient demographics affect viral susceptibility and COVID-19 disease phenotype. We found increased SARS-CoV-2 susceptibility in patients with haematological cancers (leukaemia, lymphoma, or myeloma). Patients with haematological cancers had a more severe COVID-19 disease trajectory and required more intensive clinical support, with additional risk conferred by recent chemotherapy use. As observed in the general population, age and sex were predominant risk factors for SARS-CoV-2 infection and severity of COVID-19 disease for most patients with cancer.
Implications of all the available evidence
Our data indicate that patients with cancer with different tumour types have variable SARS-CoV-2 susceptibility and COVID-19 disease phenotypes, with notable increased SARS-CoV-2 susceptibility in patients with haematological cancers. We generated individualised risk tables for patients with cancer, accounting for age, sex, and tumour subtype. These tables will be useful for physicians to have a more informed risk–benefit discussion to explain COVID-19 risk to patients with cancer.
As a consequence of generic advice given to people who are clinically susceptible to COVID-19, patients with cancer (of any age, sex, tumour subtype, and stage) have been labelled as at high risk from COVID-19, which has led to sweeping changes in management for all cancer types during the past few months, including shortening of radiotherapy, switching from intravenous to oral chemotherapy regimens, and modifications in immunotherapy use.5, 6, 7, 8 These changes were instigated with little evidence to support them, perhaps reasonably in an acute pandemic situation. There has been little attempt to define the individualised risk for a given patient with cancer, considering their primary tumour subtype, age, and sex, because of a paucity of evidence.
From the UK Coronavirus Cancer Monitoring Project (UKCCMP),9 we report the first analysis, to our knowledge, of the complex interaction between patient demographics and tumour subtype, to more accurately estimate the risk of SARS-CoV-2 infection and COVID-19 in patients with cancer. We aimed to describe the clinical outcomes of COVID-19 in patients with cancer entered into the UKCCMP registry and compare primary cancer subtype prevalence and case fatality with UK Office for National Statistics cancer incidence data.
Methods
Study design and participants
The UKCCMP database of UK patients with COVID-19 who have cancer was designed as a public health surveillance registry for the COVID-19 pandemic and a prospective, observational cohort study.10 The database was set up to support rapid clinical decision making, in accordance with the UK Policy Framework for Health and Social Care Research, the UK National Research Ethics Service, and the UK Governance Arrangement for Research Ethics Committees. Ethical approval was not specifically required, as confirmed after consultation with the Health Research Authority.
This cohort study involved collection of clinical and outcome data as routinely recommended for good clinical practice. Each of the 61 participating centres was required to obtain local approvals and follow local information governance processes. Written, informed consent was not required for inclusion in this study. Eligibility criteria for enrolment on the registry were as follows: adult patients (aged ≥18 years); active cancer; presenting to the UKCCMP network between March 18 and May 8, 2020; and a positive SARS-CoV-2 RT-PCR test from a nose or throat swab. Patients with active cancer were defined as those with metastatic cancer or those undergoing anticancer treatment in any setting (curative, radical, adjuvant, or neoadjuvant) or those treated within the past 12 months with surgery, systemic anticancer therapy, or radiotherapy. Patients with skin cancer were not included in these analyses because most are not managed in an oncological setting, so they are not representative as a comparison group. All patients attended secondary care to be reviewed for potential admission to hospital and were not part of a proactive surveillance programme. Management of patients with cancer with COVID-19 was directed by the patient's clinician team without input from the UKCCMP and was based on local policies and standard UK clinical practice at the time of this study. Decisions about intensive care unit admission and ventilation were guided by the UK National Health Service National Institute of Health and Care Excellence COVID-19 rapid guidelines.11 This study was done in accordance with the STROBE statement.
Data collection and analysis
Data collection was performed as previously outlined.10 Case reporting of patient tumour stage and demographics was lead by a COVID-19 emergency response reporting individual (ERRI), using data from clinical records or from direct assessment. The ERRI was an oncologist who was trained or in training. In a few centres, data entry was done by data managers, but with direct oversight by the ERRI. Tumour types were classified according to International Classification of Diseases, 10th Revision (ICD-10) codes. Tumour subtype and demographic analyses used the latest release of the Cancer Registration Statistics, England, 2017,12 which is the most recent cancer registration database in England and involves registrations of patients from Jan 1 to Dec 31, 2017. Cancer registrations in England can take many years to reach nationally validated quality control measures for robustness of analyses because of continuous accrual of registrations.
Outcomes
The primary outcome of interest was the association between primary tumour subtype, age, and sex and the likelihood of SARS-CoV-2 infection and all-cause inpatient case–fatality rate (during the COVID-19 episode). All-cause inpatient case fatality included death as a direct result of COVID-19 and death from any other cause, such as cancer progression or treatment toxicity.
Statistical analysis and data visualisation
We compared adult patients with cancer enrolled in the UKCCMP and a parallel non-COVID-19 UK cancer control population cohort (the UK Office for National Statistics [ONS] cancer control dataset, from 2017), investigating the effect of tumour subtype and patient demographics (age and sex) on the prevalence of and mortality from COVID-19 using univariable Fisher's exact tests and multivariable logistic regression.
Analyses were done without an a priori power calculation, since this was not possible because of the lack of information about the effect size and interactions and the nature and rapid evolution of the pandemic. Patients who had missing datapoints required for an analysis were excluded. We used a two-sided Fisher's exact test to compare categorical data from different categories. We used multivariable logistic regression13 to estimate odds ratios [ORs] and 95% CIs for tumour subtype and recent chemotherapy treatment after adjustment for the potential confounders patient age and sex. Case–fatality rate for each primary tumour subtype was compared with cancers of the digestive organs (non-colorectal) as a reference group. A p value threshold of 0·05 was used to indicate a significant difference. Analyses were done with R (version 3.6.3).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. LYWL, J-BC, TS, RA, VB, NAC, HMC, SH, CPM, CP, AS-O, CDT, and CV had access to the raw data. LYWL, J-BC, TS, GM, and RK had final responsibility for the decision to submit for publication.
Results
1044 patients with active cancer and documented SARS-CoV-2 infection or COVID-19 disease were registered in the UKCCMP database from March 18, 2020, with outcomes censored on May 8, 2020. 87 patients were excluded from the analysis because they had an unspecified tumour site or malignant neoplasia of the skin. 595 (56·9%) of 1044 patients were men and the median age was 70 years (IQR 60–77; table 1 ). Patients were followed up from the point of COVID-19 diagnosis to either discharge from hospital or death. Median follow-up was 6 days (IQR 2–11).
Table 1.
Demographics and tumour subtype representation in the UKCCMP COVID-19 cohort compared with the ONS cancer control population
| UKCCMP cohort (n=1044) | ONS cohort (n=282 878) | Odds ratio (95% CI) | p value | ||
|---|---|---|---|---|---|
| Sex | .. | .. | 1·26 (1·12–1·43) | 0·0002 | |
| Male | 595 (56·9%) | 145 034 (51·3%) | .. | .. | |
| Female | 445 (42·6%) | 137 844 (48·7%) | .. | .. | |
| Other | 4 (0·4%) | 0 | .. | .. | |
| Age, years | 70 (60–77) | NA* | .. | .. | |
| Cancer subtype (ICD-10 code) | |||||
| Breast (C50) | 143 (13·7%) | 46 109 (16·3%) | 0·82 (0·68–0·98) | 0·026 | |
| Colorectal (C18–C21) | 124 (11·9%) | 36 039 (12·7%) | 0·93 (0·76–1·12) | 0·46 | |
| Prostate (C61) | 114 (10·9%) | 41 200 (14·6%) | 0·72 (0·59–0·88) | 0·0008 | |
| Lung (C34) | 111 (10·6%) | 38 878 (13·7%) | 0·75 (0·61–0·91) | 0·0033 | |
| Digestive organs (non–colorectal) (C15–C17, C22–C26) | 95 (9·1%) | 30 096 (10·6%) | 0·84 (0·68–1·04) | 0·12 | |
| Urinary tract (C64–C68) | 77 (7·4%) | 19 333 (6·8%) | 1·09 (0·85–1·38) | 0·46 | |
| Female genital organs (C51–C58) | 56 (5·4%) | 17 969 (6·4%) | 0·84 (0·63–1·10) | 0·23 | |
| Lip, oral cavity, and pharynx (C00–C14) | 33 (3·2%) | 7558 (2·7%) | 1·19 (0·82–1·69) | 0·33 | |
| CNS (C69–C72) | 25 (2·4%) | 5038 (1·8%) | 1·36 (0·87–2·02) | 0·13 | |
| Mesothelial and soft tissue (C45–C49) | 16 (1·5%) | 4682 (1·7%) | 0·93 (0·53–1·52) | 0·90 | |
| Respiratory and intrathoracic organs (not lung; C30–C39) | 11 (1·1%) | 2780 (1·0%) | 1·08 (0·53–1·94) | 0·75 | |
| Bone and articular cartilage (C40–C41) | 4 (0·4%) | 376 (0·1%) | 2·90 (0·78–7·50) | 0·053 | |
| Male genital organs (C60–C63) | 4 (0·4%) | 2435 (0·9%) | 0·44 (0·12–1·14) | 0·13 | |
| Endocrine glands (C73–C75) | 4 (0·4%) | 3374 (1·2%) | 0·32 (0·09–0·82) | 0·0096 | |
| Lymphoma (C81–C85) | 79 (7·6%) | 13 537 (4·8%) | 1·63 (1·28–2·06) | <0·0001 | |
| Leukaemia (C91–C95) | 79 (7·6%) | 8018 (2·8%) | 2·82 (2·21–3·55) | <0·0001 | |
| Myeloma (C90) | 37 (3·5%) | 5033 (1·8%) | 2·03 (1·42–2·83) | 0·0001 | |
| Other haematological (C86, C88, C96) | 29 (2·8%) | 423 (0·1%) | 19·14 (12·59–28·05) | <0·0001 | |
Data are n (%) or median (IQR). Univariable analysis was done. p values were determined by Fisher's exact test and unadjusted for age and sex. NA=not available. ONS=Office for National Statistics. UKCCMP=UK Coronavirus Cancer Monitoring Project. ICD-10=International Classification of Diseases, 10th Revision.
Individual ages not available in the ONS dataset.
We compared the demographics and cancer subtype of patients with cancer with COVID-19 from the UKCCMP registry with those from the population of patients represented in the ONS cancer census, which was used as a control group. We found that patients with cancer with COVID-19 were significantly more likely to be men (595 [56·9%] of 1044 UKCCMP patients vs 145 034 [51·3%] of 282 878 ONS patients; OR 1·26, 95% CI 1·12–1·43; p=0·0002) compared with the ONS control population, but the age distribution of patients with cancer who contracted COVID-19 did not differ between the two groups (appendix p 2).
We found that some tumour subtypes were overrepresented in the UKCCMP patient cohort compared with the ONS control population. Patients with haematological malignancies appeared to be at significantly increased risk of COVID-19 infection, including those with leukaemia (OR 2·82, 95% CI 2·21–3·55; p<0·0001), myeloma (2·03, 1·42–2·83; p=0·0001), and lymphoma (1·63, 1·28–2·06; p<0·0001; table 1). By contrast, patients with lung cancer and prostate cancer were relatively under-represented in the UKCCMP population compared with the control ONS population (table 1).
319 (30·6%) of 1044 patients in the UKCCMP cohort died, 295 (92·5%) of whom had a cause of death recorded as due to COVID-19. The all-cause case–fatality rate in patients with cancer after COVID-19 infection was significantly associated with increasing age (figure 1 ; appendix pp 2–3). Additionally, the all-cause case–fatality rate in patients with cancer once they had contracted COVID-19 was significantly associated with sex (212 [35·6%] of 595 male patients vs 105 [23·6%] of 445 female patients; OR 1·92, 95% CI 1·51–2·45; p<0·0001).
Figure 1.
Age and all-cause case–fatality rate of patients with cancer after presenting with COVID-19 in the UK Coronavirus Cancer Monitoring Project cohort
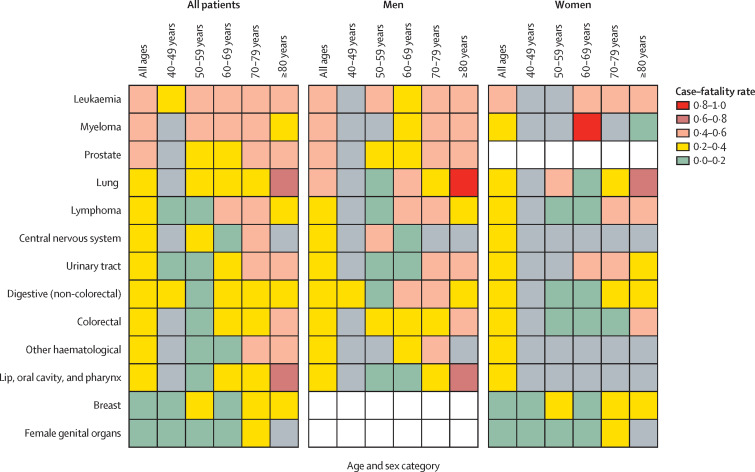
We compared the case–fatality rate for each primary tumour subtype in the UKCCMP to a reference group, cancers of digestive organs (ICD-10 codes C15–C17, C22–C26), which had the median fatality rate. On univariable analysis, we observed a significantly higher risk of death from COVID-19 in patients with prostate cancer (OR 2·14, 95% CI 1·17–3·96; p=0·014) and leukaemia (2·03, 1·04–3·97; p=0·038), and a significantly lower risk of death from COVID-19 for patients with breast cancer (0·53, 0·28–1·00; p=0·049) and female genital cancers (0·36, 0·13–0·87; p=0·031; table 2 ; figure 2 ; appendix p 3). We did a multivariable correction for the clinically relevant confounders age and sex. In this multivariable analysis, compared with the rest of the UKCCMP cohort, patients with leukaemia still showed a significantly increased case–fatality rate (OR 2·25, 95% CI 1·13–4·57; p=0·023; table 2, appendix p 4). After multivariable correction, prostate cancer was no longer associated with an increased case–fatality rate, and breast and female genital cancers were no longer associated with a reduced case–fatality rate, highlighting the effect of patient age and sex on case–fatality rate. In multivariable analyses, we found no increased case–fatality rate due to COVID-19 in patients with lung cancer compared with the rest of the UKCCMP population (table 2).
Table 2.
All-cause case fatality following COVID-19 by tumour subtype (ICD-10 codes), before and after age and sex correction
| Deaths (n) | Case–fatality rate | Univariable OR (95% CI) | p value | Multivariable adjusted OR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Prostate (C61) | 49 | 0·43 | 2·14 (1·17–3·96) | 0·014 | 1·09 (0·51–2·33) | 0·82 |
| Lung (C34) | 43 | 0·387 | 1·62 (0·89–3·00) | 0·118 | 1·41 (0·75–2·67) | 0·29 |
| Mesothelial and soft tissue (C45–C49) | 6 | 0·375 | 1·18 (0·37–3·51) | 0·772 | 1·52 (0·43–5·30) | 0·51 |
| Urinary tract (C64–C68) | 23 | 0·299 | 1·08 (0·54–2·13) | 0·834 | 0·87 (0·41–1·81) | 0·72 |
| Colorectal (C18–C21) | 35 | 0·282 | 1·03 (0·56–1·90) | 0·934 | 0·85 (0·44–1·64) | 0·63 |
| CNS (C69–C72) | 7 | 0·28 | 1·15 (0·39–3·18) | 0·797 | 1·87 (0·57–6·05) | 0·29 |
| Respiratory organs and intrathoracic organs (not lung; C30–C39) | 3 | 0·273 | 0·84 (0·17–3·29) | 0·813 | 0·96 (0·18–4·10) | 0·95 |
| Lip, oral cavity, and pharynx (C00–C14) | 8 | 0·242 | 0·75 (0·28–1·85) | 0·542 | 0·77 (0·25–2·27) | 0·64 |
| Breast (C50) | 26 | 0·182 | 0·53 (0·28–1·00) | 0·049 | 0·97 (0·40–2·52) | 0·94 |
| Female genital organs (C51–C58) | 7 | 0·125 | 0·36 (0·13–0·87) | 0·031 | 0·79 (0·24–2·63) | 0·70 |
| Myeloma (C90) | 16 | 0·432 | 1·85 (0·81–4·22) | 0·142 | 1·65 (0·71–3·85) | 0·24 |
| Leukaemia (C91–C95) | 33 | 0·418 | 2·03 (1·04–3·97) | 0·038 | 2·25 (1·13–4·57) | 0·023 |
| Lymphoma (C81–C85) | 25 | 0·316 | 1·60 (0·80–3·19) | 0·184 | 1·72 (0·81–3·68) | 0·16 |
| Other haematological (C86, C88, C96) | 7 | 0·241 | 0·81 (0·28–2·12) | 0·675 | 0·81 (0·26–2·33) | 0·70 |
| Digestive organs, non-colorectal (C15–C17, C22–C26) | 28 | 0·295 | 1 (ref) | .. | 1 (ref) | .. |
OR=odds ratio. ICD-10=International Classification of Diseases, 10th Revision. OR was calculated with digestive organs (non-colorectal; ICD-10 codes C15–C17, C22–C26) as a reference. Multivariable corrections were done, correcting for patient age and sex.
Figure 2.
Case–fatality rate after presentation with COVID-19, by tumour subtype, age, and sex
Grey boxes represent where the number of cases was less than four, and case–fatality rate was not estimable. White boxes mean not applicable.
We undertook a specific detailed analysis of 227 patients with haematological malignancies who were diagnosed with COVID-19. Compared with the rest of the UKCCMP cohort (817 patients with non-haematological cancers), we found that these patients presented with similar symptoms (appendix p 5). After adjustment for potential confounding variables of age and sex, patients with haematological malignancies were significantly more likely to require high flow oxygen (OR 1·82, 95% CI 1·11–2·94; p=0·015), non-invasive ventilation (2·10, 1·14–3·76; p=0·014), intensive care unit admission for ventilation (2·73, 1·43–5·11; p=0·0019), and have a severe or critical disease course (1·57, 1·15–2·15; p=0·0043; appendix p 5). 108 (47·6%) of 227 patients with haematological malignancies had received recent chemotherapy within 4 weeks of COVID-19 presentation, compared with 241 (29·5%) of 817 patients with non-haematological malignancies (appendix p 5). In univariable analyses, recent use of chemotherapy in patients with haematological cancers was not associated with an increased risk of death compared with those who had no recent chemotherapy (data not shown). However, after correction for age and sex, patients with haematological malignancies who had recent chemotherapy had an increased risk of death during COVID-19-associated hospital admission (OR 2·09, 95% CI 1·09–4·08; p=0·028).
Discussion
Our results show that patients with cancer with different tumour types have differing susceptibility to SARS-CoV-2 and differing COVID-19 disease phenotypes, with notable increased SARS-CoV-2 hospital presentations in patients with haematological cancers. We generated individualised risk tables for patients with cancer, displaying the effect of age and sex and tumour subtype.
There are variations and challenges in determining if COVID-19 was the direct cause of death for a patient, or if death was caused by a terminal event in a patient who was approaching the end of their cancer care. We analysed the all-cause case–fatality rate in patients with COVID-19 and cancer, which was a strength of this study, in addition to the comparison with a general cancer population control group.
Patients with haematological malignancies (leukaemia, lymphoma, and myeloma) are overrepresented, which is perhaps suggestive of an a priori increased susceptibility to viral infection. Patients with extranodal natural killer/T-cell lymphoma (ICD-10 code C86), Waldenström macroglobulinaemia (C88), and unspecified neoplasm of lymphoid, haematopoietic and related tissue (C96) were greatly overrepresented. The reasons for this are unclear because of the small number of patients involved and stochastic effects.
We compared the case–fatality rate to the median reference group (digestive organs [non-colorectal] C15–C17, C22–C26) to avoid exaggerating the OR and to take the most conservative approach. Patients with haematological malignancies were at a greater risk of having a more severe COVID-19 clinical phenotype, to require more intensive supportive interventions, and to suffer an increased risk of death compared with patients with non-haematological malignancies. In multivariable analyses, patients with leukaemia had a significantly higher risk of COVID-19-related death, after taking into account age and sex. Admittedly, there are challenges in interpretation of our findings, as this study relied on ICD-10 cancer subtype codes and leukaemia encompasses a heterogeneous group of conditions. However, the increased case–fatality rate in patients with haematological malignancies is similar to that observed in a preprint article from the UK14 and several Chinese cohorts.15, 16 However, this finding contrasts with the results of an American cohort study,17 which did not suggest increased mortality from COVID-19 in patients with haematological malignancies.
Large COVID-19 cancer cohorts of predominantly solid organ tumours have shown no significant excess mortality risk from recent chemotherapy.10, 17 In this study, we identified in multivariable analyses that risk appears to be heightened by recent (within 4 weeks) or current chemotherapy in patients with haematological malignancies, similar to findings in other cohorts.18
There could be several reasons for these observations. The immunological disruption observed in patients with leukaemia and the use of intensely myelosuppressive treatment regimens might result in a combination of risks, in terms of the likelihood of initial SARS-CoV-2 infection, its ability to gain a foothold in the host, and in terms of the downstream disease course and likelihood of severe consequences, such as cytokine storm and multi-organ failure. Therefore, further validation of these findings and more in-depth research into the possible causes is important.
In contrast to findings from a Chinese series19 and data from a European registry,20 we found that patients with lung cancer were relatively under-represented in the UKCCMP cohort compared with ONS data. Additionally, once COVID-19 was established in patients with lung cancer, we did not observe an increased case–fatality rate compared with the remaining population with cancer and COVID-19 in the UKCCMP cohort, suggesting that patients with lung cancer are not a specifically susceptible group. There could be various reasons for this difference in findings. First, there are methodological differences in our study; we compared patients with lung cancer and COVID-19 to patients with cancer without COVID-19, rather than to people who did not have cancer. Second, there might have been effective shielding of patients with lung cancer at an early stage in the pandemic in the UK, when they were designated as clinically susceptible. Third, lung cancer is the most common cancer in China, and hence would be over-represented in Chinese patients with COVID-19 and cancer. Finally, the European registry20 did not use a control group, highlighting the importance of our intrapopulation controlled study.
Patients with prostate cancer were relatively underrepresented in the UKCCMP cohort compared with ONS data. In terms of the risk of death once COVID-19 was established, patients with prostate cancer initially appeared to have an increased case–fatality rate, but multivariable analysis showed that their risk was no greater than other patients with COVID-19 and cancer in the UKCCMP cohort, reflecting again the importance of age and sex as confounding factors.
Patients with breast cancers or malignancies of the female genital tract appeared to be at a much lower risk of contracting or dying from COVID-19. However, multivariable analysis showed that this protection was due to patients being women, rather than an inherently lower risk associated with these tumour types.
Our study has some limitations. Our analyses were based on patients with symptomatic cancer who seek help from cancer centres. Therefore, the cohort might not be entirely representative of all patients with cancer, and we observed a high proportion of patients with advanced or metastatic disease and patients who were having ongoing active oncological follow-up. Patients on an end-of-life care pathway or residing in nursing homes or hospices would be unlikely to be reported or included in this registry. There are also potential limitations with the use of the ONS control population of patients with cancer as our comparator. We reported on patients with active cancer, whereas the ONS control population is a control cohort comprising all patients with a diagnosis of cancer in 2017. Prognoses and therapeutic choices might have changed in the past few years. Therefore, it would be useful to analyse more contemporaneous controls when these data become available. Other possible limitations are that performance status, patient comorbidity scale or index, and ethnicity data were not initially collected as part of the study data entry form, and therefore could not be analysed in multivariable models. However, COVID-19 prevalence in patients with cancer remains low overall and the age distribution of patients in the UKCCMP reflects the age distribution in the ONS dataset, suggesting that our comparator population is as appropriate as possible at this stage of the pandemic.
Additionally, we observed a low admission rate of patients with cancer to the intensive care unit, which could affect COVID-19 outcomes in patients with cancer in the UK.10 Reasons for the low intensive care admission rate, which could be due to perceived futility of intensive support in patients with cancer, warrant further investigation.
Despite these limitations, our study is unique in comparing a large COVID-19 population with cancer to an accurate and geographically appropriate cancer population control dataset. Morbidity and case–fatality rates from COVID-19 in UK patients with cancer who attend hospital are relatively high, particularly in older patients and those with haematological malignancies, but not all cancer patients are affected equally. This important finding could allow clinicians some ability to risk stratify their patients and make informed decisions on appropriate levels of social isolation and shielding. Future work by the UKCCMP, in collaboration with international consortia, will define risk in much greater granularity, including different subtypes of a given tumour.
This online publication has been corrected. The corrected version first appeared at thelancet.com/oncology on September 3, 2020
Acknowledgments
Acknowledgments
We thank the patients and their families affected by COVID-19, oncologists, acute physicians, and health-care staff working tirelessly on the frontlines of the COVID-19 pandemic. We thank all members of the UK Coronavirus Cancer Monitoring Project reporting network and emergency response reporting individuals for their hard work in contributing data at a challenging time as listed in the appendix (p 1). We acknowledge the University of Birmingham and the University of Oxford for funding this study. SB is funded by a Medical Research Council Clinical Research Training Fellowship (MR/P001106/1). ADMB is funded by the National Institute for Health Research (NIHR) Applied Research Collaboration West Midlands. LYWL is supported by the NIHR Oxford Biomedical Research Centre.
Contributors
LYWL, J-BC, SEWB, SB, RA, GC, VWTC, HMC, DJH, DK, AJXL, ACO-B, CP, KP, ADMB, GM, and RK were involved in study design. LYWL, J-BC, MWF, SG, AJXL, RJL, SML, NM, TN-D, ACO-B, TP, KP, OT, SS, GM, and RK were involved in data collection. LYWL, J-BC, TS, RA, VB, NAC, VWTC, HMC, PE, AG, SH, DJH, AJXL, HM, CPM, ACO-B, CP, EAP, KP, AS-O, AJS, CV, GM, and RK were involved in data acquisition and management. LYWL, J-BC, SEWB, TS, CDT, ACO-B, GM, and RK were involved in data analysis and interpretation. LYWL, J-BC, KP, TS, SEWB, ACO-B, GM, and RK were involved in manuscript writing. RK made the decision to submit for publication.
Declaration of interests
ACO-B received grant support from Roche, Bristol-Myers Squibb, Eli Lily, Novartis, and UCB Pharma, and personal fees from Roche and Bristol-Myers Squibb, all outside the submitted work. NM has advisory board roles for Pfizer, Roche, and Boehringer Ingelheim, and has received speakers' bureau fees from Merck, Pfizer, and Roche, all outside the submitted work. TN-D has received personal fees from AstraZeneca, Amgen, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp and Dohme (MSD), Eli Lily, Novartis, Pfizer, Roche, and Takeda, and non-financial support from Bristol-Myers Squibb, MSD, Roche, and Takeda, all outside the submitted work. All other authors declare no competing interests.
Contributor Information
UK Coronavirus Cancer Monitoring Project Team:
Abigail Gault, Michael Agnieszka, Ahmed Bedair, Aisha Ghaus, Akinfemi Akingboye, Alec Maynard, Alexander Pawsey, Ali Abdulnabi Suwaidan, Alicia Okines, Alison Massey, Amy Kwan, Ana Ferreira, Angelos Angelakas, Anjui Wu, Ann Tivey, Anne Armstrong, Annet Madhan, Annet Pillai, Ashley Poon-King, Bartlomiej Kurec, Caroline Usborne, Caroline Dobeson, Christina Thirlwell, Christian Mitchell, Christopher Sng, Christopher Scrase, Christopher Jingree, Clair Brunner, Claire Fuller, Clare Griffin, Craig Barrington, Daniel Muller, Diego Ottaviani, Duncan Gilbert, Eliana Tacconi, Ellen Copson, Emily Renninson, Emma Cattell, Emma Burke, Fiona Smith, Francesca Holt, Gehan Soosaipillai, Hayley Boyce, Heather Shaw, Helen Hollis, Helen Bowyer, Iris Anil, Jack Illingworth, Jack Gibson, Jaishree Bhosle, James Best, Jane Barrett, Jillian Noble, Joseph Sacco, Joseph Chacko, Julia Chackathayil, Kathryn Banfill, Laura Feeney, Laura Horsley, Lauren Cammaert, Leena Mukherjee, Leonie Eastlake, Louise Devereaux, Lucinda Melcher, Lucy Cook, Mabel Teng, Madeleine Hewish, Madhumita Bhattacharyya, Mahbuba Choudhury, Mark Baxter, Martin Scott-Brown, Matthew Fittall, Michael Tilby, Michael Rowe, Michael Agnieszka, Mohammed Alihilali, Myria Galazi, Nadia Yousaf, Neha Chopra, Nicola Cox, Olivia Chan, Omar Sheikh, Paul Ramage, Paul Greaves, Pauline Leonard, Peter S Hall, Piangfan Naksukpaiboon, Pippa Corrie, Rahul Peck, Rachel Sharkey, Rachel Bolton, Rebecca Sargent, Rema Jyothirmayi, Robert Goldstein, Roderick Oakes, Rohan Shotton, Ruhi Kanani, Ruth Board, Ruth Pettengell, Ryan Claydon, Sam Moody, Samah Massalha, Sangary Kathirgamakarthigeyan, Saoirse Dolly, Sarah Derby, Sarah Lowndes, Sarah Benafif, Sarah Eeckelaers, Sarah Kingdon, Sarah Ayers, Sean Brown, Shawn Ellis, Shefali Parikh, Sian Pugh, Simon Shamas, Simon Wyatt, Simon Grumett, Sin Lau, Yien Ning Sophia Wong, Sophie McGrath, Stephanie Cornthwaite, Stephen Hibbs, Tania Tillet, Taslima Rabbi, Tim Robinson, Tom Roques, Vasileios Angelis, Victoria Woodcock, Victoria Brown, YingYing Peng, Yvette Drew, and Zoe Hudson
Supplementary Material
References
- 1.WHO Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) Feb 28, 2020. https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19)
- 2.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.04.006. published online April 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ESMO Cancer patient management during the COVID-19 pandemic. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic
- 6.UK NHS Specialty guides for patient management during the coronavirus pandemic. Clinical guide for the management of non-coronavirus patients requiring acute treatment: cancer. March 23, 2020. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf
- 7.Banna G, Curioni-Fontecedro A, Friedlaender A, Addeo A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5(suppl 2) doi: 10.1136/esmoopen-2020-000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribal MJ, Cornford P, Briganti A, et al. European Association of Urology Guidelines Office Rapid Reaction Group: an organisation-wide collaborative effort to adapt the European Association of Urology Guidelines recommendations to the coronavirus disease 2019 era. Eur Urol. 2020;78:21–28. doi: 10.1016/j.eururo.2020.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The UK Coronavirus Cancer Monitoring Project team The UK Coronavirus Cancer Monitoring Project: protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020;21:622–624. doi: 10.1016/S1470-2045(20)30230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NICE COVID-19 rapid guideline: critical care in adults. March 20, 2020. https://www.nice.org.uk/guidance/ng159/resources/covid19-rapid-guideline-critical-care-in-adults-pdf-66141848681413 [PubMed]
- 12.Office for National Statistics Cancer registration statistics, England: 2017. April 26, 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/2017
- 13.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 14.The OpenSAFELY Collaborative. Williamson E, Walker AJ, et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020 doi: 10.1101/2020.05.06.20092999. published online May 7. (preprint) [DOI] [Google Scholar]
- 15.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;20:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.