Abstract
Improving evidence for action is crucial to tackle antimicrobial resistance. The number of interventions for antimicrobial resistance is increasing but current research has major limitations in terms of efforts, methods, scope, quality, and reporting. Moving the agenda forwards requires an improved understanding of the diversity of interventions, their feasibility and cost–benefit, the implementation factors that shape and underpin their effectiveness, and the ways in which individual interventions might interact synergistically or antagonistically to influence actions against antimicrobial resistance in different contexts. Within the efforts to strengthen the global governance of antimicrobial resistance, we advocate for the creation of an international One Health platform for online learning. The platform will synthesise the evidence for actions on antimicrobial resistance into a fully accessible database; generate new scientific insights into the design, implementation, evaluation, and reporting of the broad range of interventions relevant to addressing antimicrobial resistance; and ultimately contribute to the goal of building societal resilience to this central challenge of the 21st century.
The threat of antimicrobial resistance has risen on the global agenda with a recognition that collaboration across countries and sectors (eg, human, animal, plant, and environmental health) is needed to enhance the effectiveness and sustainability of current efforts.1, 2, 3 Governments have agreed to set out national action plans to address antimicrobial resistance, but increased action is needed both nationally and internationally.4 The COVID-19 pandemic further stresses the urgent need for ambitious action to tackle infectious diseases. Interventions to deal with antimicrobial resistance, from simple actions to complex ones,5 from regulatory to behavioural approaches, and from strategies focusing on infection prevention to those focusing on responsible use of antimicrobials,6 are crucial to consolidate an evidence-based approach to the challenge.7 Within the ongoing efforts, we discuss the important gaps regarding interventions for antimicrobial resistance and the need for, and main features of, a One Health learning platform that will help to address these gaps. This online platform would synthesise evidence for action on antimicrobial resistance into a fully accessible database; generate new insights into the design, implementation, evaluation, and reporting of the broad range of interventions for antimicrobial resistance; and ultimately help to build resilience to this central challenge.8
Fundamental gaps in knowledge hinder action against antimicrobial resistance, but the limitations of research into interventions for antimicrobial resistance serve as even bigger obstacles. Although systematic reviews have synthesised the effectiveness of some types of these interventions,9, 10, 11, 12 a broader search and assessment of published interventions for antimicrobial resistance provides insights into the current state of knowledge (panel ). First, the number of published interventions and systematic reviews is increasing, with an increasing number of reports coming from low-income and middle-income countries,13 but the understanding that informs many interventions is still biased towards those implemented in high-income countries. Additionally, evidence originates mainly from studies that reported efficacious interventions for antimicrobial resistance and were done in hospitals, farms, or in the human health sector and focused on the surveillance or reduction of antimicrobial use. Second, the overall quality of evidence reported in several systematic reviews is low.10, 11, 14 Third, most of the publications insufficiently reported on the contextual factors that are related to intervention outcomes.15, 16, 17 Fourth, some implementation barriers have been identified for interventions for antimicrobial resistance and health-care associated infections.18, 19, 20, 21 However, the importance and generalisability of these insights is still not fully understood. Finally, interventions to improve sanitation, hand hygiene, and immunisation are effective to prevent and control infections but their effect on antimicrobial resistance has not been sufficiently evaluated, except for some vaccines in human health.22
Panel. Additional information about the search strategy.
Within the scope of the AMResilience consortium, we did a scoping review of the published literature on interventions for antimicrobial resistance between June 1, 2018, and Feb 28, 2019. The search was restricted to peer-reviewed documents that were written in English. The search sought to identify interventions that targeted antimicrobial resistance in human health, animal health, including veterinary health and aquaculture, or the environment. We searched PubMed, Google Scholar, Embase, Scopus, and Cochrane Library with search terms that included keywords related to interventions (eg, “awareness campaign”, “training”, “education”, “stewardship”, “campaign”, “communication”, “regulation”, “policy”, and “legislation”) in combination with terms related to antimicrobial resistance. Interventions comparing the efficacy of new clinical treatments were not considered. Additionally, a snowball method was used to retrieve interventions already included in published systematic reviews about interventions for antimicrobial resistance. After title and abstract screening, 669 individual scientific studies describing or assessing interventions for antimicrobial resistance were identified and organised by use of a qualitative analysis software. Finally, a literature review was done on One Health, implementation science, and complex interventions to further inform the selection of the variables that should be evaluated within the scope of a One Health learning platform.
Key messages.
-
•
Fundamental gaps in knowledge hinder effective action against antimicrobial resistance, but the limitations of research on interventions serve as even bigger obstacles. Moving the agenda forward requires a better understanding of the diversity of interventions for antimicrobial resistance, their feasibility and cost–benefit, and the factors that shape and underpin their effectiveness.
-
•
To foster learning across goals, regions, levels, and sectors, information about interventions for antimicrobial resistance can be consolidated into a fully accessible and continuously updated One Health online platform. Its main added value would be to provide searchable evidence about what works, for whom, and under what conditions.
-
•
An open access learning platform on interventions for antimicrobial resistance should be useful to a broad range of stakeholders, including health-care professionals, public health practitioners, policy makers, industries, and consumer groups. It would not only provide the possibility of complementing published sources with new information, but also enable the exchange of ideas through online community tools.
-
•
By working towards amplifying the generation of science-based and actionable knowledge, the platform would be a timely contribution to the goal of building societal resilience to the complex challenge of antimicrobial resistance. The integration of the One Health learning platform within the governance mechanism will help to maximise its usefulness and sustainability.
Addressing the gaps described here requires an increase in the number and diversity of interventions for antimicrobial resistance. Efforts should be encouraged in low-income and middle-income countries where international research collaborations can foster capacity building.23 An additional need is to increase the number of interventions that seek to address antimicrobial resistance across sectors, countries, or the five objectives of the 2015 WHO Global Action Plan.1 These five objectives focus on awareness and education, surveillance and research, infection prevention, responsible use of antimicrobials, and innovation for new technologies to tackle antimicrobial resistance. Increasing the number of interventions should be done in parallel with improving their quality. First, the design of interventions, particularly multifaceted ones, can be strengthened by adopting a framework for complex adaptive systems that captures the context of implementation.24, 25, 26 The burden of diseases, levels of sanitation and immunisation, financial and institutional capacities, awareness of the problem, and attitudes toward risks are factors often mentioned in the literature as affecting whether a country can respond to antimicrobial resistance.27 How these factors interact to produce resilience to antimicrobial resistance (ie, the capacity of health, food production, agriculture, aquaculture, and environmental systems to cope and adapt to antimicrobial resistance) is a subject of ongoing research.8, 28 Second, the incorporation of guidelines for implementation and stakeholders' involvement can enhance the implementation of interventions.29, 30 Third, more attention should be devoted to the cost-effectiveness of interventions for antimicrobial resistance, their effect on Sustainable Development Goals in terms of outcomes, and factors affecting how well the intervention can be implemented.
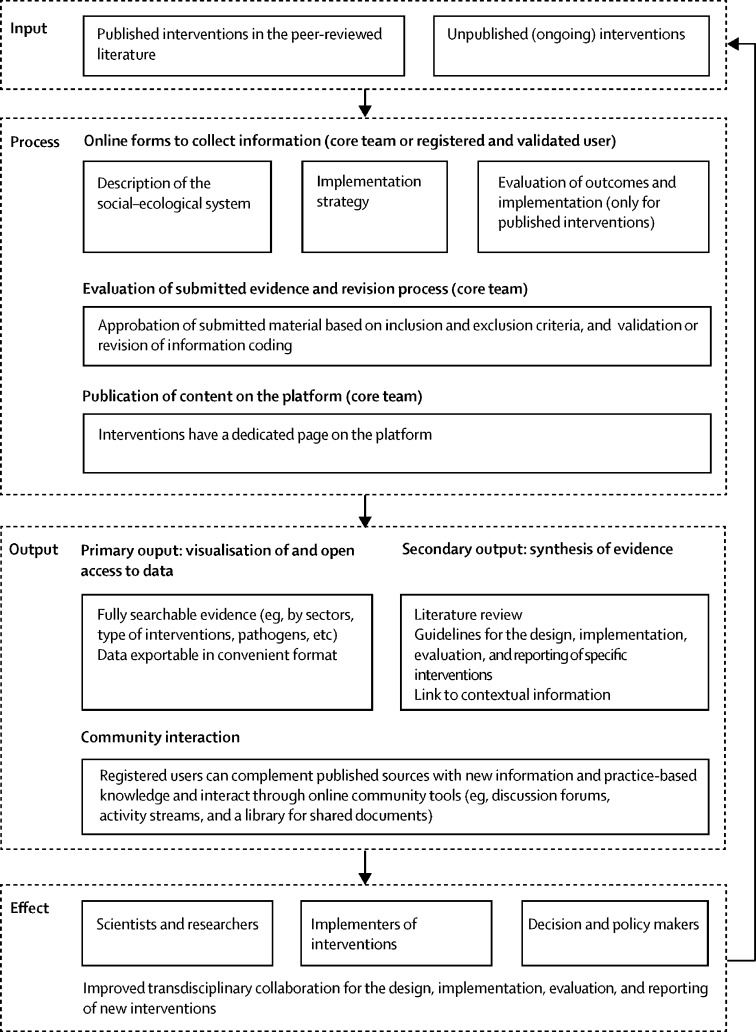
These actions to improve the number, diversity, and quality of interventions will help to enhance the successful design, implementation, evaluation, and reporting of interventions for antimicrobial resistance. Given that collaborative approaches between researchers, industry, and policy makers are crucial to move the agenda forward, there is an additional need to foster the synthesis of information across goals, regions, levels, and sectors while encouraging learning and sharing of information about interventions for antimicrobial resistance. Current efforts can be consolidated into a fully accessible and continuously updated online learning platform. Its main added value would be to provide searchable evidence about what works, for whom, and under what conditions, recognising that interventions that work in high-income countries might not work in low-income and middle-income countries,31 or even in other high-income countries. When an intervention works in one context but not in another, the platform would allow examination of potentially relevant sources of disparity between the contexts. The platform might also help to predict the conditions that are most likely to favour the emergence and spread of antimicrobial resistance. The platform should serve as an evidence repository, facilitate systematic reviews of interventions gathered from the platform, and be a source of information to model the effect of interventions. Reported information can inspire new interventions and facilitate adaptation of existing interventions to other contexts. The learning platform will provide guidance on how to effectively design, implement, evaluate, and report various types of interventions for antimicrobial resistance.
A One Health framework with relevant variables about the biological and social components, implementation process, and evaluation of interventions should guide the systematic assessment of the content inputted on the learning platform. In addition to various sectors, the platform should cover various interventions for antimicrobial resistance in terms of strategies, countries, settings, and target populations. Including interventions for common pathogens and for tuberculosis, malaria, and HIV/AIDS could help to identify synergies and trade-offs between different issues regarding antimicrobial resistance. Given the broad scope of antimicrobial resistance, focusing on high-priority resistance in bacteria would be a reasonable starting point.32 To complement the information gained from published interventions, the platform should encourage sharing of knowledge about the challenges associated with implementation, which are often under-reported in the literature. Finally, the learning platform would benefit from linking interventions to the available data about policies and situations of antimicrobial resistance in the participating countries or regions.33, 34
The platform should be primarily managed by a small team, but users with expertise on interventions for antimicrobial resistance should be able to contribute relevant information. Given that extracting information about interventions is time consuming, the learning platform would also ideally take advantage of the progress in computer science (eg, natural language processing to extract relevant information from interventions). To ensure quality, the platform requires clear guidelines and a revision system for the validation of interventions submitted from researchers in the field of antimicrobial resistance. Most importantly, studies should be graded in terms of the quality of evidence that they provide on the basis of the Grading of Recommendations Assessment, Development and Evaluation system,35 while considering the diversity of different sources of evidence.36, 37, 38, 39 In particular, evaluating the complexity of interventions and the quality of reporting would be beneficial to move the science of interventions for antimicrobial resistance forward.40, 41
An open access learning platform on interventions for antimicrobial resistance might be useful to a broad range of stakeholders, including health-care professionals, public health practitioners, policy makers, industries, and consumer groups.42 The capacities to fully search the database and to export data in a convenient format are essential to make the platform a valuable tool for those involved in addressing antimicrobial resistance. Additional outputs related to the platform include new systematic reviews; the provision of guidelines for the design, implementation, evaluation, and reporting of specific interventions for antimicrobial resistance; and policy briefs summarising important findings about interventions. As a tool to build our collective capacity to tackle antimicrobial resistance,43 the learning platform would enable the exchange of ideas and practice-based knowledge through online community tools (eg, user profiles, discussion forums, activity streams, and a shared library). Interviews with people involved in the implementation of interventions for antimicrobial resistance or surveys of unpublished or grey literature might be a suitable way to collect further information about the challenges associated with implementing interventions.
The AMResilience consortium, which is funded by the fifth call of the Joint Programming Initiative on Antimicrobial Resistance, is building a database of interventions for antimicrobial resistance. The large scope of the envisioned One Health learning platform (figure ), associated with the growth of the literature and non-published information, requires further institutionalisation and support. A reasonable grant from a national, international, or non-governmental source of research funding would make the development of the platform possible. As several research groups are already working on interventions for antimicrobial resistance, a core team of researchers should provide the brainpower to cover the diversity of interventions and keep the platform up to date. The team and entity responsible for the development of the project should work with existing international networks. Within the ongoing discussion about the need for an independent panel of evidence for action against antimicrobial resistance,6 we further advocate for the integration of the One Health learning platform within the governance mechanism to maximise its usefulness and sustainability.44 By amplifying the generation and synthesis of evidence-informed action on antimicrobial resistance, the platform would be a timely contribution to the goal of building societal resilience to the complex challenge of addressing infectious diseases.
Figure.
One Health online learning platform
Acknowledgments
Acknowledgments
We thank all the participants in the two workshops in September and October, 2019, in Stockholm, Sweden, and Penang, Malaysia. This project is funded through the fifth call of the Joint Programming Initiative on Antimicrobial Resistance by the Swedish Research Council, the Canadian Institutes of Health Research, and the Swiss National Science Foundation. CVM, JD-D, and PJGH were supported by the CGIAR Research Program on Fish Agri-Food Systems led by WorldFish. BW was supported by the CGIAR Research Program on Agriculture for Nutrition and Health.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
DW initiated and drafted the Personal View, including the figure. All other authors provided comments and suggestions on the manuscript. DW did a narrative review of the literature on interventions for antimicrobial resistance from a One Health perspective for his PhD thesis. AL did a search of the literature on published interventions for antimicrobial resistance as part of the first working package of the AMResilience consortium (principal investigator is DW). PSJ and TG organised two workshops in September and October, 2019, during which, among other topics, the need for, and main features of, an online learning platform about interventions for antimicrobial resistance were discussed. PSJ coordinates the AMResilience consortium.
Declaration of interests
SM reports grants from Canadian Institutes for Health Research during the conduct of the study; and personal fees from Epidemiology and Infection (Associate Editor) and the Attorney General of Canada (expert evidence), outside of the submitted work. SH reports personal fees from Sandoz and grants from the European Commission, outside of the submitted work. AJHS reports grants from Wellcome Trust, outside of the submitted work. All other authors declare no competing interests.
References
- 1.WHO . World Health Organization; Geneva: 2015. Global Action Plan on Antimicrobial Resistance. [DOI] [PubMed] [Google Scholar]
- 2.UN . United Nations; New York, NY: 2016. Resolution 71/3 political declaration of the high-level meeting of the General Assembly on antimicrobial resistance.https://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/71/3 [Google Scholar]
- 3.Hernando-Amado S, Coque TM, Baquero F, Martínez JL. Defining and combating antibiotic resistance from One Health and global health perspectives. Nat Microbiol. 2019;4:1432–1442. doi: 10.1038/s41564-019-0503-9. [DOI] [PubMed] [Google Scholar]
- 4.World Health Assembly . World Health Organization; Geneva: 2019. Resolution 72·5–antimicrobial resistance. [Google Scholar]
- 5.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337 doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dar OA, Hasan R, Schlundt J, et al. Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet. 2016;387:285–295. doi: 10.1016/S0140-6736(15)00520-6. [DOI] [PubMed] [Google Scholar]
- 7.Woolhouse M, Farrar J. Policy: an intergovernmental panel on antimicrobial resistance. Nature. 2014;509:555–557. doi: 10.1038/509555a. [DOI] [PubMed] [Google Scholar]
- 8.Jørgensen PS, Wernli D, Carroll SP, et al. Use antimicrobials wisely. Nature. 2016;537:159–161. doi: 10.1038/537159a. [DOI] [PubMed] [Google Scholar]
- 9.Tang KL, Caffrey NP, Nóbrega DB, et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017;1:e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price L, Gozdzielewska L, Young M, et al. Effectiveness of interventions to improve the public's antimicrobial resistance awareness and behaviours associated with prudent use of antimicrobials: a systematic review. J Antimicrob Chemother. 2018;73:1464–1478. doi: 10.1093/jac/dky076. [DOI] [PubMed] [Google Scholar]
- 11.Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database of Syst Rev. 2017;2 doi: 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers Van Katwyk S, Grimshaw JM, Nkangu M, et al. Government policy interventions to reduce human antimicrobial use: a systematic review and evidence map. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dijck C, Vlieghe E, Cox JA. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull World Health Organ. 2018;96:266–280. doi: 10.2471/BLT.17.203448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweitzer VA, van Heijl I, van Werkhoven CH, et al. The quality of studies evaluating antimicrobial stewardship interventions: a systematic review. Clin Microbiol Infect. 2019;25:555–561. doi: 10.1016/j.cmi.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci. 2007;2:40. doi: 10.1186/1748-5908-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez D, Van der Stuyft P, Zabala MC, Castro M, Lefèvre P. A modified theoretical framework to assess implementation fidelity of adaptive public health interventions. Implement Sci. 2016;11:91. doi: 10.1186/s13012-016-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth A, Moore G, Flemming K, et al. Taking account of context in systematic reviews and guidelines considering a complexity perspective. BMJ Glob Health. 2019;4(suppl 1) doi: 10.1136/bmjgh-2018-000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pakyz AL, Moczygemba LR, VanderWielen LM, Edmond MB, Stevens MP, Kuzel AJ. Facilitators and barriers to implementing antimicrobial stewardship strategies: results from a qualitative study. Am J Infect Control. 2014;42(suppl):S257–S263. doi: 10.1016/j.ajic.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Santillo M, Sivyer K, Krusche A, et al. Intervention planning for antibiotic review kit (ARK): a digital and behavioural intervention to safely review and reduce antibiotic prescriptions in acute and general medicine. J Antimicrob Chemother. 2019;74:3362–3370. doi: 10.1093/jac/dkz333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broom J, Broom A, Kirby E, Gibson AF, Post JJ. How do hospital respiratory clinicians perceive antimicrobial stewardship (AMS)? A qualitative study highlighting barriers to AMS in respiratory medicine. J Hosp Infect. 2017;96:316–322. doi: 10.1016/j.jhin.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Zingg W, Holmes A, Dettenkofer M, et al. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Dis. 2015;15:212–224. doi: 10.1016/S1473-3099(14)70854-0. [DOI] [PubMed] [Google Scholar]
- 22.Buckley BS, Henschke N, Bergman H, et al. Impact of vaccination on antibiotic usage: a systematic review and meta-analysis. Clin Microbiol Infect. 2019;25:1213–1225. doi: 10.1016/j.cmi.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Wang Y, Hulth A, et al. Study protocol for One Health data collections, analyses and intervention of the Sino-Swedish integrated multisectoral partnership for antibiotic resistance containment (IMPACT) BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-017832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunderson LH, Holling CS. Island Press; Washington, DC: 2001. Panarchy: understanding transformations in human and natural systems. [Google Scholar]
- 25.Levin S, Xepapadeas T, Crépin A-S, et al. Social-ecological systems as complex adaptive systems: modeling and policy implications. Environ Dev Econ. 2013;18:111–132. [Google Scholar]
- 26.Norris SL, Rehfuess EA, Smith H, et al. Complex health interventions in complex systems: improving the process and methods for evidence-informed health decisions. BMJ Glob Health. 2019;4(suppl 1) doi: 10.1136/bmjgh-2018-000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018;2:e398–e405. doi: 10.1016/S2542-5196(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 28.Jørgensen PS, Wernli D, Folke C, Carroll SP. Changing antibiotic resistance: sustainability transformation to a pro-microbial planet. Curr Opin Environ Sustain. 2017;25:66–76. [Google Scholar]
- 29.Pinnock H, Barwick M, Carpenter CR, et al. Standards for reporting implementation studies (StaRI) statement. BMJ. 2017;356 doi: 10.1136/bmj.i6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider A, Coope C, Michie S, Puleston R, Hopkins S, Oliver I. Implementing a toolkit for the prevention, management and control of carbapenemase-producing Enterobacteriaceae in English acute hospitals trusts: a qualitative evaluation. BMC Health Serv Res. 2019;19:689. doi: 10.1186/s12913-019-4492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakkar M, Chatterjee P, Chauhan AS, et al. Antimicrobial resistance in south east Asia: time to ask the right questions. Glob Health Action. 2018;11 doi: 10.1080/16549716.2018.1483637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 33.Anderson M, Schulze K, Cassini A, Plachouras D, Mossialos E. A governance framework for development and assessment of national action plans on antimicrobial resistance. Lancet Infect Dis. 2019;19:e371–e384. doi: 10.1016/S1473-3099(19)30415-3. [DOI] [PubMed] [Google Scholar]
- 34.Wernli D, Jørgensen PS, Harbarth S, et al. Antimicrobial resistance: the complex challenge of measurement to inform policy and the public. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328 doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rychetnik L, Frommer M, Hawe P, Shiell A. Criteria for evaluating evidence on public health interventions. J Epidemiol Community Health. 2002;56:119–127. doi: 10.1136/jech.56.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownson RC, Baker EA, Deshpande AD, Gillespie KN. 3rd edn. Oxford University Press; New York, NY: 2018. Evidence-based public health. [Google Scholar]
- 38.Frieden TR. Evidence for health decision making—beyond randomized, controlled trials. N Engl J Med. 2017;377:465–475. doi: 10.1056/NEJMra1614394. [DOI] [PubMed] [Google Scholar]
- 39.Rutter H, Savona N, Glonti K, et al. The need for a complex systems model of evidence for public health. Lancet. 2017;390:2602–2604. doi: 10.1016/S0140-6736(17)31267-9. [DOI] [PubMed] [Google Scholar]
- 40.Lewin S, Hendry M, Chandler J, et al. Assessing the complexity of interventions within systematic reviews: development, content and use of a new tool (iCAT_SR) BMC Med Res Methodol. 2017;17:76. doi: 10.1186/s12874-017-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers Van Katwyk S, Grimshaw JM, Nkangu M, Mendelson M, Taljaard M, Hoffman SJ. Study reporting quality among interventions to reduce antibiotic use is a barrier to evidence-informed policymaking on antimicrobial resistance: systematic review. J Antimicrob Chemother. 2020;75:1091–1098. doi: 10.1093/jac/dkz540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majowicz SE, Parmley EJ, Carson C, Pintar K. Identifying non-traditional stakeholders with whom to engage, when mitigating antimicrobial resistance in foodborne pathogens (Canada) BMC Res Notes. 2018;11:170. doi: 10.1186/s13104-018-3279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young OR. The MIT Press; Cambridge, MA: 2017. Governing complex systems: social capital for the anthropocene. [Google Scholar]
- 44.Interagency Coordination Group on Antimicrobial Resistance No time to wait: securing the future from drug-resistant infections. Report to the Secretary-General of the United Nations. April, 2019. https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1