Highlights
-
•
As Add-on therapy, IFN β-1b shortened the time to clinical improvement.
-
•
IFN β-1b significantly increased the discharge rate at day 14.
-
•
IFN β-1b reduced overall 28-day mortality.
-
•
IFN β-1b related adverse effects were mild and did not cause treatment interruptions.
Keywords: Interferon β, COVID19, SARS-COV-2, Iran
Abstract
In this study, efficacy and safety of interferon (IFN) β-1b in the treatment of patients with severe COVID-19 were evaluated.
Among an open-label, randomized clinical trial, adult patients (≥18 years old) with severe COVID-19 were randomly assigned (1:1) to the IFN group or the control group. Patients in the IFN group received IFN β-1b (250 mcg subcutaneously every other day for two consecutive weeks) along with the national protocol medications while in the control group, patients received only the national protocol medications (lopinavir/ritonavir or atazanavir/ritonavir plus hydroxychloroquine for 7–10 days). The primary outcome of the study was time to clinical improvement. Secondary outcomes were in-hospital complications and 28-daymortality.
Between April 20 and May 20, 2020, 80 patients were enrolled and finally 33 patients in each group completed the study. Time to clinical improvment in the IFN group was significantly shorter than the control group ([9(6–10) vs. 11(9–15) days respectively, p = 0.002, HR = 2.30; 95% CI: 1.33–3.39]). At day 14, the percentage of discharged patients was 78.79% and 54.55% in the IFN and control groups respectively (OR = 3.09; 95% CI: 1.05–9.11, p = 0.03). ICU admission rate in the control group was significantly higher than the IFN group (66.66% vs. 42.42%, p = 0.04). The duration of hospitalization and ICU stay were not significantly different between the groups All-cause 28-day mortality was 6.06% and 18.18% in the IFN and control groups respectively (p = 0.12).
IFN β-1b was effective in shortening the time to clinical improvement without serious adverse events in patients with severe COVID-19. Furthermore, admission in ICU and need for invasive mechanical ventilation decreased following administration of IFN β-1b. Although 28-day mortality was lower in the IFN group, further randomized clinical trials with large sample size are needed for exact estimation of survival benefit of IFN β-1b.
1. Introduction
Coronavirus disease 2019 (CoVID-19) was reported from Wuhan for the first time in late December 2019. Causing severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) [1], it rapidly spread throughout the world to the extent that the World Health Organization (WHO) stated it as pandemic in March 2020 [2]. Until July 5, 2020, more than 15 million confirmed cases of CoVID-19 were reported worldwide. Furthermore, more than 600.000 deaths were recorded [3].
Until now, there is no definite antiviral treatment for CoVID-19 and attempts continue for finding effective treatments worldwide. However, from the beginning of the pandemic, various treatments such as antiretrovirals, anti-malaria agents, favipiravir, remdesivir, and corticosteroids, immunoglobulin and cytokine blockers as adjunctive therapies were suggested for the treatment of CoVID-19 [4]. Except for the remdesivir which has had acceptable results, the efficacy of other drugs has not been significant on the outcomes of the patients with CoVID-19 [5], [6], [7], [8], [9].
Interferons (IFNs) have a key role in defense against viral infections as a component of innate immune system [10]. Invitro activity of IFN β has been shown against severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) [10], [11], [12], [13]. Although IFN β was used less than IFN α for the treatment of SARS-COV and MERS-CoV in human studies, it was effective in the treatment of MERS-CoV in retrospective studies and case series [14], [15]. The efficacy of IFN β-1b is being assessed in the treatment of MERS in a randomized clinical trial [16]. According to the presence of this evidence, IFN β was considered as a promising option for the treatment of CoVID-19.
In this open-label, randomized clinical trial, efficacy and safety of IFN β-1b in the treatment of patients with severe CoVID-19 were assessed.
2. Materials and methods
2.1. Study design
This open-label, randomized clinical trial was designed to evaluate the efficacy and safety of IFN β-1b in the treatment of patients with CoVID-19. Patients with severe CoVID-19 who were hospitalized during April 20 to May 20, 2020, in Imam Khomeini Hospital Center, one of the largest referral hospitals in Tehran, Iran were included.
The protocol of the study was approved by Ethics Committee of Tehran University of Medical Sciences (Reference number: IR.TUMS.VCR.REC.1398.1053). Furthermore, the study was registered as a clinical trial (register ID: IRCT20100228003449N27). The study protocol was described for participants and written informed consents were obtained from all patients or their first-degree family members.
2.2. Eligibility criteria
SARS-CoV-2 in patients’ nasopharyngeal swabs was detected using Real-Time Polymerase Chain Reaction (RT-PCR). Total RNA extraction was done applying Viral Nucleic Acid Extraction kit (Cat. No. YVN50/YVN100) from RBC Bioscience, Taipei, Taiwan. The Novel Coronavirus (2019-nCOV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) of Sansure Biotech (S3102E) (Changsha, China) was used for RT-PCR.
Adult patients (≥18 years old) with positive PCR and clinical symptoms/signs of pneumonia (including dyspnea, cough and fever), peripheral oxygen saturation (SPO2) ≤ 93% in ambient air or arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) < 300 or SPO2/FiO2 < 315 and lung involvement in chest imaging were included. These criteria indicated severe form of the disease [17]. At baseline, patients with serious allergic reactions to IFN, history of suicide thoughts and attempts, alanine amino transferase (ALT) > 5× the upper limit of the normal range, uncontrolled underlying diseases such as neuropsychiatric disorders, thyroid disorders, cardiovascular diseases and also pregnant and lactating women were not included.
Recruitment was considered during the first 48-hour of the hospital admission. During the study period, patients who received less than 4 doses of IFN β-1b were excluded. If patients were discharged before fulfilment of the treatment course, the treatment was applied at home.
2.3. Procedures
Eligible patients were recruited in the IFN group or the control group according to the permuted block randomization. Patients in the IFN group received IFN β-1b along with the national protocol medications, while in the control group, patients received only the national protocol medications. IFN β-1b (Ziferon®, Zist Daru Daneh Co., Iran) was administrated as 250 mcg subcutaneously every other day for two consecutive weeks. The national protocol consisted lopinavir/ritonavir (400/100 mg BD) or atazanavir/ritonavir (300/100 mg daily) plus hydroxychloroquine (400 mg BD in first day and then 200 mg BD) for 7–10 days. Other supportive cares such as fluid therapy, stress ulcer prophylaxis, deep vein thrombosis, treatment of electrolyte disorders and antibiotic therapy were considered according to the hospital protocols. The duration of the study was two weeks. A 4-week follow-up period was considered for all patients.
Patients’ demographic data, baseline diseases, symptoms at the time of disease presentation, vital signs and laboratory data at the time of hospital admission were recorded. Patients were daily monitored in terms of changes in the vital signs, hemodynamic parameters, oxygenation status, laboratory data and treatment strategies. Clinical status of the patients was assessed by the six-category ordinal scale at days 0, 7, 14 and 28 of the randomization [18]. Need for supplemental oxygen therapy and also invasive or non-invasive respiratory supports were evaluated regularly.
2.4. Outcomes
Time to clinical improvement was considered as primary outcome of study. Clinical improvement was defined as improvement of at least two points from the baseline status on the six-category ordinal scale [18]. This scale contains the subsequent categories: (1) death (2) hospital admission requiring invasive mechanical ventilation (3) hospital admission, requiring non-invasive positive pressure ventilation (4) hospital admission, requiring oxygen (5) hospital admission, not requiring oxygen (6) discharge. Secondary outcomes were clinical status of patients at day 7, 14 and 28, ICU admission and intubation rates, length of hospitalization and ICU stay, and 28-day mortality.
Side effects related to IFN therapy and other adverse events during the study period were monitored and recorded as the safety outcomes. Categorization of adverse events was done according to the common terminology criteria for adverse events (CTCAE), National Institutes of Health and National Cancer Institute, 2017.
Also serious complications during the hospitalization course such as acute respiratory distress syndrome (ARDS), nosocomial infections, septic shock, acute kidney injury (AKI) and acute hepatic injury (AHI) were considered.
2.5. Statistical analysis and randomization
Continuous variables are demonstrated as median (interquartile range (IQR)) and categorical variables as frequencies and percentages. Continuous variables were compared between the groups by Mann-Whitney U test. The Fisher’s exact test was applied for comparison of categorical variables.
The Hazard Ratio (HR) and 95% CI for clinical improvement were estimated by Cox proportional hazards regression analysis. The effect of ischemic heart disease, lymphocyte count, Aspartate aminotransferase (AST) and C-reactive protein (CRP) on the primary outcome was evaluated by the adjusted Cox regression models as potential confounding factors. Time to clinical improvement was estimated by Kaplan-Meier plot and compared with a log-rank test. All statistical analysis was done by SPSS software (version 21.0).
Time to clinical improvement was estimated to be approximately 10 days and sample size was calculated by following equation:
According to the above equation, at least 28 patients in each group were expected to make a difference of 4 days in time to clinical improvement with power of 85%. Patients were randomly recruited (1:1) to the IFN group or the control group. The method of randomization was the permuted block randomization (6 patients per block). A biostatistician who was not involved in patients’ care did this process.
3. Results
3.1. Patients
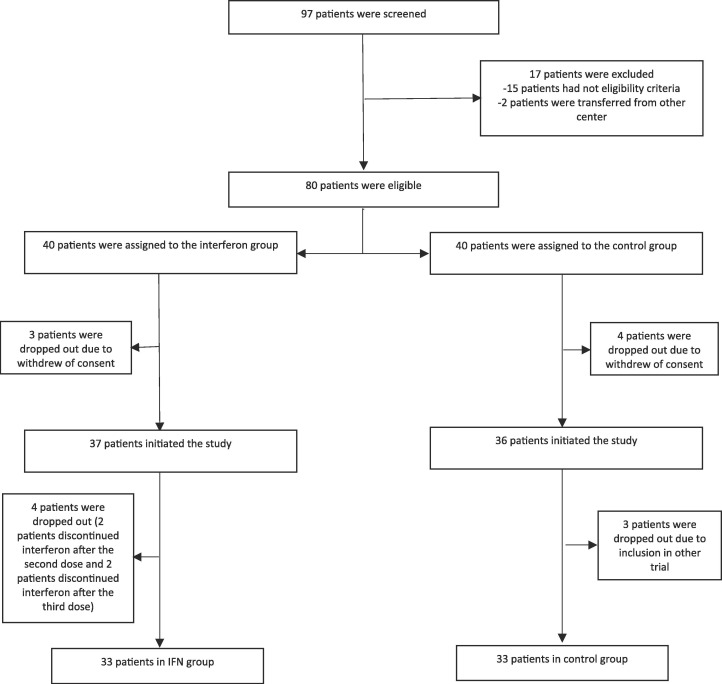
A total of 97 patients were screened. Of them, 15 patients did not have the eligibility criteria of study and 2 patients were referred from another hospital. Three and four patients withdrew the consent during the study in the IFN group and control groups, respectively. Four patients did not adhere to IFN injection after second or third dose. Also three patients in the control group were enrolled in another trial. Finally, 33 patients in each group completed the study (Fig. 1 ).
Fig. 1.
Consort flowchart of the study.
The median (IQR) age of patients was 60(50–71) years and 59.09% of them were male. No significant difference in terms of the patients’ demographic data was detected between the groups. The most common comorbidities were hypertension, diabetes mellitus and ischemic heart disease. Dyspnea, fever and cough were the most frequent symptoms at the time of hospital admission. The median (IQR) time from onset of the symptoms to hospital admission was 7(5–9) and 7(4–8) days in the IFN group and control groups respectively. The time from onset of the symptoms to randomization was not statistically significant between the groups. All of patients required respiratory support at the time of randomization. Oxygenation through facemask was required for more than 80 percent of patients. None of the patients in both groups were intubated at baseline (Table 1 ). Vital signs and laboratory data of patients at the time of recruitment were comparable between the groups (Table 2 ). During the hospitalization course, oxygen saturation dropped in 6.06% and 18.18% of patients in the IFN and control groups respectively. All of those patients were intubated. At least one antibiotic was administrated for 45.45% and 57.57% of patients in the IFN group and control groups respectively. Methylprednisolone was administered for 15.15% of patients in the IFN group and 27.27% of patients in the control group. The dose of methylprednisolone was 250 mg daily for 3 days. Methylprednisolone was considered during the cytokine or hyperinflammation phase (days 14–21 of onset of the symptoms). Approximately 6% and 18% of patients in the INF and control groups needed vasopressors during the hospitalization course respectively (Table 3 ).
Table 1.
Baseline characteristics of patients.
| Parameter; Median (IQR) or n (%) | Interferon group (n = 33) | Control group (n = 33) |
|---|---|---|
| Age | 60(47–73) | 61(50–71) |
| Sex | ||
| Male | 20(60.60) | 19(57.57) |
| Female | 13(39.39) | 14(42.42) |
| Comorbid conditions: n(%) | ||
| Hypertension | 18(54.54) | 19(57.57) |
| Diabetes mellitus | 9(27.27) | 12 (36.36) |
| Ischemic heart disease | 7(21.21) | 13(39.39) |
| Asthma | 1(3.03) | 2(6.06) |
| COPD | 2(6.06) | 1(3.03) |
| Malignancy | 1 (3.03) | 1(3.03) |
| Transplantation | 1(3.03) | 0 |
| Symptoms at admission: n(%) | ||
| Dyspnea | 20(60.60) | 23(69.69) |
| Fever | 18(54.54) | 21(63.63) |
| Cough | 18(54.54) | 20(60.60) |
| Chills | 17(51.51) | 11(33.33) |
| Duration of symptoms before admission, median (IQR) (days) | 7(5–9) | 7(4–8) |
| Time from symptom onset to randomization, median (IQR) (days) | 8(7–11) | 8(5–9) |
| Six category scale at day 0 of intervention | ||
| 3-hospital admission, requiring high-flow nasal cannula or non-invasive mechanical ventilation | 1(3.03) | 0 |
| 4- hospital admission, requiring supplemental oxygen | 32(96.97) | 33(100) |
Table 2.
Patients’ vital signs and laboratory data at the time of hospital admission.
| Parameter; Median (IQR) | Interferon group (n = 33) | Control group (n = 33) |
|---|---|---|
| Temperature (°C) | 37.5(37.2–38.5) | 37.5(37.2–38.3) |
| Heart rate (beats /minute) | 88(80–100) | 94(80–100) |
| Respiratory rate (breaths/min) | 19(18–23) | 20(19–22) |
| Systolic blood pressure (mm Hg) | 120(110–131) | 120(110–140) |
| SPO2 (%) | 88(83–89) | 88(85–92) |
| Laboratory data | ||
| White Blood Cell (cells /μl) | 5400(4025–8250) | 5900(4050–7650) |
| Acute Lymphocyte count (cells/μl) | 924(520–1400) | 869(670–1000) |
| Hemoglobin (g/dl) | 12.9(11.5–14.2) | 13.0(11.4–14.1) |
| Platelet count (cells × 103/μl) | 195(155–267) | 172(138–257) |
| Blood Urea Nitrogen (mg/dl) | 22(15–37) | 15(10–25) |
| Creatinine (mg/dl) | 1.0(0.8–1.2) | 1.2(1–1.4) |
| Aspartate aminotransferase (u/l) | 27(16–40) | 38(28–50) |
| Alanine aminotransferase (u/l) | 21(15–40) | 32(19–46) |
| Alkaline phosphatase (u/l) | 183(139–237) | 172(131–247) |
| Total bilirubin(mg/dl) | 0.5(0.4–0.8) | 0.7(0.5–0.9) |
| C-reactive protein (mg/dl) | 87(47–116) | 84(47–179) |
| Erythrocyte sedimentation rate (mm/h) | 66(32–89) | 65(50–90) |
| Lactate dehydrogenase (u/l) | 594(325–639) | 618(378–777) |
Table 3.
Respiratory support and medications.
| Parameter; n (%) | Interferon group (n = 33) | Control group (n = 33) |
|---|---|---|
| Respiratory support | ||
| Nasal cannula | 2(6.06) | 2(6.06) |
| Face mask | 28(84.84) | 25(75.75) |
| NIPPV | 1(3.03) | 0 |
| IMV | 2(6.06) | 6(18.18) |
| Antibiotics (meropenem, piperacillin-tazobactam, ceftriaxone, FQs, vancomycin, azithromycin and Colistin), n (%) | 15(45.45) | 19(57.57) |
| Corticosteroids | 5(15.15) | 9(27.27) |
| Vitamin C | 13(39.39) | 8(24.24) |
| Vasopressors | 2(6.06) | 6(18.18) |
| Diphenhydramine | 11(33.33) | 17(51.51) |
| Cardiovascular drugs | ||
| Statins | 13(39.39) | 12(36.36) |
| ARBs | 6(18.18) | 7(21.21) |
| Beta-blockers | 4(12.12) | 5(15.15) |
| ACEIs | 3(9.09) | 2(6.06) |
NIPPV: noninvasive positive pressure ventilation, IMV: invasive mechanical ventilation, FQs: fluoroquinolones, ARB: Angiotensin Π Receptor Blocker, ACEI: angiotensin converting enzyme inhibitor
3.2. Primary outcomes
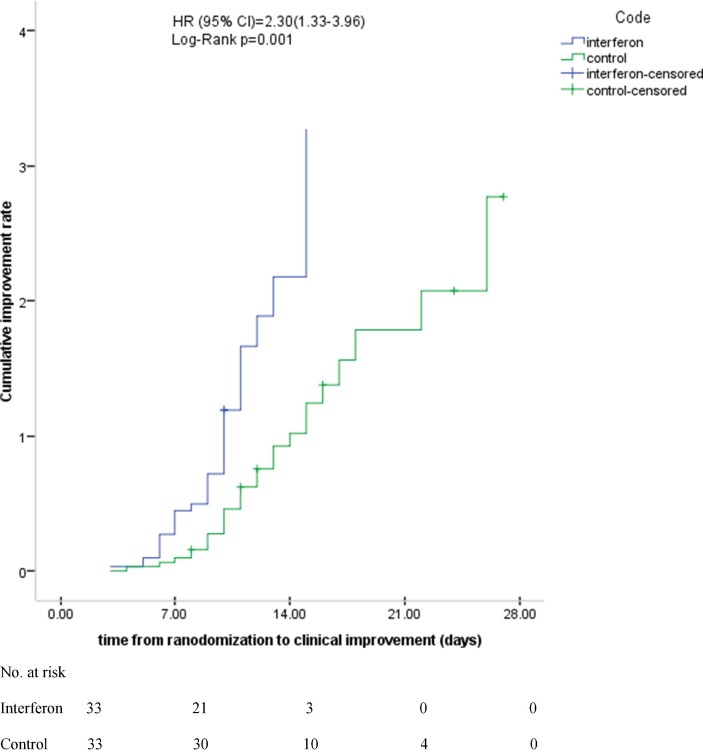
The time to clinical improvement in the IFN group was significantly shorter than the control group [9(6–10) vs. 11(9–15) days respectively, p = 0.002] (Table 4 ). Moreover, the Cox proportional hazards regression analysis showed that time difference to clinical improvement was statistically significant between the groups (HR = 2.30; 95% CI: 1.33–3.39) (Fig. 2 ). Then the model was adjusted for the confounding factors and similar results were seen (HR = 3.41; 95% CI: 1.33–8.72).
Table 4.
Outcomes and complications.
| Parameter; Median (IQR) or n (%) | Interferon group (n = 33) | Control group (n = 33) | p-value |
|---|---|---|---|
| Time to clinical response, median(IQR) (days) | 9(6–10) | 11(9–15) | 0.002 |
| ICU admission, n (%) | 14(42.42) | 22(66.66) | 0.04 |
| Intubation requirement | 2(6.06) | 6(18.18) | 0.12 |
| Length of stay in ICU (days), median (IQR) (days) | 9(6–13) | 8 (4–12) | 0.55 |
| Length of stay in hospital (days), median (IQR) (days) | 11(9–13) | 13(10–17) | 0.05 |
| All-cause mortality at day 28 | 2(6.06) | 6(18.18) | 0.12 |
| Six category scale at day 7 of intervention | OR(95% CI) | ||
| 1- Death | 0 | 1(3.03) | |
| 2- Hospital admission, requiring invasive mechanical ventilation | 2(6.06) | 4(12.12) | |
| 3- Hospital admission, requiring high-flow nasal cannula or non-invasive mechanical ventilation | 0 | 3(9.09) | |
| 4- Hospital admission, requiring supplemental oxygen | 26(78.79) | 23(69.69) | |
| 5- Hospital admission not requiring supplemental oxygen | 0 | 0 | |
| 6- Discharge | 5(15.15) | 2(6.06) | 2.76(0.49–15.42) |
| Six category scale at day 14 of intervention | |||
| 1- Death | 1(3.03) | 3(9.09) | |
| 2- Hospital admission, requiring invasive mechanical ventilation | 1(3.03) | 3(9.09) | |
| 3- Hospital admission, requiring high-flow nasal cannula or non-invasive mechanical ventilation | 1(3.03) | 0 | |
| 4- Hospital admission, requiring supplemental oxygen | 4(12.12) | 9(27.27) | |
| 5- Hospital admission not requiring supplemental oxygen | 0 | 0 | |
| 6- Discharge | 26(78.79) | 18(54.55) | 3.09(1.05–9.11) |
| Six category scale at day 28 of intervention | |||
| 1- Death | 2(6.06) | 6(18.18) | |
| 2- Hospital admission, requiring invasive mechanical ventilation | 0 | 0 | |
| 3- Hospital admission, requiring high-flow nasal cannula or non-invasive mechanical ventilation | 0 | 0 | |
| 4- Hospital admission, requiring supplemental oxygen | 0 | 0 | |
| 5- Hospital admission not requiring supplemental oxygen | 0 | 0 | |
| 6- Discharge | 31(93.94) | 27(81.82) | 3.44(0.64–18.50) |
Fig. 2.
Kaplan-Meier plot for estimation of time to clinical improvement.
3.3. Secondary outcomes
According to the six category scale, 15.15% and 6.06% of patients were discharged in the IFN and the control groups at day 7 respectively (OR = 2.76; 95% CI: 0.49–15.42, p = 0.21). Only one patient in the control group died at day 7. Also, at this time, 2 and 4 patients were intubated in the IFN and control groups respectively. At day 14, the percentage of discharged patients reached to 78.79% and 54.55% in the IFN and control groups respectively (OR = 3.09; 95% CI: 1.05–9.11, p = 0.03). Furthermore, the number of deaths increased to 1 and 3 patients the IFN and control groups respectively. Finally, at day 28 of inclusion, the proportion of discharged patients were 93.94% in the IFN group and 81.82% in the control group (OR = 3.44; 95% CI: 0.64–18.5, p = 0.12). At this time, ICU admission rate in the control group was significantly higher than the IFN group (66.66% vs. 42.42%, p = 0.04). Moreover, more patients in the control group needed invasive mechanical ventilation compared with the IFN group but the rate was not statistically different (p = 0.12). Although length of hospitalization was shorter [11 (9–13) days in the IFN group vs. 13(10–17) days in the control group p = 0.05] but length of ICU stay was not significantly different between the groups. All-cause 28-day mortality was 6.06% and 18.18% in the IFN and control groups respectively (p = 0.12) (Table 4).
3.4. Safety outcomes
A total of 47 and 62 common adverse events were recorded during the study period in the IFN and control groups respectively. Moreover, number of serious adverse events was 9 in the IFN group and 24 in the control group. The incidence of grade 3 or 4 of adverse events was higher in the control group than the IFN group. As it was expected, IFN-related common adverse effects (injection site reactions and flu-like syndrome) occurred only in the IFN group. More patients in the control group experienced ARDS, secondary infections, septic shock, AKI and AHI compared with patients in the IFN group (Table 5 ).
Table 5.
Summary of the adverse events during the study period.
| Parameter; n (%) | Interferon group (n = 33) |
Control group (n = 33) |
||
|---|---|---|---|---|
| Common adverse events | Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 |
| Leukocytosis | 5(15.15) | – | 10(33.33) | – |
| Leukopenia | 3(9.09) | – | 3(9.09) | – |
| Lymphopenia | 7(21.21) | 1(3.03) | 10(33.33) | – |
| Thrombocytopenia | 4(12.12) | – | 4(12.12) | – |
| Thrombocytosis | 0 | – | 4(12.12) | – |
| Anemia | 3(9.09) | – | 6(18.18) | 1(3.03) |
| Hyperkalemia | 3(9.09) | 1(3.03) | 10(33.33) | 3(9.09) |
| Hypokalemia | 3(9.09) | – | 0 | – |
| Hyponatremia | 1(3.03) | – | 0 | – |
| Increased creatinine | 4(12.12) | – | 4(12.12) | 3(9.09) |
| Increased aspartate aminotransferase | 2(6.06) | – | 5(15.15) | 2(6.06) |
| Nausea | 3(9.09) | – | 3(9.09) | – |
| Diarrhea | 1(3.03) | – | 1(3.03) | – |
| Abdominal pain | 2(6.06) | – | 2(6.06) | – |
| Injection site reaction | 2(6.06) | – | – | – |
| Flu-like syndrome | 4(12.12) | – | – | – |
| Serious adverse events | ||||
| ARDS | 2(6.06) | 1(3.03) | 6(18.18) | 6(18.18) |
| Nosocomial infection | 1(3.03) | – | 5(15.15) | 4(12.12) |
| Septic shock | 1(3.03) | – | 4(12.12) | 4(12.12) |
| Acute kidney injury | 3(9.09) | – | 4(12.12) | 3(9.09) |
| Acute hepatic injury | 2(6.06) | – | 5(15.15) | 2(6.06) |
ARDS: acute respiratory distress syndrome.
Nosocomial infections were detected in 6 patients (1 and 5 patients in the INF and control groups respectively). Bloodstream infection with staphylococcus aureus was detected in a patient in the INF group. Three patients in the control group experienced ventilator associated pneumonia (with klebsiella pneumonia in two patients and acinetobacter baumannii in another patient). Other patients in the control group had bloodstream infection with staphylococcus aureus.
4. Discussion
This is first randomized clinical trial that evaluated efficacy and safety of IFN β subtype 1b in patients with severe COVID-19. In this study, IFN β-1b as add-on therapy significantly shortened the time to clinical response, increased the discharge rate at day 14 and decreased need for ICU admission in these patients. However, duration of hospitalization, intubation rate, length of ICU stay and all-cause 28-day mortality were not significantly changed. Incidence rates of common and serious adverse events were higher in the control group compared with the IFN group. The sample size was calculated to assess effect of IFN β-1b on time to clinical improvement in hospitalized patients with COVID-19. However, the sample size might not have enough power to differentiate effects of IFN β-1b on the secondary endpoints.
IFN β is a subtype of the type 1 INFs that is released by the lymphocytes as the first cytokine following exposure to viruses. It activates interferon-stimulated genes (ISGs) after binding to the receptors. The antiviral effects of IFNs are regulated through these genes. Inadequate IFN response caused uncontrolled viral replication, raised viral load and led to poor outcomes in SARS-CoV infection. A strong IFN response following infection with SARS-CoV-2 was detected. [19], [20] Expression of ISGs significantly increased in patients with CoVID-19 [20]. In evaluation of transcriptional responses in various models (in vitro, ex vivo and in vivo), Balanco-Melo et al showed that the levels of IFN- I and IFN- III decreased in SARS-CoV-2 infection. In in vitro model, expressions of IFN- I and IFN- III were not detected in A549 cells (as adenocarcinomic human lung cell line) infected with SARS-CoV-2. Of note, moderate increase in the expression of ISGs was observed. Next step, the cells were treated by IFN β that caused substantially reduction in the viral replication. Furthermore, in ex vivo model, the levels of IFN- I and IFN- III were undetectable following infection of human bronchial epithelial cells with SARS-CoV-2. Finally, in vivo assessment was considered. Post-mortem lung-tissue samples were extracted from patients with COVID-19 and related transcriptional responses were compared with samples from the healthy individuals. Similar to previous models, modest expressions of ISGs were detected but not about IFNs. It is interesting that in all of the models, robust cytokine and inflammatory responses were noticed [21].
In the study of Yuan et al. the antiviral activity of 22 agents including host-based IFNs (IFN β-1a, IFN β-1b, pegylated IFN α-2a and IFN γ-1B) and virus targeting antivirals (remdesivir and lopinavir) were assessed. EC50 of these agents was determined according the plaque reduction assay. The most potent IFNs were IFN β-1b (EC50 = 31.2 IU/ml) and IFN β-1a (EC50 = 70.8 IU/ml). The EC50 values for remdesivir and lopinavir were determined as 1.04 and 11.6 µM respectively. The CC50 values of IFNs, remdesivir and lopinavir were >50.000 IU/ml, >100 µM and 102 µM respectively. Among IFNs, the most reductive effects on viral load belonged to IFN β-1a and IFN β-1b. However, IFN β-1b showed highest potency and selectivity index against SARS-COV-2 [22].
In a randomized clinical trial, 86 and 41 patients were recruited in the combination and control groups respectively. Patients in the combination group received IFN β-1b, lopinavir/ritonavir and ribavirin while those in the control group received only lopinavir/ritonavir. The primary outcome was defined as the time to reach a negative RT-PCR of respiratory secretions for SARS-CoV-2. The time to resolution of the symptoms was considered as one of the secondary outcomes. The median time to achieving a negative RT-PCR was significantly shorter in the combination group compared to the control group (7 vs. 12 days). Moreover, resolution of the symptoms occurred notably faster in the combination group than the control group (4 vs. 8 days) [23]. Similar with our study, IFN β-1b was started in the viral phase of COVID-19 i.e. within first 7 days of onset of the symptoms. In our study median time from onset of the symptoms to randomization was 8 days. In both studies, first dose of IFN β-1b was administered within 24 to 48 h of hospital admission. Initiation of antiviral agents as soon as possible following onset of the symptoms is critical in control of viral replication and prevention of tissue viral invasion. The efficacy of antivirals significantly decreased after establishment of the cytokines release phase in COVID-19 [24], [25]. Due to resource limitations, evaluation of viral clearance was not possible in our study. No patient died in Hung et al study, while in our study approximately 6% and 18% of patients died in the IFN and control groups respectively. Regarding comparison of the results, it should be considered that Hung et al evaluated IFN β-1b efficacy in patients with mild to moderate COVID-19 while in our only study patients with severe COVID-19 were included. Moreover, considering severity of the disease, incidence rates of the serious complications during the hospitalization course were much higher in our study.
Estebanez et al evaluated the efficacy of IFN β-1b in 256 patients with COVID-19. Of them, 106 and 150 patients were assigned to the IFN and control groups respectively. In-hospital mortality was considered as the primary outcome of study. The mortality rate was statistically significant in the control group than the IFN group (20.8% vs. 27.3%) [26]. Retrospective design and lack of matching of the groups in terms of receiving other antivirals should be considered when interpreting the results.
In a case series, characteristics and outcomes of five patients with severe COVID-19, who were treated with IFN β-1b, lopinavir/ritonavir and hydroxychloroquine, were described. The antiviral regimen applied for these patients was similar to our study. Treatment was successful in 3 patients while clinical status of 2 patients deteriorated during the treatment course. All patients received corticosteroids. Furthermore, all patients were initially admitted in another hospital and later transferred to the referral hospital [27]. Clinical outcomes of patients might had been affected during lag time of the transfer. Moreover, patients were different in terms of the clinical presentations and management strategies. So definite role of IFN β-1b in treatment of these patients cannot be assessed.
Payandemehr et al evaluated the efficacy of IFN β-1a in 20 patients with moderate to severe COVID-19 during a single-arm, open-label clinical trial. All patients received IFN β-1a along with hydroxychloroquine, lopinavir/ritonavir and oseltamivir. In this study, only 2 patients needed ICU admission and only one death occurred in the hospital. Fifteen of the discharged patients were followed for 5 days. No side effects were detected while in our study, some patients experienced common adverse effects such as injection site reactions and flu-like syndrome. It might be due to receiving concomitant antipyretics and analgesics that masked these reactions. Furthermore, main outcomes of the study were not well-defined in the method section. Duration of the follow-up was only 5 days [28].
The efficacy of IFN β-1a in patients with COVID-19 was assessed in another study. In this non-controlled prospective trial, 20 patients were enrolled. Five doses of 44 mcg of IFN β-1a were administrated subcutaneously on alternate days for these patients. The patients also received hydroxychloroquine and lopinavir/ritonavir for 5 days. The primary outcome of the study was symptom alleviation during 14-day period. Within 8 days, all patients became afebrile. The resolution of other symptoms gradually occurred [29]. The oxygenation status and types of respiratory supports were not exactly defined. In general, high flow nasal cannula was applied for most patients and three patients received noninvasive positive pressure ventilation (NIPPV). No serious adverse events were detected and none of the patients died. Rate of ICU admission and requirement for invasive mechanical ventilation were not reported in this study. Accounting these limitations, absence of control group and small sample size, the interpretation of the results should be done with caution.
In another study efficacy and safety of IFN β-1a were evaluated in patients with severe COVID-19 in an open label, randomized clinical trial. Forty-two and 39 patients were recruited to the IFN and control groups respectively. Time to clinical response based on the six ordinary category scale was primary endpoint of this study. Following two-week treatment with IFN β-1a, time to clinical response was not statistically different between the groups. On day 14, the numbers of discharged patients were significantly higher in the IFN group compared with the control group (66.7% vs. 43.6%). Early administration of IFN β-1a significantly reduced the mortality rate compared with late administration [30]. Absence of follow-up PCR and chest imaging along with the small sample size were the major limitations of the study.
Our study suffered from some limitations. Follow up chest imaging or virological assessment was not possible due to resources limitations, therefore the effect of IFN β-1b on viral clearance was not determined. Small sample size did not allow accurate estimation of survival benefit of IFN β-1b.
In conclusion, IFN β-1b was effective in shortening the time to clinical improvement without serious adverse events in patients with severe COVID-19. Furthermore, ICU admission rate and need for invasive mechanical ventilation significantly reduced by administration of IFN β-1b. Although compared with the control group, IFN β-1b reduced duration of hospitalization, length of ICU stay, intubation rate and 28-day mortality were not statistically different between the groups. Further randomized clinical trials with enough sample size are needed to accurately estimate survival benefit of IFN β-1b.
CRediT authorship contribution statement
Hamid Rahmani: Data Curation, Formal analysis, Investigation, Writing - original draft. Effat Davoudi-Monfared: Data Curation. Anahid Nourian: Data Curation. Hossein Khalili: Conceptualization, Methodology, Supervision, Writing - review & editing. Nooshin Hajizadeh: Project Administration. Narjes zarei Jalalabadi: Project Administration. Mohammad Reza Fazeli: Resources. Monireh Ghazaeian: Resources. Mir Saeed Yekaninejad: Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would like to thank the nurses and other staffs of Imam Khomeini Hospital Complex for their kind supports and also Ms. Ava Khalili for English proofreading the manuscript.
Funding
The authors did not receive any fund for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106903.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents. 2020;55(6):105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins Coronavirus Resource Center Home Page. July 5, 2020 (https://coronavirus.jhu.edu/map.html).
- 4.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 5.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., de Castilla D.L., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.S. Lu, Q. Zhou, L. Huang, Q. Shi, S. Zhao, Z. Wang, W. Li, Y. Tang, Y. Ma, X. Luo, T. Fukuoka, H.S. Ahn, M.S. Lee, Z. Luo, E. Liu, Y. Chen, C. Zhou, D. Peng, Effectiveness and safety of glucocorticoids to treat COVID-19: a rapid review and meta-analysis, MedRxiv. doi: 10.1101/2020.04.17.20064469. [DOI] [PMC free article] [PubMed]
- 9.Z. Shao, Z. Feng, L. Zhong, Q. Xie, M. Lei, Z. Liu, C. Wang, J. Ji, H. Liu, Z. Gu, Z. Hu, L. Su, M. Wu, Z. Liu, Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: A multicenter retrospective cohort study, MedRxiv. doi: 10.1101/2020.04.11.20061739. [DOI] [PMC free article] [PubMed]
- 10.Spiegel M., Pichlmair A., Mühlberger E., Haller O., Weber F. The antiviral effect of interferon-beta against SARS-coronavirus is not mediated by MxA protein. J. Clin. Virol. 2004;30(3):211–213. doi: 10.1016/j.jcv.2003.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sainz B., Jr, Mossel E.C., Peters C.J., Garry R.F. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) Virology. 2004;329(1):11–17. doi: 10.1016/j.virol.2004.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensley L.E., Fritz L.E., Jahrling P.B., Karp C.L., Huggins J.W., Geisbert T.W. Interferon-beta 1a and SARS coronavirus replication. Emerg. Infect. Dis. 2004;10(2):317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart B.J., Dyall J., Postnikova E., Zhou H., Kindrachuk J., Johnson R.F., Olinger G.G., Frieman M.B., Holbrook M.R., Jahrling P.B., Hensley L. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virol. 2014;95(Pt 3):571–577. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shalhoub S., Farahat F., Al-Jiffri A., Simhairi R., Shamma O., Siddiqi N., Mushtaq A. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J. Antimicrob. Chemother. 2015;70:2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Ghamdi M., Alghamdi K.M., Ghandoora Y., Alzahrani A., Salah F., Alsulami A., Bawayan M.F., Vaidya D., Perl T.M., Sood G. Treatment outcomes for patients with Middle Eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the kingdom of Saudi Arabia. BMC Infect. Dis. 2016;16:174. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S., Kojan S., Al Jeraisy M., Deeb A.M., Assiri A.M., Al-Hameed F., AlSaedi A., Mandourah Y., Almekhlafi G.A., Sherbeeni N.M., Elzein F.E., Memon J., Taha Y., Almotairi A., Maghrabi K.A., Qushmaq I., Al Bshabshe A., Kharaba A., Shalhoub S., Jose J., Fowler R.A., Hayden F.G., Hussein M.A., And the MIRACLE trial group Treatment of Middle East Respiratory Syndrome with a combination of lopinavirritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng F., Tu L., Yang Y., Hu P., Wang R., Hu Q., Cao F., Jiang T., Sun J., Xu G., Chang C. Management and treatment of COVID-19: the Chinese experience. Can. J. Cardiol. 2020 Jun;36(6):915–930. doi: 10.1016/j.cjca.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson R.L., Vock D.M., Powers J.H., Emery S., Cruz E.F., Hunsberger S., Jain M.K., Pett S., Neaton J.D. Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials. 2017;14(3):264–276. doi: 10.1177/1740774517697919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Gou L., Yang J., Wang C., Jiang S., Yang D., Zhang G., Li H., Chen F., Xu Y., Chen M., Gao Z., Yang J., Dong J., Liu B., Zhang X., Wang W., He K., Jin Q., Li M., Wang J. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan S., Chan C.C., Chik K.K., Tsang J.O., Liang R., Cao J., Tang K., Cai J.P., Ye Z.W., Yin F., To K.K., Chu H., Jin D.Y., Hung I.F., Yuen K.Y., Chan J.F. Broad-spectrum host-based antivirals targeting the interferon and lipogenesis pathways as potential treatment options for the pandemic coronavirus disease 2019 (COVID-19) Viruses. 2020;12(6):E628. doi: 10.3390/v12060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W.H., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K.L., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C.Y., Zhang R.R., Fung A.Y.F., Yan E.Y.W., Leung K.H., Ip J.D., Chu A.W.H., Chan W.M., Ng A.C.K., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W.M., Yan W.W., Chan W.M., Chan J.F.W., Lie A.K.W., Tsang O.T.Y., Cheng V.C.C., Que T.L., Lau C.S., Chan K.H., To K.K.W., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao W., Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi: 10.1038/s41422-020-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estebanez M., Ramirez-Olivencia G., Mata T., Marti D., Gutierrez C., Dios B.D., Herrero M.D., Roel A., Martinez Y., Herrero A., Nicolas F.A., Gonzalez P.F., Lopez E., Ballester L.E., Mateo-Maestre M., Campos S., Sanchez-Carrillo M.J., Fe A. Membrillo de Novales FJ. Clinical evaluation of IFN beta1b in COVID-19 pneumonia: a retrospective study. MedRxiv. 2020 doi: 10.1101/2020.05.15.20084293. [DOI] [Google Scholar]
- 27.Hong S.I., Ryu B.H., Chong Y.P., Lee S., Kim S., Kim H.C., Hong K.W., Bae I.G., Cho O.H. Five severe COVID-19 pneumonia patients treated with triple combination therapy with lopinavir/ritonavir, hydroxychloroquine, and interferon β-1b. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.106052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payandemehr P., Azhdarzadeh M., Bahrami-Motlagh H., Hadadi A., Najmeddin F., Shahmirzaei S., Pazoki M., Sotoodehnia M., Rahimian R. Interferon beta-1a as a candidate for COVID-19 treatment; an open-label single-arm clinical trial. Adv. J. Emerg. Med. 2020;4(2s) e. [Google Scholar]
- 29.Dastan F., Nadji S.A., Saffaei A., Marjani M., Moniri A., Jamaati H., Hashemian S.M., Baghaei P., Abedini A., Varahram M., Yousefian S., Tabarsi P. Subcutaneous administration of interferon beta-1a for COVID-19: a non-controlled prospective trial. Int. Immunopharmacol. 2020 doi: 10.1016/j.intimp.2020.106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davoudi-Monfared E., Rahmani H., Khalil H., Hajiabdolbaghi M., Salehi M.R., Abbasian L., Kazemzade H., Yekaninejad M.S. Efficacy and safety of interferon beta-1a in treatment of severe COVID-19: a randomized clinical trial. Antimicrob. Agents Chemother. 2020 doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.