Abstract
Adoptive cell therapy (ACT) is an approach to cancer treatment that involves the use of antitumor immune cells to target residual disease in patients after completion of chemo/radiotherapy. ACT has several advantages compared with other approaches in cancer immunotherapy, including the ability to specifically expand effector cells in vitro before selection for adoptive transfer, as well as the opportunity for host manipulation in order to enhance the ability of transferred cells to recognize and kill established tumors. One of the main challenges to the success of ACT in cancer clinical trials is the identification and generation of antitumor effector cells with high avidity for tumor recognition. Natural killer (NK) cells, cytokine‐induced killers and natural killer T cells are key innate or innate‐like effector cells in cancer immunosurveillance that act at the interface between innate and adaptive immunity, to have a greater influence over immune responses to cancer. In this review, we discuss recent studies that highlight their potential in cancer therapy and summarize clinical trials using these effector immune cells in adoptive cellular therapy for the treatment of cancer.
Keywords: adoptive cell therapy, cancer, cytokine‐induced killer cells, immunotherapy, natural killer cells, natural killer T cells
1. INTRODUCTION
Adoptive cell therapy (ACT) of cancer relies on the identification and generation of antitumor immune cells with high avidity for tumor recognition before infusion into patients. It was first described in 1988, with the use of tumor infiltrating lymphocytes (TILs) and interleukin (IL)‐2 and achieved cancer regression in some patients with metastatic melanoma. 1 Since then, the transfer of immune cells with antitumor activity whether unmodified or following in vitro stimulation, expansion or genetic engineering has shown dramatic regressions in a variety of hematological and solid cancers. ACT has the advantage that large numbers of effector cells can be grown and activated in vitro before selection for specific antitumor functions. A critical improvement in the efficacy of ACT‐based cancer immunotherapy came in 2002 with the introduction of a lymphodepletion preparative regimen of chemotherapy and/or radiation prior to adoptive transfer, which enhances the ability of transferred cells to recognize and kill established tumors through elimination of host inhibitory factors and clonal repopulation of antitumor cells. 2 , 3 Much of the clinical focus has centered on adaptive T cells, with exciting reports from clinical trials on chimeric antigen receptor (CAR)‐engineered T cells achieving impressive remission rates in patients with hematological malignancies, but there have also been some setbacks including patient experience of adverse side effects from infusion‐related toxicity and cytokine release syndrome (CRS). 4 , 5 There is now a growing body of evidence suggesting that innate immune cells share many of the attributes of adaptive immunity including antigen specificity, clonal expansion, and even memory. 6 Recent clinical studies have revived an interest in innate and innate‐like effector cells as strong candidates for different immunotherapeutic strategies in cancer with a potentially safer therapeutic profile. Here, we focus on three key effector cells; natural killer (NK) cells, cytokine‐induced killers (CIKs), and natural killer T cells (NKT), which exhibit direct antitumor activity by linking innate and adaptive immune responses. We summarize different approaches using these innate‐immune effector cells in ACT‐based immunotherapy of cancer, emphasizing those that have been clinically tested during this past decade.
2. NK CELLS
NK cells are the major cytotoxic effector cells of the innate immune system, known for their natural ability to lyse tumor cells in vitro without prior sensitization. They constitute 5% to 15% of circulating lymphocytes and belong to the recently identified group 1 innate lymphoid cells. 7 Their antitumor effector functions of cytotoxicity and cytokine secretion are determined by the balance of signals from both inhibitory and activating receptors expressed on their cell surface (Figure 1A ). Inhibitory receptors for major histocompatibility complex (MHC) class I molecules include killer immunoglobulin‐like receptors (KIRs), which bind human leukocyte antigen (HLA)‐A, B, and C, and the CD94‐NKG2A heterodimer, which recognizes HLA‐E. Downregulation of MHC Class I expression by tumor cells to escape T‐cell immunity may lower the threshold to trigger NK cell cytotoxicity through “missing‐self” recognition. 8 Non‐MHC‐binding NK cell inhibitory receptors include carcinoembryonic‐antigen‐related‐cell‐adhesion molecule 1 (CEACAM1), NK‐cell receptor protein 1 (NKRP1) family members, sialic‐acid‐binding immunoglobulin‐like lectins (SIGLECs), and T‐cell immunoglobulin and immunoreceptor tyrosine‐based inhibitory motif domain (TIGIT). 9 , 10 NK cell function also depends on the presence of activation signals through NK cell activation receptors including the low‐affinity activating receptor FcγRIIIa (CD16) that binds the Fc portion of immunoglobulin G1 (IgG1) and mediates antibody‐dependent cellular cytotoxicity, NKG2D, which binds MHC class I chain‐related proteins A and B (MICA/B), UL16 binding proteins (ULBPs) and DNAM‐1, which binds poliovirus receptor (PVR/CD155) and Nectin‐2 (CD112). 11 Many other activation receptors have been characterized, including natural cytotoxicity receptors (NCRs) like NKp30, NKp46, NKp80, 2B4, CD2, lymphocyte function‐associated antigen (LFA‐1), and NK‐T‐B antigen (NTB‐A), to play important costimulatory roles in NK cell recognition of tumors. 12 The collective engagement of several NK cell activation receptors such as the combination of LFA‐1, NKG2D and 2B4 has been shown to define a minimal requirement for the induction of NK cell natural cytotoxicity against tumor targets (Figure 1A ). 13 The use of NK cells in adoptive therapy is mainly hampered by the limited ability to generate large numbers of cells ex vivo as well as their short life span in vivo. Several protocols have been developed to overcome these challenges and expand large numbers of NK cells with strong antitumor functions, which are discussed elsewhere. 14
FIGURE 1.
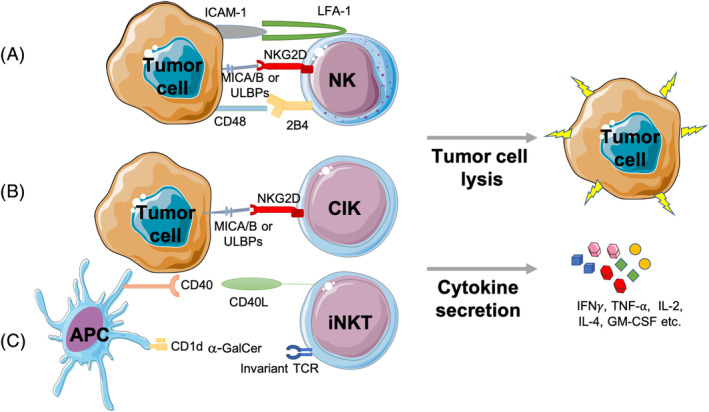
Tumor recognition by natural killer cells, cytokine‐induced killer cells, and invariant natural killer T cells. A, NK cell antitumor activity is determined by the balance of signals from inhibitory and activating receptors expressed at the cell surface to trigger cytokine secretion or direct tumor cell lysis, with the collective engagement of several activation receptors such as the combination of LFA‐1, NKG2D, and 2B4 on NK cells inducing NK cell natural cytotoxicity against tumor targets. B, CIK cell cytokine secretion and tumor lysis are mainly mediated through NKG2D‐signaling and the engagement of MHC‐related ligands MICA/B and the ULBP family of ligands. C, iNKT cells express an invariant T‐cell receptor that is specifically activated by α‐GalCer loaded on CD1d molecules expressed by antigen‐presenting cells to exert direct cytotoxicity against tumor targets or secrete large amounts of cytokines like IFN‐ γ or IL‐4. α‐GalCer; alpha‐galactosylceramide, APC; antigen‐presenting cell, GM‐CSF; granulocyte‐macrophage colony‐stimulating factor, ICAM‐1; intracellular adhesion molecule 1, IFN‐ γ; interferon‐gamma, IL‐2; interleukin‐2, LFA‐1; lymphocyte function‐associated antigen 1 MICA/B; MHC class I chain‐related protein A and B, NK; natural killer, iNKT; invariant natural killer T cell, TCR; T‐cell receptor, TNF‐α; tumor necrosis factor‐alpha, ULBP; UL16 binding protein
The transfer of autologous NK cells for the treatment of different hematological malignancies and solid tumors has shown to be remarkably safe resulting in better engraftment and persistence of cells as well as a low risk of GvHD, but with limited clinical benefit. 15 , 16 , 17 A positive clinical outcome associated with large numbers of circulating antitumor NK cells was observed in hematological cancer patients in the early days following autologous hematopoietic stem cell transplantation (HSCT). 18 Conversely, the transfer of expanded autologous NK cells has not shown positive clinical responses in metastatic melanoma, renal‐cell carcinoma, or advanced gastrointestinal cancer. 19 , 20 In many cases, circulating autologous NK cells demonstrate weak antitumor cytotoxicity without further in vitro or in vivo stimulation. Thus, recent and ongoing clinical trials focus on immunotherapeutic strategies that activate autologous NK cells with cytokines such as IL‐2/−15, or target cells like K562‐mb15‐41BBL, to reach their full antitumor potential, in a combination setting with chemotherapy, cytotoxic T cells, or monoclonal antibodies (mAbs) (Table 1). In a recent phase I clinical trial, the transfer of activated and expanded NK cells (NKAE) co‐cultured ex vivo with K562‐mb15‐41BBL before infusion into five patients with relapsed or refractory MM, showed disease stabilization in four patients and a long‐term response (>1 year) in two patients, suggesting that ex vivo expansion of autologous NK cells can be tolerated in cancer patients to result in a clinical response. 21
TABLE 1.
Summary of NK‐, CIK‐, and iNKT‐cell‐based clinical trials for adoptive cellular therapy of cancer completed on or after 1 January 2010
| Approach/treatment | Disease | Patients (total) | Trial identifier |
|---|---|---|---|
| NK | |||
| Autologous NK cell transfer (+/− chemotherapy, IL‐2/−15, K562‐mb15‐41BBL, CTLs, and/or monoclonal antibodies) | Pediatric cancers, neuroblastoma, sarcoma, brain and solid tumors, HCC, MM, prostate cancer, glioblastoma, and B‐cell lymphoma | 139 | NCT01875601, NCT0114738, NCT01884688, NCT02481934, NCT01313897, NCT00891345, NCT01422850, NCT01588769, NCT02843061 |
| Allogeneic NK cell transfer (+/− chemotherapy, HSCT, IL‐2, and/or cryosurgery) | Advanced biliary tract cancer, liver metastases of colorectal or pancreatic cancers, lymphomas, leukemias, ovarian cancer, fallopian tube carcinoma and primary peritoneal cancer, breast cancer, NSCLC, neuroblastoma, and HCC | 207 | NCT03358849, NCT02845999, NCT01212341, NCT01853358, NCT01287104, NCT01105650, NCT01181258, NCT00303667, NCT00383994, NCT02843815, NCT02008929 |
| Haploidentical NK cell infusions (+/− chemotherapy, HSCT, IL‐2/IL‐15, 41BBL, CNDO‐109, genetic modification, KIR‐ligand mismatch, and/or monoclonal antibodies) | NSCLC, neuroblastoma, lymphoma, leukemia, MDS, myeloma, ovarian cancer, fallopian tube carcinoma, primary peritoneal cancer, MM, neuroblastoma, rhabdomyosarcoma, and melanoma | 526 | NCT03366064, NCT02130869, NCT00640796, NCT00697671, NCT00660166, NCT01947322, NCT02118285, NCT01385423, NCT01795378, NCT00995137, NCT00089453, NCT00823524, NCT00187096, NCT02074657, NCT02395822, NCT00877110, NCT01576692, NCT01386619, NCT00846833, NCT00526292, NCT01593670, NCT00402558, NCT01390402, NCT01520558 |
| NK cell line NK‐92 (+/− CAR) | Leukemia, lymphoma, MM, Hodgkin's disease, and solid tumors | 21 | NCT00990717, NCT00900809, NCT03027128 |
| Unspecified NK cell transfer + cryosurgery | Advanced kidney cancer, advanced breast cancer, liver cancer, and esophageal cancer | 200 | NCT02843607, NCT02844335, NCT02849015, NCT02843802, NCT02843581 |
| CIK | |||
| Autologous CIK cell transfer (+/− surgery, stem cell transplant or RFA) | HCC, hematological malignancies, CRCLM | 499 | NCT00769106, NCT01749865, NCT00477035, NCT00394381, NCT02419677 |
| Autologous DC‐CIK (+/− surgery, chemotherapy, or γδ T cells) | Pancreatic, liver, colorectal, prostatic, renal, lung, gastric, and breast cancer | 799 | NCT02406846, NCT02416635, NCT02450422, NCT02450435, NCT02450448, NCT02412384, NCT02450357, NCT02425735, NCT02418481, NCT02425748, NCT01781520, NCT01783951, NCT01232062, NCT01395056 |
| CIK cell agent following curative resection (PEIT, RFA, or surgery) | HCC | 230 | NCT00699816 |
| Allogeneic CIK cell transfer following allogeneic stem cell transplantation | MDS, MPD, and hematologic malignancies | 142 | NCT01392989, NCT01186809, NCT00460694 |
| iNKT | |||
| Autologous iNKT cells | Malignant melanoma | 9 | NCT00631072 |
Abbreviations: CIK; cytokine‐induced killer, CRCLM; colorectal cancer liver metastases, CTL; cytotoxic T lymphocyte, HCC; hepatocellular carcinoma, HSCT; hematopoietic stem cell transplantation, iNKT; invariant natural killer, IL; interleukin, KIR; killer cell immunoglobulin‐like receptors, MDS; myelodysplastic syndromes, MM; multiple myeloma, MPD; myeloproliferative disorders, NK; natural killer, NSCLC; non‐small cell lung cancer.
An earlier study by Ruggeri et al provided the first evidence that allogeneic NK cells can be more potent effectors against tumor targets, especially in the case of KIR‐ligand incompatibility, in acute myeloid leukemia (AML) patients following allogeneic hematopoietic transplants from HLA‐mismatched donors. 22 KIR‐ligand incompatibility generates alloreactive NK cells that eliminate tumor cells through the absence of appropriate inhibitory KIRs on donor‐derived NK cells to engage their respective MHC class I ligands on patient tumor cells. In an allogeneic setting, NK cells are thought to mediate antitumor responses following adoptive transfer with limited induction of graft‐vs‐host disease (GvHD). 23 Infusion of allogeneic NK cells has not been associated with GvHD in the majority of trials and NK cells have been shown to prevent GvHD through the elimination of T cells and dendritic cells. 24 , 25 , 26 However, conflicting reports have shown that NK cell expansion and activation methods can lead to the induction of GvHD through T‐cell activation and the secretion of pro‐inflammatory cytokines. 27 , 28 , 29 The transfer of IL‐15/4‐1BBL activated NK cells following allogeneic HSCT has also been associated with incidences of acute GvHD. 30 Allogeneic NK cell transfer with IL‐2 infusions has shown some promising results in advanced cancer patients, 31 , 32 and numerous studies have reported similar results using allogeneic NK cells following HSCT. 33 , 34 , 35 , 36 , 37 , 38 , 39 A recent phase I clinical trial using allogeneic NK cells (MG4101) expanded ex vivo using IL‐2 and an mAb against CD3 to treat 17 patients with malignant lymphoma or advanced recurrent solid tumors, reported no significant adverse events, and 8 patients showing stable disease and a reduction in regulatory T cells (Tregs) and myeloid‐derived suppressive cells (MDSCs). 40 The combination of cryosurgery with infusions of KIR‐mismatched allogeneic NK cells, activated and expanded ex vivo using irradiated K562‐mb15‐41BBL cells and other cultural additives showed enhanced immune function, improved quality of life, a higher response rate (RR) and disease control rate in a phase I clinical trial for the treatment of 60 patients with advanced non‐small cell lung cancer (NSCLC). 41 In another recent study by Adotevi et al, allogeneic NK cells combined with high‐dose IL‐2 and cetuximab were used to treat liver metastasis of colorectal or pancreatic cancer, and clinical responses were observed in three of nine patients who were infused with cell products having one or two KIR ligand mismatches. 42 Similar to previous studies, IL‐2 administration resulted in the expansion of FoxP3+ Treg cells and PD‐1+ T cells.
Early clinical trials investigating the transfer of allogeneic NK cells from haploidentical donors combined with chemotherapy and IL‐2 infusions reported complete remissions in AML patients with poor prognosis. 33 More recently, haploidentical NK cells used in combination with 3F8 mAb in a phase I study for the treatment of resistant high‐risk neuroblastoma, showed complete remission in 3 out of 20 patients without GvHD or myeloablation reported. 43 Although haploidentical transplants are associated with high rates of GvHD, in a recent phase I study using IL‐2‐activated haploidentical NK cells for the treatment of high‐risk AML, myelodysplastic syndromes (MDS) or chronic myeloid leukemia (CML) patients enhanced the outcome of the hematopoietic transplantation without inducing GvHD, but patients receiving KIR‐mismatched NK cells did not show higher survival rates. 44 In another phase II study investigating the efficacy of haploidentical NK cell and IL‐2 infusions following allogeneic HCT in eight MDS/AML patients, no incidence of GvHD was observed, two patients showed a complete response but relapsed by 1.8 months, and one patient showed stable disease for 65 months. 45 In two studies using infusions of NKAE cells from haploidentical donors with low‐dose IL‐2 for the treatment of relapse or refractory acute leukemia after rescue chemotherapy, complete remission was achieved in 13 out of 18 patients, without GvHD or other serious adverse events. 46 In two phase I/II trials for the treatment of advanced AML, 42 patients received intravenous (IV) or subcutaneous (SC) recombinant human IL‐15 (rhIL‐15) infusions following chemotherapy and haploidentical NK cell transfer. 47 Although 32% and 40% of patients achieved complete remission after IV and SC dosing, respectively, cytokine release syndrome was associated with SC but not IV dosing. Two phase I studies using NK cells generated from related HLA haploidentical donors, activated using a tumor cell lysate in the absence of cytokine stimulation, were also shown to be well tolerated with no dose‐limiting toxicities in AML patients, with the infused NK cells surviving and expanding in vivo and diapedesing into the bone marrow to exert antileukemia effects. 48 , 49 Contrasting results from different studies may be explained by variations in T‐cell depletion, which can have a great influence on GvHD risk, or the nature of the transferred NK cells and the method of their activation. 50
An alternative approach is the use of NK cell lines, induced‐pluripotent stem cells (iPSCs), human embryonic stem cells (hESCs), or umbilical cord blood (CB)‐derived NK cells for adoptive transfer. 51 The NK‐92 cell line, which lacks inhibitory receptor expression, shows high cytotoxicity against a variety of tumor targets, is easy to transfect and can be expanded under good manufacturing practice conditions using recombinant IL‐2, has been investigated in numerous clinical studies, some of which have been completed in the last 10 years (Table 1). The first clinical trial using NK92 cell reported initial signs of antitumor activity, 52 and this was followed by other clinical studies reporting that unmodified NK‐92 cells can be safely infused into patients with advanced treatment‐resistant malignancies to show encouraging antitumor responses in patients with lung cancer. 53 A recent phase I clinical trial in AML investigated the safety of activated NK‐92 (aNK) cells and reported no grade 3 to 4 toxicities related to cell infusion and some signs of clinical activity, with two out of seven patients showing stable disease and one patient showing a reduction in blast percentage from 70% to 48%. 54 In another recent phase I study, irradiated NK‐92 cells were used to treat 12 lymphoma or MM patients, and the cell infusions were also well tolerated, with 2 patients achieving complete response, 2 showing minor responses, and 1 reporting clinical improvement. 55 Boyiadzis et al recently reported that the adoptive transfer of NK92 cells for the treatment of refractory/relapsed AML patients resulted in three out of six patients showing transient clinical responses. 56 To explore other sources for adoptive NK cell transfer, the first clinical trial exploring the safety and efficacy of NK cells derived from iPSCs (FT500 NK cells) in combination with immune checkpoint inhibitors for the treatment of 64 patients with advanced solid tumors including lymphoma, melanoma, gastric, colorectal, lung, cervical, and breast cancer is currently ongoing and open for recruitment (NCT03841110). Similarly, a first‐in‐human study investigating the use of umbilical CB‐derived NK cells for the treatment of 12 MM patients undergoing high‐dose chemotherapy and autologous HSCT has been reported, with no infusional toxicities or GvHD observed and 8 patients achieving near complete responses. 57 Although the widespread use of these alternative approaches is currently hampered by challenges in procurement and sourcing, encouraging results from ongoing trials, especially from NK cell lines used in adoptive transfer, is likely to result in a noticeable increase in their clinical development over the next decade.
3. CIK CELLS
CIK cells are a heterogeneous population of CD3+CD56−, CD3−CD56+, and CD3+CD56+ cells, which are expanded in vitro from peripheral blood mononuclear cells to demonstrate cytolytic activity against a wide range of tumor targets in a non‐MHC‐restricted manner. 58 The main antitumor effector cell population comprises mostly CD3+CD56+ cells, which share characteristics with NK cells and T lymphocytes. CIK cell lysis of tumors is mediated through NKG2D‐signaling and the engagement of MHC‐related ligands MICA/B and the ULBP family of ligands, the expression of which is frequently upregulated in hematologic or solid cancers (Figure 1B). 59 , 60 , 61 Several protocols for the generation of CIK cells have been reported, and typically involve the sequential incubation of peripheral blood lymphocytes with interferon (IFN)‐γ, anti‐CD3 mAb, and IL‐2. 62 The use of IL‐15 in the presence or absence of IL‐2 has also been shown to induce faster expansion of CIK cells to exhibit enhanced cytotoxicity, with the additional advantage of inducing fewer numbers of suppressive Treg cells. 63 , 64 CIK cells exhibit stronger cytolytic activity compared to lymphokine‐activated killer (LAK) cells, which are generated by incubation with interleukins. Enhanced function of CIK cells relative to LAK cells has been attributed to a higher proliferative capacity and an increase in lytic units. 64 , 65
The first phase I trial using CIK cells was conducted in the early 1990s by Schmidt‐Wolf and colleagues and demonstrated high cytotoxicity in patients with metastatic renal cancer, colorectal cancer, and lymphoma. 66 At the time of writing this manuscript, a total of 117 clinical trials using CIK cells for the treatment of cancer were registered on ClinicalTrials.gov. The majority of clinical trials completed within the last 10 years used autologous CIK cells in a combination setting, with the highest number of patients enrolled in autologous, combinatorial dendritic cell (DC)‐CIK therapy trials (Table 1). DCs are important APCs that capture and process tumor antigens, express co‐stimulatory molecules, and secrete cytokines to activate adaptive and innate immune responses, making them good candidates for cancer immunotherapy. In a recent study by Jiang et al, DC‐CIK infusions combined with chemotherapy resulted in favorable overall survival (OS) (212 vs 141 days) and progression‐free survival (PFS) (136 vs 92 days) relative to chemotherapy alone, without reported grade 3 or 4 toxicities in a total of 47 patients with clinical advanced pancreatic cancer. 67 Similarly, clinical trials for the treatment of MM and advanced gastric cancer using DC‐CIK combined with chemotherapy also provided favorable survival rates, without serious adverse events reported. 68 , 69 Although DC‐CIK infusions combined with chemotherapy for the treatment of 23 patients with negative metastatic breast cancer resulted in enhanced survival rates, serious adverse events including neutropenia and anemia were commonly observed. 70
The use of autologous and allogeneic CIK cells in combination with curative therapy has thus far yielded mixed responses. For the treatment of 60 patients with colorectal liver metastases, autologous CIK cell transfer 1 week following RFA showed higher median PFS (23 vs 18.5 months), 3‐year progression‐free rates (20.3% vs 13.3%), and 3‐year survival rates (81.0% vs 64.6%) relative to the control group not receiving CIK cell transfer. 71 In a phase 3 clinical trial for the treatment of hepatocellular carcinoma (HCC), autologous CIK cell transfer after liver resection did not improve disease‐free survival (DFS) or OS, but prolonged the median time in therapeutic range (TTR) in a total of 200 patients (13.6 vs 7.8 months). 72 Another phase 3 trial treating 230 HCC patients with autologous CIK cells after RFA, surgery or percutaneous ethanol injection, demonstrated higher median RFS (44 vs 30 months), but a higher number of patients receiving CIK cell infusions experienced adverse events (62% vs 41%) relative to the control group. 73 In an allogeneic setting, a one‐time infusion of CIK cells (1 × 108/kg) administered after nonmyeloablative allogeneic transplantation for patients with myeloid neoplasms did not significantly change relapse or survival rates, compared to data from a retrospective cohort of 100 patients. 74 In another phase I study using allogeneic CIK cells to treat 11 patients relapsing from AML, ALL, MDS, or Hodgkin's disease after allogeneic HSCT, a median number of two CIK cell infusions at 12.4 × 106/kg were administered, with GvHD observed in four cases. 75 Otherwise, three patients achieved complete responses, one had stable disease, and one patient showed hematologic improvement. Similarly, in five leukemia patients relapsing after cord blood transplantation, treatment with cord blood‐derived CIK cells ex vivo expanded using the washouts of cord blood units remaining at the end of the transplant, one partial response was observed who also developed acute grade II GvHD. 76 Overall the data show potential for clinical responses achieved from adjuvant CIK cell therapy and feasibility of using cord blood CIK cells for patients who could not otherwise benefit from donor lymphocyte infusion, but not without concern for toxicity. The next decade is likely to see novel CIK‐based combination strategies in the clinic based on in vitro studies using CIK cells with the addition of other cytokines, CARs, immune checkpoint inhibitors, or antibodies. 62
4. NKT CELLS
Natural killer T (NKT) cells are a heterogeneous subset of lymphocytes that share properties of both NK cells and T lymphocytes by co‐expressing NK cell lineage markers and the T‐cell receptor (TCR). The NKT cell receptor recognizes lipid antigens in the context of antigen‐presenting nonclassical MHC class I molecule CD1d expressed on B cells, DCs, monocytes, and macrophages. There are three types of NKT cells, type‐I NKT cells, type II NKT cells, and NKT‐like cells. Type I NKT cells are also known as invariant NKT (iNKT) cells because of their highly restricted TCR repertoire. iNKT cells are the most prevalent type of NKT cells with the highest levels of antitumor activity and will thus be the focus of this review. Human iNKT cells express the invariant Vα24‐Jα18 chain coupled with Vβ11 chain and are specifically activated by the glycolipid α‐galactosylceramide (GalCer). 77 , 78 Once activated, iNKT cells can exert direct cytotoxicity against CD1d‐expressing tumors or indirectly initiate antitumor responses by secreting large amounts of cytokines like IFN‐γ, tumor necrosis factor (TNF)‐α, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), IL‐2, −6, −10, −17, and −21 to affect a broad spectrum of innate and adaptive immune cells, including macrophages, dendritic cells, NK cells, B cells, and T cells (Figure 1C ). 79 Activated iNKT cells can also induce DC maturation through direct engagement of CD40 on DCs to its ligand CD40L on iNKT cells. In turn, mature DCs secrete IL‐12 and upregulate their expression of costimulatory molecules such as CD40, CD70, CD80, CD86, and NKG2D ligands to enhance antitumor immune responses. 80 Studies have reported iNKT dysfunction in cancer as evidenced by decreased iNKT cell proliferation and lower levels of IFN‐γ secretion. 81 , 82 , 83 A reduction in circulating iNKT cells has also been reported in a wide range of cancers, including MDS, MM, and prostate cancer, and high numbers of circulating iNKT cells is associated with better clinical outcome in advanced cancer patients. 84 , 85 , 86 , 87 This has led to different therapeutic strategies aiming to reconstitute or activate the deficient iNKT cell population in clinical trials for the treatment of cancer.
At the time of writing this manuscript, seven clinical trials using iNKT cells in cancer were registered on ClinicalTrials.gov, with only one completed in the last 10 years (Table 1). Early clinical trials administering synthetic α‐GalCer (KRN‐7000) to activate iNKT cells in patients with solid tumors showed that it can be tolerated over a wide range of doses (50‐4800 μg/m2) to increase serum cytokine levels of TNF‐α and GM‐CSF and transiently enhance NK cell numbers and cytotoxicity. 88 Another approach that has been the focus of clinical trials using iNKT cells in the treatment of cancer is the use of α‐GalCer‐pulsed APCs to activate innate and adaptive immune responses. In a phase I clinical trial, Nieda et al reported enhanced NK and T‐cell function as well as increased serum levels of IL‐12 and IFN‐γ using α‐GalCer‐pulsed DCs. 89 Chang et al also showed a 100‐fold expansion of iNKT cells and increased levels of IL‐12p40 and IP‐10 in advanced cancer patient sera following injection with α‐GalCer‐pulsed DCs. 90 Expanded iNKT cells could still be detected 6 months following vaccination, and memory cytomegalovirus‐specific CD8+ T cells were also expanded in patients treated with α‐GalCer‐pulsed DCs, but not unpulsed DCs. The use of α‐GalCer‐pulsed APCs in patients with lung cancer resulted in better infiltration and activation of iNKT cells in the tumor microenvironment. 91 In patients with advanced non‐small lung cancer, α‐GalCer‐pulsed APCs from PBMCs cultured with GM‐CSF/IL‐2 induced an increase in the numbers of NKT and NK cells and higher levels of IFN‐γ in peripheral blood, which was significantly associated with prolonged median survival times in 10 out of 17 patients. 92 ex vivo expansion of autologous NKT cells using IL‐2 and CD3 mAb before infusion induced in vivo expansion of NKT cells and enhanced IFN‐γ production in melanoma patients. 93 The combination of NKT expansion and α‐GalCer‐pulsed APCs used in a phase II clinical trial for the treatment of head and neck squamous cell carcinoma activated iNKT cells to induce antitumor functions associated with positive clinical outcomes, with 5 of 10 patients achieving tumor regression. 94 Studies in mice have shown that iPSCs can be differentiated into NKT cells to suppress tumor growth in vivo, an approach that is yet to be tested for NKT cell‐based therapy in humans. 95
5. GENETIC MODIFICATION OF KILLER CELLS FOR ADOPTIVE CELL THERAPY
The genetic modification of killer cells can induce more sustained changes to their function for adoptive transfer. CAR‐modified NK cell development has been largely limited to the preclinical stage, with studies showing promising results for the use of CAR‐NK cells in vitro and in animal models against some hematological malignancies and solid tumors as well as potential advantages in CAR‐NK cell production compared to CAR‐T cells. 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 One clinical trial assessing anti‐CD19 CAR‐NK cells for the treatment of patients with relapsed or refractory B‐lineage ALL has been completed, awaiting results (NCT00995137). In this study, a second‐generation CAR design with 4‐1BB co‐stimulatory domain linked to the CD3 zeta chain was used, which is identical to CAR constructs used in other clinical trials currently underway (eg, NCT01974479). 106 Third‐generation CAR designs incorporating CD28 and 4‐1BB costimulatory domains linked to the CD3 zeta chain are also being tested on different targets, including CD7, CD33, and MUC1‐positive tumors (eg, NCT02742727, NCT02944162, NCT02839954). The safety and efficacy of umbilical and cord‐blood‐derived CAR‐NK cell infusions administered after chemotherapy in stem cell transplant patients with lymphoid malignancies will be tested in a clinical trial that is currently recruiting (NCT03056339).
CAR‐modified NK cells may provide several advantages compared to their T‐cell counterparts. NK cells have a shorter life span and secrete a safer cytokine profile compared to T cells, which might lower the risk and severity of adverse effects relating to autoimmunity and CRS. 107 A shorter life span might also negate the need for a suicide or off‐switch to clear the cells after infusion. Their limited time in vivo however, also raises concern about their potential efficacy. Other concerns relating to the use of CAR‐NK cells in cancer therapy include uncertainty about their ability to migrate to tumor sites, their sensitivity to cryopreservation, low transfection efficiency, and the challenge of acquiring large numbers in vitro. 108 Some of these challenges can be addressed using NK cell lines for genetic modification. The delivery of IL‐2, IL‐12, IL‐15, and stem cell factor (SCF) genes into human NK cell lines like NK‐92, results in cells with a higher capacity for proliferation, cytokine production and cytolysis against a wider range of targets compared to parental cells in the absence of exogenous cytokines in vitro and in animal models. 109 , 110 , 111 , 112 , 113 , 114 CAR‐modified NK92 cells have shown promising results in preclinical studies. 115 , 116 Anti‐CD19 CAR‐NK‐92 cells were shown to exert enhanced cytotoxicity against CD19 positive leukemic cell lines and primary leukemia cells. 117 Preclinical studies in mice on receptor tyrosine‐protein kinase ErbB2 (HER2)‐CAR‐modified NK‐92 cells showed encouraging results against solid tumors like glioblastoma. 118 , 119 , 120 Since NK‐92 cells require irradiation to render them replication incompetent prior to administration, a recent study assessed the effects of irradiation on genetically modified NK‐92 cells and demonstrated that ErbB2‐CAR NK cells tested in a mouse model for lung metastasis retain their cytolytic capability after irradiation. Similarly, NK‐92 cells do not express CD16 to mediate antibody‐dependent cellular cytotoxicity (ADCC) or IL‐2, which is necessary for their proliferation and survival. Thus, a clinical study using NK‐92 cells genetically modified to express CD16 and retain IL‐2, high‐affinity NK (haNK) cells combined with monocloncal antibody therapy is currently underway (NCT03027128). Several clinical studies using CAR‐NK92 cells are ongoing, and the next decade should yield important clinical results that will help us assess their overall potential for efficacy against cancer.
Preclinical studies investigating the antitumor activity of CAR‐CIK cells have shown promising results against different cancer targets. 121 , 122 , 123 , 124 , 125 Preclinical evaluation of allogeneic CD19 specific CAR‐CIK cells modified with a nonviral gene delivery approach showed encouraging results for the treatment of ALL, 126 which is now being tested in an ongoing phase I/II clinical trial treating ALL patients after allogeneic stem cell transplant (NCT03389035). Preclinical CAR‐NKT cell studies have also yielded promising results on the potential of these cells to be used as autologous and allogeneic off‐the‐shelf cancer treatment, but no clinical trials involving CAR‐NKT cells have been conducted to date. 127
6. CONCLUSION
ACT‐based clinical trials involving killer cells have delivered remarkable clinical outcomes in some cancer patients particularly in hematological malignancies over the last few decades, and recent reports on novel approaches using NK‐, CIK‐, and NKT‐cell‐based therapies have shown encouraging signs of clinical efficacy. The genetic modification of killer cells, particularly CAR‐NK cells has attracted much attention based on their potential ability to overcome some of the shortcomings of T‐cell‐based toxicities. Still, there remain many challenges in identifying the therapeutic conditions that harness the full antitumor potential of these effector cells in vivo and render them more optimal “off‐the‐shelf” products to a wider range of donors. The coming decade is likely to see accelerated clinical development of combination strategies and therapeutic conditions that influence the tumor microenvironment, as well as new cell sources that mitigate some of the challenges of blood‐derived killer cells. Ultimately, the results of these studies will give important insights that will hopefully lead to novel therapeutic options that prevent recurrence and improve survival and quality of life in cancer patients.
CONFLICT OF INTEREST
M.S. declared consultancy with INmuneBio Inc., a company developing NK‐related therapy. M.L. declared consultancy role and ownership interest in INmuneBio Inc.
AUTHOR CONTRIBUTIONS
M.S.: manuscript writing; M.W.L.: conception and design, manuscript writing.
Sabry M, Lowdell MW. Killers at the crossroads: The use of innate immune cells in adoptive cellular therapy of cancer. STEM CELLS Transl Med. 2020;9:974–984. 10.1002/sctm.19-0423
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor‐infiltrating lymphocytes and interleukin‐2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676‐1680. [DOI] [PubMed] [Google Scholar]
- 2. Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with anti‐tumor lymphocytes. Science. 2002;298(5594):850‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non‐myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346‐2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361‐1365. [DOI] [PubMed] [Google Scholar]
- 5. Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T‐cells forward. Nat Rev Clin Oncol. 2016;13(6):370‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lanier LL. Shades of grey—the blurring view of innate and adaptive immunity. Nat Rev Immunol. 2013;13(2):73‐74. [DOI] [PubMed] [Google Scholar]
- 7. Spits H, Artis D, Colonna M, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145‐149. [DOI] [PubMed] [Google Scholar]
- 8. Karre K, Ljunggren HG, Piontek G, et al. Selective rejection of H‐2‐deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675‐678. [DOI] [PubMed] [Google Scholar]
- 9. Kumar V, McNerney ME. A new self: MHC‐class‐I‐independent natural‐killer‐cell self‐tolerance. Nat Rev Immunol. 2005;5(5):363‐374. [DOI] [PubMed] [Google Scholar]
- 10. He Y, Tian Z. NK cell education via nonclassical MHC and non‐MHC ligands. Cell Mol Immunol. 2017;14(4):321‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev. 2001;181:158‐169. [DOI] [PubMed] [Google Scholar]
- 12. Malmberg KJ, Carlsten M, Bjorklund A, et al. Natural killer cell‐mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20‐29. [DOI] [PubMed] [Google Scholar]
- 13. Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114(13):2657‐2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Becker PS, Suck G, Nowakowska P, et al. Selection and expansion of natural killer cells for NK cell‐based immunotherapy. Cancer Immunol Immunother. 2016;65(4):477‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burns LJ, Weisdorf DJ, DeFor TE, et al. IL‐2‐based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32(2):177‐186. [DOI] [PubMed] [Google Scholar]
- 16. Krause T, Anders J, von Renteln‐Kruse W. Treatment of pressure sores: rarely based on guidelines. Z Arztl Fortbild Qualitatssich. 2004;98(9–10):769‐774. [PubMed] [Google Scholar]
- 17. Lundqvist A, Berg M, Smith A, Childs RW. Bortezomib treatment to potentiate the anti‐tumor immunity of ex‐vivo expanded adoptively infused autologous natural killer cells. J Cancer. 2011;2:383‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rueff J, Medinger M, Heim D, Passweg J, Stern M. Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transplant. 2014;20(6):896‐899. [DOI] [PubMed] [Google Scholar]
- 19. Sakamoto N, Ishikawa T, Kokura S, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17(19):6287‐6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leivas A, Perez‐Martinez A, Blanchard MJ, et al. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti‐myeloma drugs for multiple myeloma. Onco Targets Ther. 2016;5(12):e1250051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097‐2100. [DOI] [PubMed] [Google Scholar]
- 23. Lupo KB, Matosevic S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers (Basel). 2019;11(6). 10.3390/cancers11060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olson JA, Leveson‐Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115(21):4293‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noval Rivas M, Hazzan M, Weatherly K, et al. NK cell regulation of CD4 T cell‐mediated graft‐versus‐host disease. J Immunol. 2010;184(12):6790‐6798. [DOI] [PubMed] [Google Scholar]
- 26. Asai O, Longo DL, Tian ZG, et al. Suppression of graft‐versus‐host disease and amplification of graft‐versus‐tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101(9):1835‐1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xun CQ, Thompson JS, Jennings CD, et al. The effect of human IL‐2‐activated natural killer and T cells on graft‐versus‐host disease and graft‐versus‐leukemia in SCID mice bearing human leukemic cells. Transplantation. 1995;60(8):821‐827. [PubMed] [Google Scholar]
- 28. Xun C, Brown SA, Jennings CD, et al. Acute graft‐versus‐host‐like disease induced by transplantation of human activated natural killer cells into SCID mice. Transplantation. 1993;56(2):409‐417. [DOI] [PubMed] [Google Scholar]
- 29. Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood. 2005;106(13):4370‐4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah NN, Baird K, Delbrook CP, et al. Acute GVHD in patients receiving IL‐15/4‐1BBL activated NK cells following T‐cell‐depleted stem cell transplantation. Blood. 2015;125(5):784‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller JS, Soignier Y, Panoskaltsis‐Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051‐3057. [DOI] [PubMed] [Google Scholar]
- 32. Szmania S, Lapteva N, Garg T, et al. Ex vivo‐expanded natural killer cells demonstrate robust proliferation in vivo in high‐risk relapsed multiple myeloma patients. J Immunother. 2015;38(1):24‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Curti A, Ruggeri L, D'Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand‐mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118(12):3273‐3279. [DOI] [PubMed] [Google Scholar]
- 34. Stern M, Passweg JR, Meyer‐Monard S, et al. Pre‐emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant. 2013;48(3):433‐438. [DOI] [PubMed] [Google Scholar]
- 35. Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Passweg JR, Tichelli A, Meyer‐Monard S, et al. Purified donor NK‐lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18(11):1835‐1838. [DOI] [PubMed] [Google Scholar]
- 37. Koehl U, Sorensen J, Esser R, et al. IL‐2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2004;33(3):261‐266. [DOI] [PubMed] [Google Scholar]
- 38. Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL‐2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855‐3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bachanova V, Burns LJ, McKenna DH, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59(11):1739‐1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y, Lim O, Kim TM, et al. Phase I study of random healthy donor‐derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res. 2016;4(3):215‐224. [DOI] [PubMed] [Google Scholar]
- 41. Lin M, Liang SZ, Wang XH, et al. Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non‐small cell lung cancer. Immunol Res. 2017;65(4):880‐887. [DOI] [PubMed] [Google Scholar]
- 42. Adotevi O, Godet Y, Galaine J, et al. In situ delivery of allogeneic natural killer cell (NK) combined with Cetuximab in liver metastases of gastrointestinal carcinoma: a phase I clinical trial. Onco Targets Ther. 2018;7(5):e1424673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cologne2014 . 2014. Phase I study of haploidentical natural killer (NK) cells plus monoclonal antibody 3F8 for resistant high‐risk neuroblastoma (HR‐NB). Adv Neuroblastoma Res.
- 44. Lee DA, Denman CJ, Rondon G, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a Phase I trial. Biol Blood Marrow Transplant. 2016;22(7):1290‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaffer BC, Le Luduec JB, Forlenza C, et al. Phase II study of Haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(4):705‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vela M, Corral D, Carrasco P, et al. Haploidentical IL‐15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia. Cancer Lett. 2018;422:107‐117. [DOI] [PubMed] [Google Scholar]
- 47. Cooley S, He F, Bachanova V, et al. First‐in‐human trial of rhIL‐15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019;3(13):1970‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fehniger TA, Miller JS, Stuart RK, et al. A Phase 1 trial of CNDO‐109‐activated natural killer cells in patients with high‐risk acute myeloid leukemia. Biol Blood Marrow Transplant. 2018;24(8):1581‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kottaridis PD, North J, Tsirogianni M, et al. Two‐stage priming of allogeneic natural killer cells for the treatment of patients with acute myeloid leukemia: a Phase I trial. PLoS One. 2015;10(6):e0123416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin‐like receptor. Blood. 2002;100(10):3825‐3827. [DOI] [PubMed] [Google Scholar]
- 51. Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front Immunol. 2017;8:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arai S, Meagher R, Swearingen M, et al. Infusion of the allogeneic cell line NK‐92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10(6):625‐632. [DOI] [PubMed] [Google Scholar]
- 53. Tonn T, Schwabe D, Klingemann HG, et al. Treatment of patients with advanced cancer with the natural killer cell line NK‐92. Cytotherapy. 2013;15(12):1563‐1570. [DOI] [PubMed] [Google Scholar]
- 54. Boyiadzis M, Whiteside TL. Plasma‐derived exosomes in acute myeloid leukemia for detection of minimal residual disease: are we ready? Expert Rev Mol Diagn. 2016;16(6):623‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams BA, Law AD, Routy B, et al. A phase I trial of NK‐92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget. 2017;8(51):89256‐89268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boyiadzis M, Agha M, Redner RL, et al. Phase 1 clinical trial of adoptive immunotherapy using "off‐the‐shelf" activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy. 2017;19(10):1225‐1232. [DOI] [PubMed] [Google Scholar]
- 57. Shah N, Li L, McCarty J, et al. Phase I study of cord blood‐derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177(3):457‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schmidt‐Wolf IG, Lefterova P, Mehta BA, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine‐induced killer cells. Exp Hematol. 1993;21(13):1673‐1679. [PubMed] [Google Scholar]
- 59. Pende D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I‐related chain a and UL16‐binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D‐dependent natural killer cell cytotoxicity. Cancer Res. 2002;62(21):6178‐6186. [PubMed] [Google Scholar]
- 60. Nwangwu CA, Weiher H, Schmidt‐Wolf IGH. Increase of CIK cell efficacy by upregulating cell surface MICA and inhibition of NKG2D ligand shedding in multiple myeloma. Hematol Oncol. 2017;35(4):719‐725. [DOI] [PubMed] [Google Scholar]
- 61. He JY, Jia ZX, Cai XH, et al. Roles of NKG2D in cytokine‐induced killer (CIK) against hematological malignant cells lines. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21(6):1380‐1384. [DOI] [PubMed] [Google Scholar]
- 62. Gao X, Mi Y, Guo N, et al. Cytokine‐induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. 2017;8:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rettinger E, Meyer V, Kreyenberg H, et al. Cytotoxic capacity of IL‐15‐stimulated cytokine‐induced killer cells against human acute myeloid leukemia and Rhabdomyosarcoma in humanized preclinical mouse models. Front Oncol. 2012;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bremm M, Pfeffermann LM, Cappel C, et al. Improving clinical manufacturing of IL‐15 activated cytokine‐induced killer (CIK) cells. Front Immunol. 2019;10:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schmidt‐Wolf IG, Lefterova P, Johnston V, et al. Propagation of large numbers of T cells with natural killer cell markers. Br J Haematol. 1994;87(3):453‐458. [DOI] [PubMed] [Google Scholar]
- 66. Schmidt‐Wolf IG, Finke S, Trojaneck B, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin‐2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81(6):1009‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jiang N, Qiao G, Wang X, et al. Dendritic cell/cytokine‐induced killer cell immunotherapy combined with S‐1 in patients with advanced pancreatic cancer: a prospective study. Clin Cancer Res. 2017;23(17):5066‐5073. [DOI] [PubMed] [Google Scholar]
- 68. Qiao G, Wang X, Zhou L, et al. Autologous dendritic cell‐cytokine induced killer cell immunotherapy combined with S‐1 plus Cisplatin in patients with advanced gastric cancer: a prospective study. Clin Cancer Res. 2019;25(5):1494‐1504. [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, Lv B, Li K, Zhang A, Liu H. Adjuvant immunotherapy of dendritic cells and cytokine‐induced killer cells is safe and enhances chemotherapy efficacy for multiple myeloma in China: a meta‐analysis of clinical trials. Drug des Devel Ther. 2017;11:3245‐3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang X, Ren J, Zhang J, et al. Prospective study of cyclophosphamide, thiotepa, carboplatin combined with adoptive DC‐CIK followed by metronomic cyclophosphamide therapy as salvage treatment for triple negative metastatic breast cancers patients (aged <45). Clin Transl Oncol. 2016;18(1):82‐87. [DOI] [PubMed] [Google Scholar]
- 71. Li X, Dai X, Shi L, et al. Phase II/III study of radiofrequency ablation combined with cytokine‐induced killer cells treating colorectal liver metastases. Cell Physiol Biochem. 2016;40(1–2):137‐145. [DOI] [PubMed] [Google Scholar]
- 72. Xu L, Wang J, Kim Y, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine‐induced killer cells after curative resection for hepatocellular carcinoma. Onco Targets Ther. 2016;5(3):e1083671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine‐induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383‐1391. e1386. [DOI] [PubMed] [Google Scholar]
- 74. Narayan R, Benjamin JE, Shah O, et al. Donor‐derived cytokine‐induced killer cell infusion as consolidation after nonmyeloablative allogeneic transplantation for myeloid neoplasms. Biol Blood Marrow Transplant. 2019;25(7):P1293‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Introna M, Borleri G, Conti E, et al. Repeated infusions of donor‐derived cytokine‐induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92(7):952‐959. [DOI] [PubMed] [Google Scholar]
- 76. Introna M, Pievani A, Borleri G, et al. Feasibility and safety of adoptive immunotherapy with CIK cells after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(11):1603‐1607. [DOI] [PubMed] [Google Scholar]
- 77. Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d‐dependent NKT cells. J Clin Invest. 2004;114(10):1379‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kawano T, Cui J, Koezuka Y, et al. CD1d‐restricted and TCR‐mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626‐1629. [DOI] [PubMed] [Google Scholar]
- 79. Coquet JM, Chakravarti S, Kyparissoudis K, et al. Diverse cytokine production by NKT cell subsets and identification of an IL‐17‐producing CD4‐NK1.1‐ NKT cell population. Proc Natl Acad Sci U S A. 2008;105(32):11287‐11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bae EA, Seo H, Kim IK, Jeon I, Kang CY. Roles of NKT cells in cancer immunotherapy. Arch Pharm Res. 2019;42(7):543‐548. [DOI] [PubMed] [Google Scholar]
- 81. Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN‐gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167(7):4046‐4050. [DOI] [PubMed] [Google Scholar]
- 82. Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha‐galactosylceramide. J Immunol. 2002;168(12):6494‐6499. [DOI] [PubMed] [Google Scholar]
- 83. Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, V. Dhodapkar M. Severe and selective deficiency of interferon‐gamma‐producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122(4):617‐622. [DOI] [PubMed] [Google Scholar]
- 84. Molling JW, Kolgen W, van der Vliet HJ, et al. Peripheral blood IFN‐gamma‐secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116(1):87‐93. [DOI] [PubMed] [Google Scholar]
- 85. Exley MA, Lynch L, Varghese B, Nowak M, Alatrakchi N, Balk SP. Developing understanding of the roles of CD1d‐restricted T cell subsets in cancer: reversing tumor‐induced defects. Clin Immunol. 2011;140(2):184‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schneiders FL, Scheper RJ, von Blomberg BM, et al. Clinical experience with alpha‐galactosylceramide (KRN7000) in patients with advanced cancer and chronic hepatitis B/C infection. Clin Immunol. 2011;140(2):130‐141. [DOI] [PubMed] [Google Scholar]
- 87. Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25(7):862‐868. [DOI] [PubMed] [Google Scholar]
- 88. Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T‐cell ligand alpha‐galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702‐3709. [PubMed] [Google Scholar]
- 89. Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103(2):383‐389. [DOI] [PubMed] [Google Scholar]
- 90. Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen‐specific T cells after injection of alpha‐galactosyl‐ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201(9):1503‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nagato K, Motohashi S, Ishibashi F, et al. Accumulation of activated invariant natural killer T cells in the tumor microenvironment after alpha‐galactosylceramide‐pulsed antigen presenting cells. J Clin Immunol. 2012;32(5):1071‐1081. [DOI] [PubMed] [Google Scholar]
- 92. Motohashi S, Nagato K, Kunii N, et al. A phase I‐II study of alpha‐galactosylceramide‐pulsed IL‐2/GM‐CSF‐cultured peripheral blood mononuclear cells in patients with advanced and recurrent non‐small cell lung cancer. J Immunol. 2009;182(4):2492‐2501. [DOI] [PubMed] [Google Scholar]
- 93. Exley MA, Friedlander P, Alatrakchi N, et al. Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: a Phase I clinical trial. Clin Cancer Res. 2017;23(14):3510‐3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yamasaki K, Horiguchi S, Kurosaki M, et al. Induction of NKT cell‐specific immune responses in cancer tissues after NKT cell‐targeted adoptive immunotherapy. Clin Immunol. 2011;138(3):255‐265. [DOI] [PubMed] [Google Scholar]
- 95. Watarai H, Fujii S, Yamada D, et al. Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J Clin Invest. 2010;120(7):2610‐2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL‐15 and a CD19‐targeted CAR show long‐term persistence and potent anti‐tumor activity. Leukemia. 2018;32(2):520‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oelsner S, Friede ME, Zhang C, et al. Continuously expanding CAR NK‐92 cells display selective cytotoxicity against B‐cell leukemia and lymphoma. Cytotherapy. 2017;19(2):235‐249. [DOI] [PubMed] [Google Scholar]
- 98. Murakami T, Nakazawa T, Natsume A, et al. Novel human NK cell line carrying CAR targeting EGFRvIII induces anti‐tumor effects in Glioblastoma cells. Anticancer Res. 2018;38(9):5049‐5056. [DOI] [PubMed] [Google Scholar]
- 99. Muller N, Michen S, Tietze S, et al. Engineering NK cells modified with an EGFRvIII‐specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF‐1alpha‐secreting Glioblastoma. J Immunother. 2015;38(5):197‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Esser R, Muller T, Stefes D, et al. NK cells engineered to express a GD2 ‐specific antigen receptor display built‐in ADCC‐like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16(3):569‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Muller T, Uherek C, Maki G, et al. Expression of a CD20‐specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK‐resistance of lymphoma and leukemia cells. Cancer Immunol Immunother. 2008;57(3):411‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kruschinski A, Moosmann A, Poschke I, et al. Engineering antigen‐specific primary human NK cells against HER‐2 positive carcinomas. Proc Natl Acad Sci U S A. 2008;105(45):17481‐17486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chu Y, Hochberg J, Yahr A, et al. Targeting CD20+ aggressive B‐cell non‐Hodgkin lymphoma by anti‐CD20 CAR mRNA‐modified expanded natural killer cells in vitro and in NSG mice. Cancer Immunol Res. 2015;3(4):333‐344. [DOI] [PubMed] [Google Scholar]
- 105. Altvater B, Landmeier S, Pscherer S, et al. 2B4 (CD244) signaling by recombinant antigen‐specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15(15):4857‐4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saudemont A, Jespers L, Clay T. Current status of gene engineering cell therapeutics. Front Immunol. 2018;9:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Glienke W, Esser R, Priesner C, et al. Advantages and applications of CAR‐expressing natural killer cells. Front Pharmacol. 2015;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang L, Dou M, Ma Q, Yao R, Liu J. Chimeric antigen receptor (CAR)‐modified NK cells against cancer: opportunities and challenges. Int Immunopharmacol. 2019;74:105695. [DOI] [PubMed] [Google Scholar]
- 109. Nagashima S, Mailliard R, Kashii Y, et al. Stable transduction of the interleukin‐2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood. 1998;91(10):3850‐3861. [PubMed] [Google Scholar]
- 110. Zhang J, Sun R, Wei H, Zhang J, Tian Z. Characterization of interleukin‐15 gene‐modified human natural killer cells: implications for adoptive cellular immunotherapy. Haematologica. 2004;89(3):338‐347. [PubMed] [Google Scholar]
- 111. Jiang W, Zhang J, Tian Z. Functional characterization of interleukin‐15 gene transduction into the human natural killer cell line NKL. Cytotherapy. 2008;10(3):265‐274. [DOI] [PubMed] [Google Scholar]
- 112. Tam YK, Maki G, Miyagawa B, Hennemann B, Tonn T, Klingemann HG. Characterization of genetically altered, interleukin 2‐independent natural killer cell lines suitable for adoptive cellular immunotherapy. Hum Gene Ther. 1999;10(8):1359‐1373. [DOI] [PubMed] [Google Scholar]
- 113. Konstantinidis KV, Alici E, Aints A, Christensson B, Ljunggren HG, Dilber MS. Targeting IL‐2 to the endoplasmic reticulum confines autocrine growth stimulation to NK‐92 cells. Exp Hematol. 2005;33(2):159‐164. [DOI] [PubMed] [Google Scholar]
- 114. Zhang J, Sun R, Wei H, Zhang J, Tian Z. Characterization of stem cell factor gene‐modified human natural killer cell line, NK‐92 cells: implication in NK cell‐based adoptive cellular immunotherapy. Oncol Rep. 2004;11(5):1097‐1106. [PubMed] [Google Scholar]
- 115. Kloess S, Kretschmer A, Stahl L, Fricke S, Koehl U. CAR‐expressing natural killer cells for cancer retargeting. Transfus Med Hemother. 2019;46(1):4‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Klingemann H. Are natural killer cells superior CAR drivers? Onco Targets Ther. 2014;3:e28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Romanski A, Uherek C, Bug G, et al. CD19‐CAR engineered NK‐92 cells are sufficient to overcome NK cell resistance in B‐cell malignancies. J Cell Mol Med. 2016;20(7):1287‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Han J, Chu J, Keung Chan W, et al. CAR‐engineered NK cells targeting wild‐type EGFR and EGFRvIII enhance killing of glioblastoma and patient‐derived glioblastoma stem cells. Sci Rep. 2015;5:11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhang C, Burger MC, Jennewein L, et al. ErbB2/HER2‐specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst. 2016;108(5). djv375 10.1093/jnci/djv375. [DOI] [PubMed] [Google Scholar]
- 120. Genssler S, Burger MC, Zhang C, et al. Dual targeting of glioblastoma with chimeric antigen receptor‐engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances anti‐tumor activity and survival. Onco Targets Ther. 2016;5(4):e1119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tettamanti S, Marin V, Pizzitola I, et al. Targeting of acute myeloid leukaemia by cytokine‐induced killer cells redirected with a novel CD123‐specific chimeric antigen receptor. Br J Haematol. 2013;161(3):389‐401. [DOI] [PubMed] [Google Scholar]
- 122. Schlimper C, Hombach AA, Abken H, et al. Improved activation toward primary colorectal cancer cells by antigen‐specific targeting autologous cytokine‐induced killer cells. Clin Dev Immunol. 2012;2012:238924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ren X, Ma W, Lu H, et al. Modification of cytokine‐induced killer cells with chimeric antigen receptors (CARs) enhances anti‐tumor immunity to epidermal growth factor receptor (EGFR)‐positive malignancies. Cancer Immunol Immunother. 2015;64(12):1517‐1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Merker M, Pfirrmann V, Oelsner S, et al. Generation and characterization of ErbB2‐CAR‐engineered cytokine‐induced killer cells for the treatment of high‐risk soft tissue sarcoma in children. Oncotarget. 2017;8(39):66137‐66153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hombach AA, Rappl G, Abken H. Arming cytokine‐induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28‐OX40 "super‐stimulation". Mol Ther. 2013;21(12):2268‐2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Magnani CF, Mezzanotte C, Cappuzzello C, et al. Preclinical efficacy and safety of CD19CAR cytokine‐induced killer cells transfected with sleeping beauty transposon for the treatment of acute lymphoblastic leukemia. Hum Gene Ther. 2018;29(5):602‐613. [DOI] [PubMed] [Google Scholar]
- 127. Kriegsmann K, Kriegsmann M, von Bergwelt‐Baildon M, Cremer M, Witzens‐Harig M. NKT cells—new players in CAR cell immunotherapy? Eur J Haematol. 2018;101(6):750‐757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


