Abstract
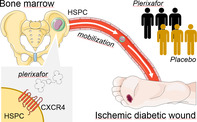
Bone marrow‐derived cells contribute to tissue repair, but traffic of hematopoietic stem/progenitor cells (HSPCs) is impaired in diabetes. We therefore tested whether HSPC mobilization with the CXCR4 antagonist plerixafor improved healing of ischemic diabetic wounds. This was a pilot, phase IIa, double‐blind, randomized, placebo‐controlled trial (NCT02790957). Patients with diabetes with ischemic wounds were randomized to receive a single subcutaneous injection of plerixafor or saline on top of standard medical and surgical therapy. The primary endpoint was complete healing at 6 months. Secondary endpoints were wound size, transcutaneous oxygen tension (TcO2), ankle‐brachial index (ABI), amputations, and HSPC mobilization. Twenty‐six patients were enrolled: 13 received plerixafor and 13 received placebo. Patients were 84.6% males, with a mean age of 69 years. HSPC mobilization was successful in all patients who received plerixafor. The trial was terminated after a preplanned interim analysis of 50% of the target population showed a significantly lower healing rate in the plerixafor vs the placebo group. In the final analysis data set, the rate of complete healing was 38.5% in the plerixafor group vs 69.2% in the placebo group (chi‐square P = .115). Wound size tended to be larger in the plerixafor group for the entire duration of observation. No significant difference was noted for the change in TcO2 and ABI or in amputation rates. No other safety concern emerged. In conclusion, successful HSPC mobilization with plerixafor did not improve healing of ischemic diabetic wounds. Contrary to what was expected, outside the context of hematological disorders, mobilization of diabetic HSPCs might exert adverse effects on wound healing.
Keywords: angiogenesis, CD34+, clinical translation, diabetes, hematopoietic stem cells, mobilization
We randomized patients with diabetic ischemic wounds to receive stem cell mobilization with the CXCR4 antagonist plerixafor or placebo. Despite the fact that plerixafor successfully mobilized hematopoietic stem cells, no significant difference was observed between the two groups in the rates of wound healing and in surrogate measures of perfusion.
Lessons learned.
Bone marrow‐derived cells contribute to tissue repair.
Traffic of hematopoietic stem/progenitor cells (HSPCs) is impaired in diabetes.
This study tested whether HSPCs mobilization with plerixafor improved the healing of ischemic diabetic wounds.
Successful HSPC mobilization with plerixafor did not improve healing of ischemic diabetic wounds.
Mobilization of diabetic HSPCs might exert adverse effects on tissue repair.
Significance statement.
The role of hematopoietic stem cells in diabetic complications is well characterized. In the setting of limb ischemia, cell therapy trials with a variety of cell products yielded variable results. There is an unmet need for therapies for the diabetic foot syndrome beyond the state of the art. This study has hypothesized that the CXCR4 antagonist plerixafor could be repurposed to treat ischemic diabetic wounds. In this phase IIa clinical trial, successful stem cell mobilization with plerixafor did not improve healing of ischemic diabetic wounds. Contrary to what was expected, mobilization of diabetic stem cells might exert adverse effects on tissue repair.
1. INTRODUCTION
Impaired healing of diabetic wounds is due to a combination of atherosclerosis obliterans, local microangiopathy, neuropathy, and infection. 1 The resulting diabetic foot syndrome is a devastating complication, driving morbidity and mortality. Despite surgical treatments and advanced medications, many patients undergo amputations, highlighting the need for additional therapies. 2
Diabetes impairs tissue repair, at least in part by altering stem cell biology. Hematopoietic stem/progenitor cells (HSPCs) are reduced in the peripheral blood (PB) of patients with diabetes. 3 Shortage of PB‐HSPCs has been attributed to impaired mobilization from the bone marrow (BM),4, 5 a condition deemed mobilopathy. 6 The diabetic BM is characterized by inflammation, neuropathy, and microvascular remodeling,7, 8 which are more profound in patients with limb ischemia.9, 10 In addition to impairing HSPC mobilization from the BM to PB, diabetes hampers the traffic of BM‐derived cells to sites of delayed wound healing. 11 Among patients with diabetes, those with lower PB‐HSPCs display a worse outcome of micro‐ and macrovascular complications.12, 13 This set of notions suggest that increasing the levels of circulating HSPCs could counter diabetic complications. 14
Granulocyte‐colony stimulating factor (G‐CSF) is most commonly used to mobilize HSPCs, and it might be useful as adjunctive therapy for diabetic wound healing. 15 However, the HSPC mobilizing capacity of G‐CSF is significantly compromised by diabetes.5, 16 On the other hand, the CXCR4 antagonist plerixafor induces rapid and effective HSPC mobilization in patients with diabetes as in nondiabetic controls. 17 Plerixafor is a small chemical approved for HSPC mobilization in myeloma and lymphoma. By blocking interaction of the chemokine CXCL12 with its receptor CXCR4, plerixafor desensitizes HSPCs to the intrinsically high levels of CXCL12 that keep them attached to the BM stroma. 18 The resulting surge of PB‐HSPCs is rapid and transient because once plerixafor is cleared, CXCR4 again senses CXCL12 levels and HSPCs return to the BM or home where CXCL12 concentrations are high. Because tissue ischemia stimulates the release of CXCL12, that guides homing of BM‐derived cells, 19 we hypothesized that HSPCs mobilized by plerixafor could reach ischemic wounds and aid healing. In diabetic mice, plerixafor was able to mobilize stem/progenitor cells and improved wound healing,20, 21 supporting a potential repurposing of this drug for the treatment of diabetic wounds. Therefore, we designed a clinical trial to test the effects of HSPC mobilization with plerixafor on healing of ischemic diabetic wounds.
2. MATERIALS AND METHODS
2.1. Study design
This was a pilot, phase IIa (repurposing), single‐center, randomized, double‐blind, placebo‐controlled trial. The study protocol is registered on ClinicalTrials.gov (NCT02790957) and complies with the CONSORT (CONsolidated Standards of Reporting Trials) statement and checklist. 22 The protocol was approved by the Ethical Committee of the University Hospital of Padova (no. 3694/Ao/15).
2.2. Participants
Patients were recruited from June 2016 to April 2019 at the University Hospital of Padova, Italy. All consecutive patients with diabetes presenting at the inpatient or outpatient clinics with signs of ischemic wound(s) were screened for eligibility. Inclusion criteria were as follows: a diagnosis of type 1 or type 2 diabetes; age 18‐85 years for men or postmenopausal status (cessation of menstrual periods for at least 12 consecutive months) and age ≤85 for women; presence of neuroischemic or ischemic diabetic wound(s) of the foot(s) with a Texas University Classification (TUC) grade 2 or 3, C or D (with ischemia and with or without infection) 23 ; and ability to provide informed consent. Ischemia was defined in the presence of hemodynamically significant stenosis of limb arteries upon ultrasound examination or angiography, with transcutaneous oxygen tension (TcO2) <50 mmHg and/or ankle‐brachial index (ABI) <0.9. 24 Exclusion criteria were as follows: ongoing sepsis; dialysis; severe chronic kidney disease (defined as an estimated glomerular filtration rate [eGFR] <20 mL/minute/1.73 m2); advanced liver disease (defined as cirrhosis or liver enzyme elevation >3 times the upper limit of normality); clinically relevant abnormalities in white blood cell counts at baseline (eg, leukopenia or thrombocytopenia); hematologic disorders (lymphoma, myeloma, acute or chronic leukemia, chronic myeloproliferative disorders); known or highly suspected solid cancer; women with childbearing potential; known hypersensitivity to plerixafor or its excipients; or inability to provide informed consent.
2.3. Clinical data collection
For all patients, we recorded the following information. Demographics and anthropometrics: age, sex, duration of diabetes, weight, height, and body mass index. Prevalence of concomitant conditions: arterial hypertension (defined as a blood pressure level of 140/90 mmHg or higher or use of blood pressure‐lowering drugs); dyslipidemia (defined as a total cholesterol level >200 mg/dL or a triglycerides level >150 mg/dL or use of lipid‐lowering drugs); smoking status (defined as habitual smoking of one or more cigarettes per day); coronary artery disease (defined as a past history of acute coronary syndrome or coronary revascularization); cerebrovascular disease (defined as a past history of cerebral ischemia or evidence of carotid artery atherosclerosis); diabetic retinopathy (defined according to the Early Treatment of Diabetic Retinopathy Study 25 ); somatic or autonomic neuropathy (defined using the Michigan neuropathy screening instrument 26 and cardiovascular autonomic tests, respectively); chronic kidney disease (defined as an eGFR of <60 mL/minute/1.73 m2). We also recorded the following laboratory test results, all performed at the same core laboratory of the University Hospital of Padova: HbA1c, complete blood cell count and lymphocyte immunophenotype (CD3, CD4, CD8, CD16, CD56), HbA1c, serum creatinine (eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration [CKD‐EPI] equation 27 ), urinary albumin/creatinine ratio, and lipid profile (total cholesterol, high‐density lipoprotein cholesterol, triglycerides); low‐density lipoproteins were calculated using the Friedewald equation. 28 We recorded all details on ongoing medications for the treatment of diabetes and of concomitant cardiovascular disease and risk factors.
2.4. Wound characterization
Each patient's wound was evaluated clinically for anatomical location, area, depth, ischemia, and infection. Wound size was quantified by taking digital photos of the wound with a caliper; area was then calculated using ImageJ 1.8 (NIH, Bethesda, Maryland). Wound depth was determined using a blunt probe and the probe‐to‐bone maneuver. Infection was suspected by clinical signs (eg, purulent exudate or smell) and confirmed with microbiological culture test. Ischemia was evaluated by TcO2 and ABI. TcO2 was quantified with TCM400 (De Mori, Milan, Italy), placing the probe closest to the wound, in an area free of edema. ABI was determined using standard automatic sphygmomanometer cuffs (Meteda Srl, San Benedetto del Tronto, Italy) placed at the level of the left arm and ankle homolateral to the wound. When more than one wound was present in the same patient, the wound with the most severe grade was chosen as the index wound.
2.5. Randomization and treatment
After signing written informed consent, patients were randomized 1:1 to treatment with plerixafor or placebo, based on a computer‐generated sequence not available to study staff. To allow for a balanced distribution of patients in the two arms at interim analysis (see below), the randomization sequence had two equally distributed blocks. Concealment was guaranteed by the closed envelope system. Once a patient was ready to be randomized, the allocation envelope was opened by the investigator and the patient was irrevocably randomized to the indicated allocation. Patients, study staff, and care providers remained blinded to the assigned treatment for the entire duration of the study. Patients' follow‐up and outcome assessment was performed by staff members who were blinded to the treatment.
At 8:00 am of the day of treatment, a blood sample was drawn for complete blood cell count and quantification of circulating stem/progenitor cells. Then, the patient received a subcutaneous injection of plerixafor 0.24 mg/kg or matching volume of 0.9% saline (placebo). The injection was prepared by personnel not involved in patient care. Plerixafor and saline injections looked identical. At 2:00 pm, a second blood sample was drawn to evaluate stem/progenitor cell mobilization. Contrary to what was done in most cell therapy trials for peripheral arterial disease, 29 we did not limit to patients who could not undergo revascularization (no‐option), because lack of blood supply was considered a limiting factor for HSPC homing to the wound. In addition to plerixafor or placebo, all patients received standard of care with medical and surgical therapy, including vasodilators, antibiotics, advanced medications, negative pressure, surgical debridement, and revascularization, as deemed appropriate. For wounds on a weight‐bearing surface, offloading was achieved with removable cast walkers, fore/rear‐foot offloading shoes, custom shoes, or non‐weight‐bearing strategies, according to individual patient characteristics and needs. If the patient was scheduled for revascularization (surgical or endovascular), study treatment had to be performed within 72 hours after revascularization, to benefit from the expectedly high CXLC12 expression in the (hitherto) ischemic tissue.
2.6. Follow‐up and outcome definitions
Study participation did not interfere with routine clinical management of the patients, who were seen at intervals dictated by wound and patient characteristics, adapted over time based on healing trends. For the purpose of the study, patients were scheduled for the first follow‐up visit 1 week after treatment and then every month after treatment until wound healing, death, or completion of the maximum 6‐month follow‐up period. An intention‐to‐treat approach was used and only the first follow‐up was obliged to retain the patient in the analysis. Wound characteristics were reassessed as each visit. When one or more visits were missing, the healing status was updated at the next available visit or the patient was censored if no further visit was available.
The primary outcome was complete wound healing, defined as a TUC grade 0 (complete re‐epithelization), independently of the residual presence of ischemia or closed infection. Secondary outcomes were the changes over time in wound size, TcO2, and ABI; the need for minor or major amputations; and the comparison of CD34+ stem cell mobilization 6 hours after plerixafor administration in patients with good outcome vs those with poor outcome. At each follow‐up visit, patients were also evaluated for the occurrence of eventual adverse events (AEs).
2.7. Stem/progenitor cell quantification
Quantification of HSPCs was performed using flow cytometry on fresh blood cells as previously described in detail. 12 Briefly, after red blood cell lysis, cells were stained with monoclonal antibodies against human CD133 (Miltenyi Biotech, Bergisch Gladbach, Germany), CD34, CD45 (Becton, Dickinson and Company, Franklin Lakes, New Jersey), and KDR (R&D Systems, Minneapolis, Minnesota). After gating mononuclear cells in the morphologic gate, cells were examined for expression of CD34. CD45 diminished staining was used to confirm HSPC identity. At least 500 000 events were acquired. The absolute HSPC count per milliliter of blood was retrieved by multiplying the frequency of CD34+ cells over 1 million events by the white blood cell count (×1000 per microliter). The same trained operator performed all analyses throughout the study.
2.8. Sample size estimation
No prior study could be used to derive a prespecified effect size. In a recent cohort of 52 patients with a median TUC stage 3 wound at our center, the rate of complete healing after a median 3‐month follow‐up was 40.4%. 30 Because all patients enrolled in the present study had ischemia, we allowed for a longer observation time, setting the primary endpoint evaluation at 6 months. We calculated that, with n = 20 patients per group, the study would have 80% power to detect a threefold higher chance of healing in plerixafor‐treated patients vs controls (60% vs 20%). 31 To account for a dropout rate <15%, we planned to recruit a maximum of 46 patients.
Plerixafor is approved for HSPC mobilization in lymphoma and myeloma and has never been used before in patients with diabetes with ischemic wounds. Thus, for safety reasons, we planned an interim analysis once the follow‐up of 50% of the target number of patients was completed (n = 23). The protocol imposed study termination in case the interim analysis showed a lower probability of achieving the primary endpoint (complete wound healing) in the plerixafor group with a P value <.10.
2.9. Statistical analysis
For descriptive purposes, continuous variables are reported as mean and SD, whereas categorical variables are reported as percentage. Normality of continuous variables were checked using the Shapiro‐Wilk test and non‐normal variables were log‐transformed before analysis. Comparisons between the two treatment groups were performed using two‐tail unpaired Student's t test for continuous variables and chi‐square test for categorical variables. The Hochberg method was used to adjust for multiplicity of testing. For the primary endpoint, wound healing rates at 6 months were compared between groups using the chi‐square test. To account for time‐dependency of the event, in a separate analysis, we used the Cox proportional hazards regression model. The between‐group comparisons in the change over time of wound size, TcO2, and ABI were performed using the mixed model for repeated measures, considering the treatment group, time, and time × group interaction. The rate of amputations was analyzed using Cox regression. HSPC mobilization was within each group using two‐tail paired Student's t test; the ratio between post‐plerixafor and baseline HSPC levels were compared between patients treated with plerixafor, according to their meeting the primary endpoint. SPSS version 23 was used. Statistical significance was accepted at P < .05.
3. RESULTS
3.1. Patient disposition and characteristics
The study flowchart is shown in Figure 1. From June 2016 to April 2019, a total of 52 patients were screened; 26 were excluded for the following reasons: mistiming of revascularization (n = 10), lack of significant ischemia (n = 7), hematological disorders (n = 4), severe renal or hepatic dysfunction (n = 2), solid cancer (n = 1), or consent denial (n = 2).
FIGURE 1.
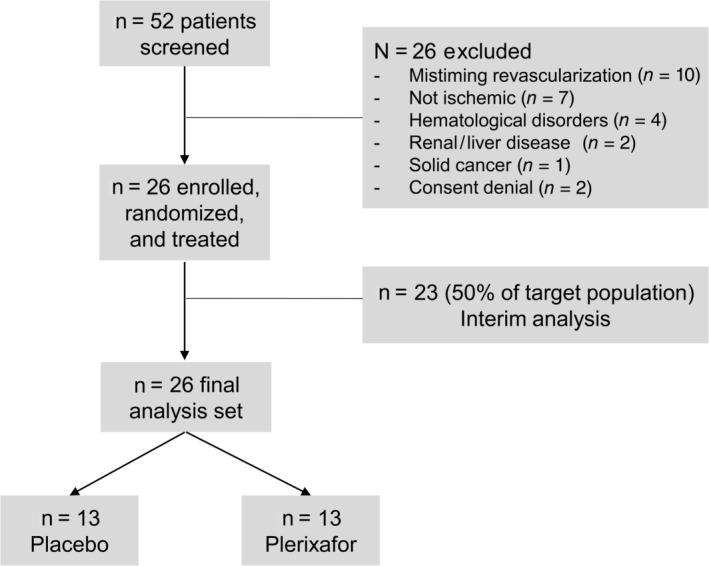
Study flowchart
An interim analysis was performed once the follow‐up of the first 23 patients was completed. Four of the 12 patients who received plerixafor vs 9 of the 11 patients who received placebo achieved complete wound healing (chi‐square P = .019). This was equal to a relative risk of nonhealing of 2.45 (95% confidence interval 1.05‐5.73) and a 49% absolute lower risk of healing in the plerixafor group. Based on such analysis, the study was terminated. While follow‐up of patients included in the interim analysis was being completed, three more patients were enrolled. Thus, the final analysis data set was composed of 26 patients, equally distributed in the two groups (13/13). Clinical characteristics of study patients are summarized in Table 1. Patients (84.6% males) were on average 69 years of age and had diabetes for ~15 years. Despite randomization, diabetes duration was longer in the plerixafor group, although such difference was not significant after adjusting for multiple testing. All enrolled patients had hemodynamically significant stenosis of leg arteries combined with a TcO2 <50 mmHg and/or ABI <0.9. Prior to randomization and treatment, surgical debridement was performed in all cases and most patients had underwent revascularization (69.2% in the plerixafor group and 76.9% in the placebo group).
TABLE 1.
Patient characteristics
| Characteristics | All patients (n = 26) | Placebo (n = 13) | Plerixafor (n = 13) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Sex male, % | 84.6 | 84.6 | 84.6 | 1.000 |
| Age, years | 69.2 ± 8.5 | 72.2 ± 8.5 | 66.3 ± 7.7 | .078 |
| Diabetes duration, years | 14.7 ± 12.1 | 9.9 ± 11.9 | 19.5 ± 10.6 | .039 a |
| Risk factors | ||||
| BMI, kg/m2 | 28.4 ± 4.5 | 27.2 ± 4.8 | 29.5 ± 4.0 | .193 |
| Hypertension, % | 100.0 | 100.0 | 100.0 | 1.000 |
| Systolic blood pressure, mmHg | 132.3 ± 15.4 | 134.6 ± 17.6 | 129.9 ± 13.1 | .449 |
| Diastolic blood pressure, mmHg | 72.7 ± 7.8 | 75.0 ± 8.4 | 70.4 ± 6.6 | .133 |
| Dyslipidemia, % | 80.8 | 76.9 | 84.6 | .635 |
| Current smoke, % | 26.9 | 38.5 | 15.4 | .165 |
| Complications | ||||
| Coronary artery disease, % | 53.8 | 61.5 | 46.2 | .452 |
| Carotid atherosclerosis, % | 84.6 | 76.9 | 92.3 | .296 |
| Retinopathy, % | 50.0 | 38.5 | 61.5 | .257 |
| Neuropathy, % | 69.2 | 53.8 | 84.6 | .096 |
| Chronic kidney disease, % | 34.6 | 30.8 | 38.5 | .695 |
| Stroke/TIA, % | 7.7 | 7.7 | 7.7 | 1.000 |
| Prior revascularization, % | 73.1 | 76.9 | 69.2 | .674 |
| Blood exams | ||||
| HbA1c, % (mmol/mol) | 9.2 ± 2.1 (75 ± 17) | 9.0 ± 2.0 (73 ± 16) | 9.4 ± 2.3 (77 ± 19) | .677 |
| Serum creatinine, μmol/L | 110.0 ± 52.4 | 98.1 ± 44.8 | 121.8 ± 58.4 | .255 |
| eGFR, mL/min/1.73 m2 | 65.2 ± 24.3 | 69.9 ± 24.0 | 60.5 ± 24.7 | .331 |
| Albumin/creatinine ratio, mg/g | 357.5 ± 845.4 | 279.0 ± 730.1 | 442.5 ± 981.2 | .639 |
| Total cholesterol, mg/dL | 136.1 ± 42.9 | 131.9 ± 49.5 | 140.3 ± 36.8 | .628 |
| HDL cholesterol, mg/dL | 36.9 ± 13.1 | 35.7 ± 11.5 | 38.1 ± 14.9 | .652 |
| LDL cholesterol, mg/dL | 74.8 ± 36.1 | 71.8 ± 43.9 | 77.8 ± 27.6 | .678 |
| Triglycerides, mg/dL | 127.5 ± 51.6 | 127.2 ± 56.7 | 127.8 ± 48.3 | .976 |
| Therapies | ||||
| Statin, % | 65.4 | 61.5 | 69.2 | .695 |
| RAS blockers, % | 65.4 | 69.2 | 61.5 | .695 |
| Other BP‐lowering drugs, % | 92.3 | 100.0 | 84.6 | .153 |
| APA, % | 88.5 | 92.3 | 84.6 | .558 |
| Insulin, % | 76.9 | 61.5 | 92.3 | .067 |
| Metformin, % | 23.1 | 30.8 | 15.4 | .372 |
| DPP‐4 inhibitors, % | 7.7 | 15.4 | 0.0 | .153 |
| SGLT‐2 inhibitors, % | 3.8 | 7.7 | 0.0 | .327 |
| Sulphonylurea, % | 11.5 | 7.7 | 15.4 | .558 |
| Wound characteristics | ||||
| TUC II/III, n | 3/23 | 1/12 | 2/10 | .652 |
| TcO2, mmHg | 46.0 ± 14.9 | 46.5 ± 17.1 | 45.6 ± 12.9 | .888 |
| ABI | 1.00 ± 0.21 | 0.96 ± 0.23 | 1.06 ± 0.19 | .304 |
Abbreviations: ABI, ankle‐brachial index; APA, antiplatelet agents; BMI, body mass index; BP, blood pressure; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RAS, renin angiotensin system; SGLT‐2, sodium‐glucose cotransporter‐2; TcO2, transcutaneous oxygen tension; TIA, transient ischemic attack; TUC, Texas University Classification.
Not significant after adjusting for multiple testing.
3.2. Wound healing
The median (interquartile range) follow‐up was 4.3 (3.3‐5.9) months in the plerixafor group and 3.9 (2.7‐4.1) months in the placebo group. The rate of complete wound healing was 38.5% (5/13 patients) in the plerixafor group vs 69.2% (9/13 patients) in the placebo group (chi‐square P = .115). To account for the time‐dependency of the event, we evaluated Kaplan‐Meier curves (Figure 2A) and the log‐rank P value was .147.
FIGURE 2.
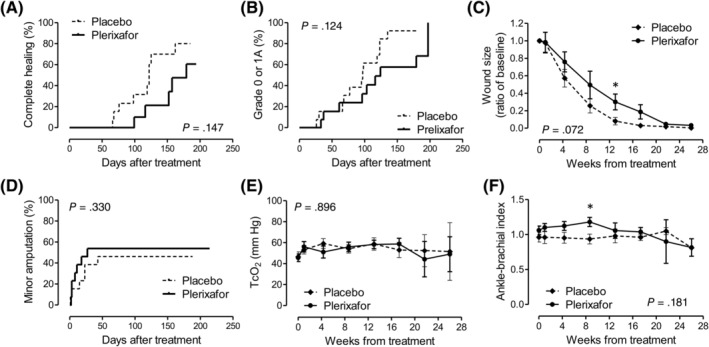
Study outcomes. A, The primary endpoint (complete wound healing). B, A modified healing endpoint (not specified in the original protocol). A, B, D, Time is shown in days because categorical events were recorded at given day after treatment; P values were obtained from the log‐rank test. C, E, F, Time is shown in weeks because continuous variables were averaged at the prespecified follow‐up time point; P values were obtained from the mixed model for repeated measures. TcO2, transcutaneous oxygen tension
Because it was difficult to distinguish between complete healing (grade 0) and superficial wounds (grade 1A) when scars were present, we explored a modified definition of the primary endpoint, which was not specified in the protocol (ie, achieving a grade 0‐1A wound). With this exploratory definition, wound outcome still tended to be worse in the plerixafor vs the placebo group (log‐rank P = .124; Figure 2B).
3.3. Secondary outcomes
Because the study was terminated prematurely and the primary endpoint was not met, the analysis of all secondary outcomes must be considered exploratory.
On average, relative wound size remained larger in the plerixafor group compared with the placebo group. According to the mixed model for repeated measures, the two curves were not significantly different (P = .072; Figure 2C), but a point‐by‐point analysis showed that wound size was significantly different between groups at 3 months (not significant after multiple testing). Minor amputations were nominally, but not significantly, more frequent in the plerixafor group than in the placebo group (Figure 2D). The change in TcO2 and ABI were not significantly different between groups (Figure 2E,F). ABI values were relatively high, likely as a reflection of uncompressible arteries in a population with a high prevalence of neuropathy, being therefore poorly indicative of the degree of ischemia. ABI values tended to be greater in the plerixafor group, which was significant at 2 months (not significant after multiple testing).
3.4. Stem cell mobilization
Baseline levels of CD34+ HSPCs was 2.3/μL in the plerixafor group and 2.5/μL in the placebo group (P = .74). Although HSPC levels remained unchanged from 8.00 am to 2.00 pm in the placebo group (fold increase 1.07 ± 0.29; Figure 3A), the increase was statistically significant in the plerixafor group (fold increase 7.72 ± 4.41; P < .001; Figure 3B,C), and all patients who received plerixafor achieved a greater than twofold increase in HSPCs compared with baseline. The degree of HSPC mobilization obtained after plerixafor was similar to that obtained in a historical series of 10 younger patients with diabetes without ischemic wounds (7.15 ± 2.23; P = .712 vs patients in the present study; Figure 3D). Among patients who received plerixafor, those who achieved complete wound healing tended to have lower fold‐increase in HSPCs compared with those who did not heal (6.08 ± 1.57 vs 8.76 ± 5.37; P = .309).
FIGURE 3.
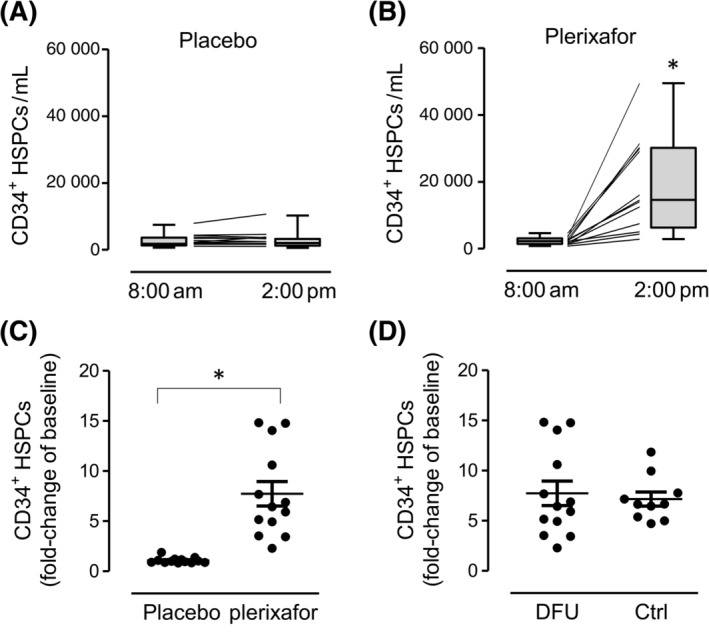
Stem cell mobilization. A, B, Levels of CD34+ HSPCs at baseline (8:00 am) and 6 hours after placebo (A) or plerixafor (B) injection. Box and whisker plots illustrate median, interquartile range (box), and range (whiskers). *P < .05 vs 8:00 am C, Fold‐increase of HSPCs after plerixafor or placebo (*P < .05). D, Fold‐increase of HSPCs after plerixafor in patients with ischemic DFUs of the present study compared with historical diabetic control patients without chronic complications. Ctrl, control; DFU, diabetic foot ulcer; HSPCs, hematopoietic stem/progenitor cells
3.5. Adverse events
One serious AE (acute coronary syndrome) occurred in one patient randomized to plerixafor, 3 days after injection, and was deemed related to pre‐existing conditions and unrelated to study drug. There were two nonserious AEs: one in a patient randomized to plerixafor (transient flushing after injection) and one in a patient randomized to placebo (petechial rash, that disappeared after aspirin withdrawal).
4. DISCUSSION
In this phase IIa, double‐blind, randomized, placebo‐controlled trial, successful HSPC mobilization with plerixafor did not improve healing of ischemic diabetic wounds. The trial was terminated because of safety concerns after completing follow‐up of 50% of the target population. The significant adverse effect of plerixafor observed in the interim analysis was not confirmed in the final analysis, but safety concerns remained because all outcomes tended to be worsened by plerixafor.
Interestingly, in response to plerixafor, patients with diabetes with ischemic wounds showed the same HSPC mobilization response as patients with diabetes without chronic complications. This finding demonstrates that although diabetes affects the BM microenvironment and impairs HSPC traffic, 4 it does not reduce HSPC bioavailability in the BM, even in the presence of neuropathy and/or vascular complications.
There are several possible explanations for the null results of this trial. First, heterogeneity of patients and of wound characteristics may have masked smaller effects of plerixafor. The degree of ischemia was variable, most patients underwent revascularization, allowing a high healing rate, and patients in the plerixafor group had longer disease duration. Second, plerixafor half‐life is 3‐5 hours and its transient effect on HSPC levels may be insufficient to drive durable benefits needed to improve healing. However, no transient favorable effect was observed at 1 week or 1 month. Third, HSPCs might not have reached ischemic tissues, owing to the impaired expression of the homing cytokine CXCL12 previously noted in experimental diabetic wounds.32, 33 Indeed, local re‐expression of CXCL12 has been recently identified as a strategy to promote diabetic wound healing in mice. 34
None of these explanations would, however, justify the adverse effects of plerixafor observed in the interim analysis. Given the small sample size, it is possible that plerixafor exerted no benefit and no harm and that results of the interim analysis occurred by chance. Mechanistic explanations include the inhibition of CXCR4 by plerixafor in the wound, which may worsen endothelial cell migration and tissue healing. 35 Alternatively, prior studies have shown that diabetic HSPCs have impaired angiogenic function and differentiation capacity.36, 37 A recent double‐blind randomized controlled trial of BM cell therapy for critical limb ischemia showed that the benefit was worse in patients with diabetes than in nondiabetic ones. 38 Furthermore, it has been shown that excess migration of CD34+ progenitor cells is associated with worse outcomes of patients with diabetes with critical limb ischemia. 39 Moreover, we have recently shown that the same signaling pathway causing inflammatory myelopoiesis is also responsible for stem cell mobilopathy in diabetes. 40 Based on these literature data, we speculate that plerixafor may have mobilized antiangiogenic or proinflammatory cells exerting detrimental effects on wound healing. Thus, mobilizing HSPCs without countering myelopoiesis might be insufficient to improve diabetes‐related outcomes.
5. CONCLUSION
Although unsuccessful, this study helps provide a better understanding of the role of stem cells in diabetic complications. The interplay among HSPC function, their traffic, and repair of peripheral tissues is complex. Acting on the downstream processes that drive HSPC mobilization (ie, CXCR4 antagonism) is not a suitable strategy to improve healing of ischemic diabetic wounds. Because adjunctive therapy for the diabetic foot syndrome remains an unmet need, a more comprehensive re‐education of diabetic hematopoiesis should be attempted.
CONFLICT OF INTEREST
G.P.F. is the major inventor of a patent, held by the University of Padova, based on a pharmacologic composition to induce stem cell mobilization in diabetes. The other authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
B.M.B.: data collection, data analysis and interpretation, manuscript writing, manuscript revision, final approval of manuscript; R.C., M. Mazzucato, M.G., M. Menegolo, A.B.: data collection, data analysis and interpretation, manuscript revision, final approval of manuscript; M.R.: data analysis and interpretation; final approval of manuscript; A.A., G.P.F.: study design; data analysis and interpretation, manuscript writing, final approval of manuscript.
ACKNOWLEDGMENTS
This study was supported by the Italian Ministry of Health grant GR‐2011‐02347600 to G.P.F.; the Italian Ministry of University grant 2015ZTT5KB to G.P.F.; and Sanofi Genzyme, which provided the free drug. The external sponsors had no role in study design, conduction, or data analysis and interpretation.
Bonora BM, Cappellari R, Mazzucato M, et al. Stem cell mobilization with plerixafor and healing of diabetic ischemic wounds: A phase IIa, randomized, double‐blind, placebo‐controlled trial. STEM CELLS Transl Med. 2020;9:965–973. 10.1002/sctm.20-0020
Funding information Italian Ministry of University, Grant/Award Number: 2015ZTT5KB; Italian Ministry of Health, Grant/Award Number: GR‐2011‐02347600
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. American Diabetes Association . Microvascular complications and foot care: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41(suppl 1):S105‐S118. [DOI] [PubMed] [Google Scholar]
- 2. Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care. 2018;41:645‐652. [DOI] [PubMed] [Google Scholar]
- 3. Fadini GP, Boscaro E, de Kreutzenberg S, et al. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care. 2010;33:1097‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fadini GP, DiPersio JF. Diabetes mellitus as a poor mobilizer condition. Blood Rev. 2018;32:184‐191. [DOI] [PubMed] [Google Scholar]
- 5. Fadini GP, Albiero M, Vigili de Kreutzenberg S, et al. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 2013;36:943‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DiPersio JF. Diabetic stem‐cell "mobilopathy". N Engl J Med. 2011;365:2536‐2538. [DOI] [PubMed] [Google Scholar]
- 7. Albiero M, Poncina N, Ciciliot S, et al. Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes. 2015;64:2957‐2968. [DOI] [PubMed] [Google Scholar]
- 8. Albiero M, Poncina N, Tjwa M, et al. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014;63:1353‐1365. [DOI] [PubMed] [Google Scholar]
- 9. Dang Z, Maselli D, Spinetti G, et al. Sensory neuropathy hampers nociception‐mediated bone marrow stem cell release in mice and patients with diabetes. Diabetologia. 2015;58:2653‐2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spinetti G, Cordella D, Fortunato O, et al. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: implication of the microRNA‐155/FOXO3a signaling pathway. Circ Res. 2013;112:510‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Albiero M, Menegazzo L, Boscaro E, et al. Defective recruitment, survival and proliferation of bone marrow‐derived progenitor cells at sites of delayed diabetic wound healing in mice. Diabetologia. 2011;54:945‐953. [DOI] [PubMed] [Google Scholar]
- 12. Fadini GP, Rigato M, Cappellari R, et al. Long‐term prediction of cardiovascular outcomes by circulating CD34+ and CD34+CD133+ stem cells in patients with type 2 diabetes. Diabetes Care. 2017;40:125‐131. [DOI] [PubMed] [Google Scholar]
- 13. Rigato M, Bittante C, Albiero M, et al. Circulating progenitor cell count predicts microvascular outcomes in type 2 diabetic patients. J Clin Endocrinol Metab. 2015;100:2666‐2672. [DOI] [PubMed] [Google Scholar]
- 14. Fadini GP, Ciciliot S, Albiero M. Concise review: perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells. 2017;35:106‐116. [DOI] [PubMed] [Google Scholar]
- 15. Cruciani M, Lipsky BA, Mengoli C, et al. Granulocyte‐colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst Rev. 2013;CD006810 10.1002/14651858.CD006810.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferraro F, Lymperi S, Mendez‐Ferrer S, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fadini GP, Fiala M, Cappellari R, et al. Diabetes limits stem cell mobilization following G‐CSF but not plerixafor. Diabetes. 2015;64:2969‐2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holmes D. Diabetes: SDF‐1 dysregulation mediates diabetic stem cell mobilopathy. Nat Rev Endocrinol. 2015;11:318. [DOI] [PubMed] [Google Scholar]
- 19. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nat Med. 2004;10:858‐864. [DOI] [PubMed] [Google Scholar]
- 20. Nishimura Y, Ii M, Qin G, et al. CXCR4 antagonist AMD3100 accelerates impaired wound healing in diabetic mice. J Invest Dermatol. 2012;132:711‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tepper OM, Carr J, Allen RJ Jr, et al. Decreased circulating progenitor cell number and failed mechanisms of stromal cell‐derived factor‐1alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes. 2010;59:1974‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oyibo SO, Jude EB, Tarawneh I, et al. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care. 2001;24:84‐88. [DOI] [PubMed] [Google Scholar]
- 24. Boulton AJM, Armstrong DG, Kirsner RS, et al. Diagnosis and Management of Diabetic Foot Complications. Arlington, VA: American Diabetes Association; 2018. [PubMed] [Google Scholar]
- 25. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie house classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786‐806. [PubMed] [Google Scholar]
- 26. Bax G, Fagherazzi C, Piarulli F, et al. Reproducibility of Michigan Neuropathy Screening Instrument (MNSI). A comparison with tests using the vibratory and thermal perception thresholds. Diabetes Care. 1996;19:904‐905. [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499‐502. [PubMed] [Google Scholar]
- 29. Rigato M, Monami M, Fadini GP. Autologous cell therapy for peripheral arterial disease: systematic review and meta‐analysis of randomized, nonrandomized, and noncontrolled studies. Circ Res. 2017;120:1326‐1340. [DOI] [PubMed] [Google Scholar]
- 30. Fadini GP, Menegazzo L, Rigato M, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes. 2016;65:1061‐1071. [DOI] [PubMed] [Google Scholar]
- 31. Sealed Envelope Ltd . Power Calculator for Binary Outcome Superiority Trial; 2012. https://www.sealedenvelope.com/power/binary-superiority/. Accessed February 24, 2020.
- 32. Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO‐mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF‐1 alpha. J Clin Invest. 2007;117:1249‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fadini GP, Sartore S, Schiavon M, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia‐reperfusion injury in rats. Diabetologia. 2006;49:3075‐3084. [DOI] [PubMed] [Google Scholar]
- 34. Vagesjo E, Ohnstedt E, Mortier A, et al. Accelerated wound healing in mice by on‐site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci U S A. 2018;115:1895‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salcedo R, Wasserman K, Young HA, et al. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal‐derived factor‐1alpha. Am J Pathol. 1999;154:1125‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loomans CJ, van Haperen R, Duijs JM, et al. Differentiation of bone marrow‐derived endothelial progenitor cells is shifted into a proinflammatory phenotype by hyperglycemia. Mol Med. 2009;15:152‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schatteman GC, Hanlon HD, Jiao C, et al. Blood‐derived angioblasts accelerate blood‐flow restoration in diabetic mice. J Clin Invest. 2000;106:571‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy MP, Ross C, Kibbe MR et al. Administration of autologous bone marrow cells for limb salvage in patients with critical limb ischemia: results of the multicenter phase III MOBILE Trial AHA 2016 Late breaking clinical trials; 2016. http://www.abstractsonline.com/pp8/#!/4096/presentation/58438. Accessed May 2020.
- 39. Spinetti G, Specchia C, Fortunato O, et al. Migratory activity of circulating mononuclear cells is associated with cardiovascular mortality in type 2 diabetic patients with critical limb ischemia. Diabetes Care. 2014;37:1410‐1417. [DOI] [PubMed] [Google Scholar]
- 40. Albiero M, Ciciliot S, Tedesco S, et al. Diabetes‐associated myelopoiesis drives stem cell mobilopathy through an OSM‐p66Shc signaling pathway. Diabetes. 2019;68:1303‐1314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


