Abstract
One hallmark of neurodegenerative diseases is the intracellular accumulation of hyperphosphorylated tau protein, a neuronal microtubule-associated protein, into structures known as neurofibrillary tangles. Tauopathies are heterogeneous neurodegenerative diseases caused by the misfolding of the tau protein. It has been previously shown that the tau protein can spread from cell to cell in a prion-like manner. Tauopathies can be sporadic or familial, with the identification of pathogenic mutations in the microtubule-associated protein tau gene on chromosome 17 in the familial cases. Different frontotemporal dementia with parkinsonism-17 (FTDP-17) cases are associated with varying clinical presentations and types of neuropathology. We previously demonstrated that insoluble tau extracted from sporadic tauopathy human brains contain distinct tau strains, which underlie the heterogeneity of these diseases. Furthermore, these tau strains seeded tau aggregates that resemble human tau neuropathology in nontransgenic and 6hTau mice in vivo. Here, we show insoluble tau from human brains of FTDP-17 cases transmit different patterns of neuronal and glial tau pathology in vivo, similar to the sporadic tauopathies. This suggests that each of these tau mutations has unique properties that underlie the heterogeneity of FTDP-17 cases.
Keywords: FTDP-17, Human tauopathies, MAPT, Tau mouse model, Tau transmission
INTRODUCTION
Tauopathies are heterogeneous neurodegenerative diseases characterized by the abnormal misfolding of tau protein. Tauopathies vary greatly in clinical symptoms and in the distribution of tau neuropathology, although the molecular basis of this heterogeneity is unknown. Tau is normally a highly soluble protein, with the full-length tau containing 2N-terminus domains, a proline-rich domain, 4 microtubule-binding repeat domains (R1–R4), and a C-terminus domain. It is encoded by a microtubule-associated protein tau (MAPT) gene, which has 14 exons, with exons 9–12 each encoding a microtubule-binding repeat domain. The alternative splicing of exon 10 results in 3R or 4R tau isoforms (1). In healthy adult brains, equal amount soluble 3R and 4R tau isoforms are expressed, but in tauopathy brains, tau becomes misfolded, forming larger aggregates as intracellular inclusions with distinct isoform compositions (2).
Although most cases of tauopathies are sporadic, ∼10% of cases are familial, found to have pathogenic mutations in the MAPT gene on chromosome 17 (FTDP-17) (3). Different FTDP-17 cases are associated with varying clinical presentations and types of neuropathology, although tau aggregation is the primary pathology. For example, 2 cases with the L266V mutation showed Pick-like pathology, with excessive atrophy in the frontal and temporal lobes and the subcortical nuclei and brainstem. Moreover, the tau pathology consisted of neuronal and glial pretangle lesions and extensive threads throughout the brain, including Pick-like bodies in the dentate gyrus, astrocytic inclusions in the neocortex, and oligodendroglial coiled bodies in subcortical white matter (4). In contrast, the P301L mutation cases have been previously described as having similar patterns and severity of neuropathological features to globular glial tauopathy (GGT) (5). It was reported that the P301L mutation leads to neuronal loss in addition to globular glial inclusions varying throughout the gray and white matter of affected cortical regions, demonstrating that various pathologies of GGT subtypes are present in these cases. Lastly, the E10 + 16 mutation cases have prototypical frontotemporal dementia phenotype with prominent disinhibition and affective disturbances (6). Additionally, oligodendroglial coil-like structures are observed, and low numbers of neurofibrillary tangles and other tau-positive neuronal inclusions were found in the hippocampus (6).
It has been previously shown that the tau protein can spread from cell to cell in a prion-like manner (1). We previously found that insoluble tau extracted from sporadic human tauopathy brains have different biochemical properties, suggestive of tau strains; now, cryo-EM studies have further confirmed unique structural conformations of aggregated tau from different human tauopathies (7–11). Furthermore, we showed unique transmission properties of these human tau strains in vivo, in both nontransgenic (nonTg) and 6hTau mice (expressing all 6 human tau isoforms similar to human brains) (12, 13). Based on these studies, we hypothesized that insoluble tau from human FTDP-17 cases represent distinct tau strains with unique transmission properties in vivo. To test this hypothesis, insoluble tau from 3 different FTDP-17 cases (P301L, E10 + 16, and L266V) were intracerebrally injected into 6hTau and nonTg mouse brain to compare their spatiotemporal propagation.
MATERIALS AND METHODS
Selection and Sequential Extraction of Insoluble Tau From FTDP-17 Cases
The amount of tau pathology in the midfrontal cortex of all FTDP-17 cases in the CNDR brain bank was scored from 0 to 3+ in order to differentiate the amount of tau pathology in each of the human cases. Three cases with the highest score (i.e. most abundant tau pathology in the frontal cortex) were selected for extraction as previously described (Table) (14, 15).
TABLE.
Demographics of Human Cases Used for the Study
| Mutation Case | Neuropathological Diagnosis | Gender | Age at Death (Yrs) | Postmortem Interval (Hour) | Insoluble Tau Concentration (µg/µL) | Final Insoluble Tau Yield (µg/g Human Tissue) | Description of Neuropathology |
|---|---|---|---|---|---|---|---|
| MAPT L266V | Pick disease (familial) | F | 34 | 12 | 0.1 | 1.25 | Numerous tau-positive neuronal and glial inclusions including Pick bodies, ballooned neurons and threads are present in almost all regions of the brain, but most abundant in limbic structures and neocortical brain areas. Extensive neuron loss and spongiosis in the brain regions |
| MAPT P301L | FTDP-17 | F | 64 | – | 0.162 | 3.02 | Prominent neuronal tau-positive inclusions in gray matter of frontotemporal neocortex with severe neuron loss and gliosis in cortical regions. Astrocytic glial tau inclusions and white matter oligodendrocyte tau pathology also present |
| MAPT E10 + 16 | FTLD-tau, tauopathy unclassifiable | F | 64 | 10 | 0.206 | 4.68 | Widespread neuronal tau pathology in form of pretangles, rare globose tangles and threads and glial tau pathology in astrocytes and oligodendrocytes in neocortical regions, subcortical nuclei, brainstem, and cerebellar nuclei |
Extraction of insoluble tau from human cases was performed as previously described (7). Briefly, frontal cortical gray and white matter was homogenized in 9 volumes (v/w) of high-salt buffer (10 mM Tris with 0.8 M NaCl, pH7.4) with 0.1% sarkosyl and 10% sucrose added, and centrifuged at 10 000g for 10 minutes at 4°C. Pellets were re-extracted twice using the same high-salt buffer and the supernatants from all 3 extractions were filtered and pooled. Additional sarkosyl was added to the pooled supernatants to reach 1% and the samples were rotated for 1 hour at RT. The samples were centrifuged at 300 000g for 60 minutes at 4°C and the resulting 1% sarkosyl-insoluble pellets containing pathological tau were resuspended in PBS. The resuspended sarkosyl-insoluble pellets were further purified by a brief sonication using a handheld probe (QSonica, Newtown, CT), followed by centrifugation at 100 000g for 30 minutes at 4°C. The pellets were resuspended in PBS at 1/2–1/5 of the precentrifugation volume, sonicated, and spun at 10 000g for 30 minutes at 4°C to remove large debris (14). The final purified supernatants contained insoluble, pathological tau.
The different fractions from each purification were analyzed by Western blot (see below). The Western blots for tau in the final supernatant were used to estimate tau concentration from each mutation case (12).
Western Blotting
Samples were loaded on 7.5% SDS-PAGE gels, transferred to 0.2 µm nitrocellulose membranes, and blocked with 5% milk diluted in TBS. Blots were incubated in appropriate primary anti-tau antibodies overnight (RD3 [3R tau antibody, mouse monoclonal, 1:1k Millipore, Burlington, MA], RD4 [4R tau antibody, mouse monoclonal, 1:5k Millipore], 17025 [Peter Davies], and PHF-1 [Peter Davies] all at a concentration of 0.05 µg/µL) samples were then incubated with IRDye-labeled secondary antibodies in Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE), and scanned using an ODY-2816 Imager (Li-Cor Biosciences).
Stereotaxic Injections
To test whether different FTDP-17 cases would lead to varying patterns of endogenous mouse tau aggregation in vivo, comparable concentrations of tau from the 3 FTDP-17 cases (1 µg/mL of P301L, 1 µg/mL of E10 + 16, and 0.6 µg/mL of L266V) were injected into the dorsal hippocampus of both male and female nonTg (C57Bl/6) and 6hTau mice (a transgenic mouse line expressing all 6 isoforms of human tau that does not express endogenous mouse tau [mouse tau KO]). Briefly, 2–3-month-old mice were deeply anesthetized with ketamine/xylazine/acepromazine and immobilized in a stereotaxic frame. The mice were aseptically inoculated with human brain extracts in the dorsal hippocampus (bregma: –2.5 mm; lateral: +2 mm; depth: 2.4 mm from the skull). The hippocampus received 5–6 µL of inoculum. Concentrations of tau per injection site for the 3 cases were determined based on data from Western blots (Table) (12). All animal protocols were approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee (IACUC) and complied with all relevant ethical regulations for animal testing and research.
Histology and Immunohistochemistry
Injected mice were killed and analyzed via immunohistochemistry (IHC) at 3- or 6-months post injection (p.i.). Brains and spinal cords were fixed in 10% neutral buffered formalin overnight and then embedded in paraffin and microtome-sectioned at 6-µm thickness, followed by IHC for various antitau primary antibodies: AT8 (pS202/205 tau Ab, mouse monoclonal, 1:10k, Thermo Scientific, Waltham, MA), RD3, RD4. The next day, the sections were developed using a polymer horseradish peroxidase detection system (BioGenex, Fremont, CA) with counterstaining for hematoxylin. Stained slides were scanned using the Lamina Multilabel Slide Scanner (PerkinElmer, Waltham, MA).
Experimental Design and Statistical Analysis
For the in vivo experiments in nonTg mice, mice were injected once per lysate in the dorsal hippocampus, and were then incubated for 3 and 6 months (for 6hTau mice, n = 4 mice were injected per lysate for each time point and for nonTg mice, n = 5 mice were injected per lysate for each time point). For quantification of IHC, AT8-positive tau pathology was defined as either neuron, astrocyte, or oligodendrocyte with positive staining as seen in the insets of Figures 2 and 3. The extent of AT8-positive pathology at 3 and 6 months p.i. for each lysate was performed for coronal sections aligning with bregma 0.98 mm, –0.22 mm, –1.22 mm, –2.18 mm, –2.92 mm, –3.52 mm, –4.48 mm, –5.52 mm. The total number of AT8-positive cells per mouse at each bregma were counted using the HALO software (Indica Labs, Albuquerque, NM) by manually clicking each tau-positive neuron (in the hippocampus of all coronal sections ipsilateral/contralateral to side of injection), oligodendrocyte (in the fimbria and corpus callosum of all coronal sections ipsilateral/contralateral to side of injection), or astrocyte (in the hippocampus of all coronal sections ipsilateral/contralateral to side of injection). The manual counts were performed by 2 independent reviewers to verify the accuracy of quantification.
FIGURE 2.
Differential propagation of neuronal and glial tau pathology by FTDP-17 cases in 6hTau mouse model. (A) Representative images of neuronal pathology in the dorsal hippocampus using IHC with antihyperphosphorylated tau antibody AT8 on brain tissues from 6hTau mice injected with E10 + 16 case (3 months p.i.: 1 μg/site, n = 4 mice and 6 months p.i.: 1 μg/site, n = 4 mice), P301L case (3 months p.i.: 0.97 μg/site, n = 4 mice and 6 months p.i.: 0.97 μg/site, n = 4 mice), and L266V case (3 months p.i.: 0.6 μg/site, n = 4 mice and 6 months p.i.: 0.96 μg/site, n = 4 mice). Insets show example of AT8-positive neuronal tau pathology. Scale bar: 50 μm, Inset: 10 μm. (B) Quantification of neuronal tau pathology in the hippocampus of the 3 FTDP-17 cases. One-way ANOVA with Tukey post hoc analysis was performed (mean ± SEM plotted). (C) Representative images of oligodendrocyte pathology in the fimbria using IHC with AT8 on same mice as in panel A. Insets show example of AT8-positive oligodendroglial tau pathology. Scale bar: 50 μm, inset: 10 μm. (D) Quantification of oligodendrocyte pathology in fimbria region for each region on the 3 FTDP-17 cases. One-way ANOVA with Tukey post hoc analysis was performed (mean ± SEM plotted). (E) Quantification of oligodendrocyte pathology in corpus callosum region on the 3 FTDP-17 cases. One-way ANOVA with Tukey post hoc analysis was performed (mean ± SEM plotted). (F) Representative images of oligodendrocyte pathology in the corpus callosum using IHC with AT8 on same mice as in panel A. Scale bar: 50 μm, inset: 10 μm. (G) Representative images of astrocytic pathology in the dorsal hippocampus using IHC with AT8 on same mice as in panel A. Insets show example of AT8-positive astrocytic tau pathology. Scale bar: 50 μm, inset: 10 μm. (H) Representative images of neuronal pathology in the dorsal hippocampus using IHC with anti-3R tau isoform antibody RD3, anti-4R tau isoform antibody RD4, and antihyperphosphorylated tau antibody AT8 on adjacent brain tissue slices in panel A to compare 3R and 4R tau recruitment in the 6hTau mice. Scale bar: 50 µm.
FIGURE 3.
Differential propagation of neuronal and glial tau pathology by FTDP-17 cases in nonTg mouse model. (A) Representative images of neuronal pathology in the dorsal hippocampus using IHC with AT8 on nonTg mice injected with E10 + 16 case (3 months p.i.: 1 μg/site, n = 5 mice and 6 months p.i.: 1 μg/site, n = 5 mice), P301L case (3 months p.i.: 0.97 μg/site, n = 5 mice and 6 months p.i.: 0.97 μg/site, n = 5 mice), and L266V case (3 months p.i.: 0.6 μg/site, n = 5 mice and 6 months p.i.: 0.96 μg/site, n = 5 mice). Insets show an example of AT8-positive neuronal tau pathology. Scale bar: 50 μm, inset: 10 μm. (B) Quantification performed for neuronal tau pathology in the hippocampus on the 3 FTDP-17 cases. One-way ANOVA with Tukey post hoc analysis was performed (mean ± SEM plotted). (C) Representative images of oligodendrocyte pathology in the fimbria using IHC with AT8 on same mice as in panel A. Insets show example of AT8-positive oligodendroglial tau pathology. Scale bar: 50 μm, inset: 10 μm. (D) Quantification of oligodendrocyte pathology in fimbria region for each region on the FTDP-17 cases. One-way ANOVA with Tukey post hoc analysis was performed (mean ± SEM plotted). (E) Representative images of oligodendrocyte pathology in the corpus callosum using IHC with AT8 on same mice as in panel A. Scale bar: 50 μm, inset: 10 μm. (F) Quantification of oligodendrocyte pathology in corpus callosum region for each region on the 3 mutation cases. One-way ANOVA with Tukey post hoc analysis was performed (mean ± SEM plotted). (G) Representative images of astrocytic pathology using IHC for AT8 on same mice as in panel A. Insets show example of AT8-positive astrocytic tau pathology. Scale bar: 50 μm, inset: 10 μm.
For comparisons of absolute counts of AT8+ cells, the total counted tau-positive cells were plotted as mean ± SEM. IHC quantification results were analyzed across mice using one-way ANOVA with Tukey post hoc test with Graph-Pad Prism, with each statistical test described per figure. p values <0.05 were considered statistically significant. In the heatmaps, for neuronal tau pathology, each region was given a semiquantitative score from 0 to 3 at 10× magnification (0 = no positive cells, 1 = 0–5 positive cells, 2 = 5–10 positive cells, 3 = >10 positive cells). Then, the scores were averaged across all mice at each time point and imported into a customized software to generate color-coded heat maps of the spatial distribution of the tau pathology. For the oligodendroglial and astrocytic tau pathology, the absolute counts determined as above were divided by either 3 (for astrocytes) or 8 (for oligodendrocytes) and individual stars (for astrocytes) or ovals (for oligodendrocytes) were added on top of the neuronal heatmaps.
RESULTS
Biochemical Characterization of Insoluble Tau From FTDP-17 Cases
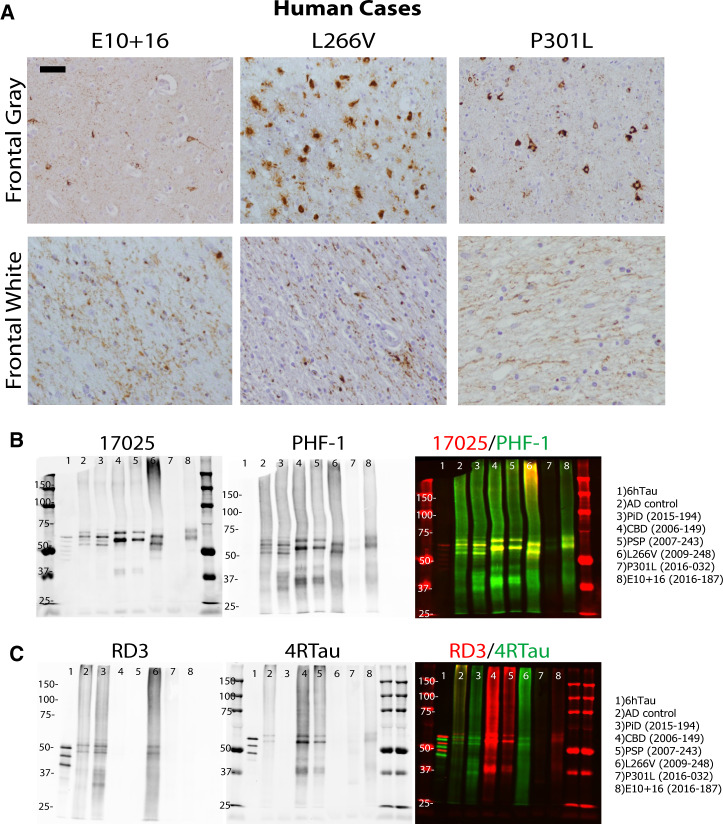
Human brain tissues from 3 FTDP-17 cases with abundant frontal tau pathology were identified and selected for this study: E10 + 16 MAPT mutation on an intron flanking exon 10; P301L MAPT mutation on exon 10; and L266V MAPT mutation on exon 9 (Table; Fig. 1A). As previously described, these cases have a much younger age of onset and shorter disease duration (Table) (4, 5). Of these cases, L266V had the greatest amount of neuronal and glial pathology in the frontal cortex, but also significant neuronal atrophy.
FIGURE 1.
Biochemical characterization of insoluble tau from FTDP-17 cases. (A) IHC with antitau antibody PHF-1 on all 3 FTDP-17 cases. (B) Western blots on final supernatants from extraction of FTDP-17 and sporadic tauopathy cases using antitau antibodies 17025 (red) and PHF-1 (green) for total tau and pS396/pS404 phosphorylated tau, respectively. (C) Western blots of same supernatants as in panel B using mouse monoclonal anti-3R isoform tau antibody RD3 (red) and rabbit polyclonal anti-4R isoform tau antibody 4RTau (green) for 3R tau and 4R tau, respectively. Scale bar: 50 µm.
Frontal cortical gray and white matter was extracted from all cases as previously described (12), and final supernatants contained insoluble, phosphorylated tau by Western blot for 17025 (total tau) and PHF-1 (phosphorylated tau) (Table; Fig. 1B). Of note, the yield of insoluble tau from the FTDP-17 cases was much lower than sporadic tauopathy cases (∼1%–2% tau in final supernatant in sporadic cases) (12, 13). The tau isoform compositions of these 3 FTDP-17 cases have been previously reported: L266V, 3R; P301L, 4R; and E10 + 16, 4R (5). Utilizing anti-3R and 4R tau antibodies, we confirmed the tau isoform composition of the insoluble tau extracted from the 3 cases and also found that the L266V mutation case contained primarily 3R tau isoform, similar to Pick disease, (RD3; Fig. 1C), and the E10 + 16 and P301L cases contained mostly 4R tau isoform, similar to progressive supranuclear palsy and corticobasal degeneration (CBD) (4RTau; Fig. 1C).
Differential Propagation of Neuronal and Glial Tau Pathology by FTDP-17 Cases In Vivo
The final supernatants with insoluble tau from the 3 FTDP-17 cases were intracerebrally injected into the dorsal hippocampus of young 6hTau mice and subsequently analyzed at 3- and 6-months p.i. for the transmission of tau pathology in vivo (13). At 3 months p.i., IHC staining with hyperphosphorylated tau antibody AT8 (pSer202/205) showed that the P301L MAPT mutation seeded the least amount of neuronal pathology, and L266V induced the greatest amount of neuronal pathology (Fig. 2A). From 3 to 6 months, the amount of neuronal pathology increased for each case in both ipsilateral and contralateral sides to the injection sites (Fig. 2B).
It has been previously shown that the 6hTau mouse model, which has equal ratios of 3R and 4R human tau isoforms, is able to recapitulate the deposition of pathological tau with varying tau isoform compositions and cell type specificities as in human tauopathies after seeded with corresponding tau strains (13). Similarly, the neuronal and glial tau pathology induced here in the 6hTau mouse model by insoluble tau from 3 FTDP-17 cases mirrored what was seen in the human cases (Fig. 1A). In comparison with sporadic cases injected into 6hTau mice, the sporadic cases seeded much larger amounts of total tau pathology than the FTDP-17 cases (13).
While the L266V case had the greatest amount of neuronal pathology in the 6hTau mouse model, the E10 + 16 case had the greatest amount of oligodendroglial tau pathology in both the fimbria and corpus callosum (Fig. 2D, E). Again, the amount of oligodendrocyte tau pathology increased from 3 to 6 months for all cases, but the total amount of oligodendroglial tau pathology for each case was much less than neuronal tau pathology in the 6hTau mouse model (Fig. 2C–E). Lastly, the greatest amount of astrocytic pathology was seen in the E10 + 16 case, and the least amount of astrocytic pathology was seen in the P301L case (Fig. 2F). When comparing these 3 FTDP-17 cases with the sporadic cases, the sporadic cases again seeded much more glial tau pathology than the FTDP-17 cases (13).
Moreover, we analyzed the 3R and 4R tau isoform compositions of the seeded pathology in the 6hTau mice (using tau isoform-specific antibodies, respectively recognizing 3R [RD3] and 4R tau [RD4] antibodies). The AT8-positive pathology from both the E10 + 16 and P301L cases were positive for RD4, but not RD3, suggesting the seeded tau aggregates were comprised of 4R tau primarily. In contrast, the AT8-positive pathology from the L266V case primarily had RD3+ staining, suggesting L266V seeded primarily 3R tau in the 6hTau mice (Fig. 2G).
In addition to the 6hTau mouse model, the insoluble tau from the 3 FTDP-17 cases was intracerebrally injected into the dorsal hippocampus of young nonTg mice and was subsequently analyzed at 3 and 6 months p.i. In comparison with the 6hTau mice, there was much less neuronal pathology (Fig. 3A, B) and oligodendroglial pathology (Fig. 3C–E) induced in nonTg mice for each mutation case, especially for the L266V case (Fig. 3A, B). In addition, much less oligodendrocyte glial tau pathology is seen both in the fimbria and corpus callosum in the nonTg mice compared with 6hTau mice for the L266V and P301L cases, but not for the E10 + 16 case (Figs. 2C–E and 3C–E). Moreover, insoluble tau from E10 + 16 case induced more astrocytic tau pathology in nonTg mice compared with 6hTau mice (Figs. 2F and 3F).
Spatiotemporal Transmission of Tau Pathologies From FTDP-17 Cases in the 6hTau Mice
Spatiotemporal transmission patterns of neuronal and glial tau pathologies in the 6hTau mouse model induced by insoluble tau from 3 FTDP-17 cases were analyzed using heatmaps (see Materials and Methods). All FTDP-17 cases transmit neuronal tau aggregates to various regions that are anatomically connected to the site of injection, as previously described for sporadic cases (12, 13).
L266V and E10 + 16 cases showed the widest spatial distribution of tau pathology, while the P301L case showed the least (Fig. 4; Supplementary Data Fig. S1). The spreading of both oligodendroglial and astrocytic tau pathology also increased over time, as described previously (12, 13). However, when comparing each FTDP-17 case, the L266V case displayed the greatest amount of neuronal tau transmission, while E10 + 16 demonstrated the greatest amount of oligodendroglial and astrocytic tau transmission.
FIGURE 4.
Spatiotemporal transmission patterns of tau pathology in FTDP-17 cases in 6hTau mouse model. Semiquantitative analyses were performed on a scale of 0 (gray) to 3 (red) for neuronal tau pathology and color coded onto heat maps at 5 bregma levels as described in the Materials and Methods section. Scores were averaged between mice for (A) E10 + 16 mutation case, (B) L266V mutation case, and (C) P301L mutation case 3 months p.i. and 6 months p.i. with n = 4 mice for each case. Absolute counts for oligodendroglial and astrocytic tau pathology at same bregma levels were divided by 8 and 3, respectively, and individual purple ovals (for oligodendrocytes) and red stars (for astrocytes) were added on top of neuronal heatmaps.
DISCUSSION
In this study, we demonstrated that insoluble tau from human FTDP-17 cases have unique transmission properties in vivo. In particular, the neuronal and glial tau pathology varied between the 3 cases, P301L, E10 + 16, and L266V, in both nonTg and 6hTau mouse brains. It has been previously shown that mutations on the MAPT gene alter varying properties and functions of tau (16). Different FTDP-17 cases are therefore associated with varying clinical presentations and types of neuropathology, even though tau aggregation is the primary pathology.
Our study used human brain tissues from the 3 FTDP-17 cases that all had abundant frontal tau pathology; however, the yield of insoluble tau was much lower than from sporadic tauopathy cases (Table) (12). It is possible that these FTDP-17 cases exhibit different biochemical properties, and the extraction method used here may not be the most efficient for their unique properties. However, this method was utilized in order to compare our prior findings on sporadic cases with the FTDP-17 mutation cases used here.
Among the 3 human cases, the L266V case had the most abundant neuronal and glial tau pathology in the frontal cortex, as well as the most neural atrophy. Consistently, the insoluble tau from L266V case seeded the highest amount of neuronal tau pathology in the 6hTau mice. Surprisingly, a similar amount of insoluble tau from P301L case compared with the other cases did not seed as much tau pathology in the 6hTau mice. One possible explanation is that the P301L mutation cannot efficiently seed wildtype tau; however, one recent study suggested there were no differences in P301L seeding wildtype or mutant tau (17). Nonetheless, that study was performed using in vitro fibrils, and there could still be cross-seeding differences in vivo.
Interestingly, insoluble tau from the L266V case has a similar transmission pattern to sporadic Pick disease as we showed recently (13). Both L266V-tau and Pick-tau are comprised predominantly of 3R tau, primarily seed 3R tau pathologies in 6hTau mice, and induce the least amount of tau pathology in the nonTg mice. The lower amount of tau pathology from L266V in nonTg mice is likely not due to the lower amount of insoluble tau extracted from the L266V case, since the same amount of insoluble tau from this case induced the greatest amount of pathology in 6hTau mice. However, one possibility is that the nonTg mice only express 4R tau isoforms (unlike human brain expressing both 3R and 4R tau), while the 6hTau mice express all 6 isoforms of human tau. We previously showed that the insoluble 3R tau from Pick disease brain does not seed 4R tau in nonTg mice efficiently due to conformation-dependent cross-seeding barriers (13). It is likely that the L266V 3R tau has a similar misfolded conformation as in Pick’s pathological tau, and thereby also does not seed 4R tau in nonTg mice efficiently (8).
Insoluble tau from E10 + 16 and P301L cases are both primarily comprised of 4R tau. Although a similar amount of insoluble tau from these 2 cases were respectively injected into 6hTau mice as well as nonTg mice, the induced in vivo pathological patterns were quite different. The transmission pattern induced by E10 + 16 case tau is quite similar to sporadic CBD tau with large amount astrocytic plaque-like tau induced as we recently observed (12, 13). However, the P301L case tau did not seed much glial tau pathology. Consistent with the latest cryo-EM structure of CBD tau, the 301st amino acid proline formed a critical folding angle (beta turn) in the core fibril region (10, 11), so it is possible that a mutation from proline to leucine could disrupt this structure and alter the seeding properties. As a result, insoluble 4R tau from E10 + 16 and P301L cases showed distinct transmission patterns in vivo.
Moreover, the sporadic tauopathy cases seeded much greater amounts of tau pathology in 6hTau mice compared with FTDP-17 cases. One explanation for this observation is the mutated tau seeding ability (16). It has been previously shown that different tau mutations have unique seeding propensities for wildtype versus mutant tau (17). These varying seeding abilities of the FTDP-17 cases can constitute the difference in pathology seen between the FTDP-17 and sporadic cases in both 6hTau and nonTg mice. Future studies using mouse models that express each of the mutations will be necessary to investigate this phenomenon further.
Additionally, in both the 6hTau and nonTg mouse models, the E10 + 16 case had the greatest amount of oligodendrocyte and astrocytic glial tau pathology. While considerable amounts of oligodendrocyte and astrocytic glial tau pathology were found in the 6hTau mice injected with all 3 FTDP-17 cases, in the nonTg mice, only the E10 + 16 case induced substantial amounts of astrocytic pathology. Similar to the neuronal pathology, there was much less glial tau pathology with the FTDP-17 cases compared with sporadic cases. We recently showed that glial tau pathology can propagate independent of neuronal tau, so it is possible that the insoluble tau from FTDP-17 cases are not able to transmit glial tau pathology as efficiently as sporadic cases (18).
In conclusion, we have demonstrated that insoluble tau from human FTDP-17 cases have unique transmission properties in vivo. This study provides the first experimental evidence suggesting FTDP-17 cases in human disease brain-derived pathological tau may have distinct properties that confer unique seeding patterns in vivo. These findings have significant implications for using transgenic mouse models that express different tau mutations, as they may not recapitulate sporadic tauopathies. Furthermore, different therapies may be needed to target the insoluble tau in FTDP-17 cases compared with sporadic disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Modupe Olufemi, Emily Maynard, and Tia Bernard-Banks for technical assistance. We also thank the patients and families for brain donation.
This study was supported by NIH grant AG10124, U19 AG062418 (J.Q.T.), and AG17586 (V.M.Y.L.), a grant from CurePSP, Woods Foundation, and BrightFocus Foundation (Z.H., A2018802S).
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. D'Souza I, Schellenberg GD.. Regulation of tau isoform expression and dementia. Biochim Biophys Acta 2005;1739:104–15 [DOI] [PubMed] [Google Scholar]
- 2. Frost B, Diamond MI.. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci 2010;11:155–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998;393:702–5 [DOI] [PubMed] [Google Scholar]
- 4. Van Deerlin VM, Forman MS, Farmer JM, et al. Biochemical and pathological characterization of frontotemporal dementia due to a Leu266Val mutation in microtubule-associated protein tau in an African American individual. Acta Neuropathol 2007;113:471–9 [DOI] [PubMed] [Google Scholar]
- 5. Forrest SL, Kril JJ, Stevens CH, et al. Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain 2018;141:521–34[published correction appears in Brain 2018;141:e30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doran M, du Plessis DG, Ghadiali EJ, et al. Familial early-onset dementia with tau intron 10 + 16 mutation with clinical features similar to those of Alzheimer disease. Arch Neurol 2007;64:1535–9 [DOI] [PubMed] [Google Scholar]
- 7. Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer's disease. Nature 2017;547:185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falcon B, Zhang W, Murzin AG, et al. Structures of filaments from Pick's disease reveal a novel tau protein fold. Nature 2018;561:137–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falcon B, Zivanov J, Zhang W, et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 2019;568:420–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arakhamia T, Lee CE, Carlomagno Y, et al. Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell 2020;180:633–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang W, Tarutani A, Newell KL, et al. Novel tau filament fold in corticobasal degeneration. Nature 2020;580:283–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narasimhan S, Guo JL, Changolkar L, et al. Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J Neurosci 2017;37:11406–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He Z, McBride JD, Xu H, et al. Transmission of tauopathy strains is independent of their isoform composition. Nat Commun 2020;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo JL, Narasimhan S, Changolkar L, et al. Unique pathological tau conformers from Alzheimer's brains transmit tau pathology in nontransgenic mice. J Exp Med 2016;213:2635–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Narasimhan S, Lee V.. The use of mouse models to study cell-to-cell transmission of pathological tau. Methods Cell Biol 2017;141:287–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strang KH, Golde TE, Giasson BI.. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Invest 2019;99:912–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strang KH, Croft CL, Sorrentino ZA, et al. Distinct differences in prion-like seeding and aggregation between Tau protein variants provide mechanistic insights into tauopathies. J Biol Chem. 2018;293:2408–21[published correction appears in J Biol Chem 2018;293:4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narasimhan S, Changolkar L, Riddle DM, et al. Human tau pathology transmits glial tau aggregates in the absence of neuronal tau. J Exp Med 2020;217: e20190783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.