Abstract
Background
Rapid infant weight gain (RIWG) is strongly related to childhood overweight and obesity, and prevention of RIWG is an approach to early years obesity prevention. This systematic review aimed to explore effectiveness, deliverers’ and recipients’ experiences of involvement, and key intervention components and processes of such prevention activities.
Methods
Key databases and websites were searched systematically for quantitative and qualitative studies covering intervention effectiveness, experiences with intervention involvement or process outcomes. After duplicate screening and quality assessment, papers were analyzed through narrative synthesis, thematic synthesis and intervention component analysis.
Results
Seven quantitative and seven qualitative studies were eligible for inclusion. Most intervention studies reported small, but significant results on infant weight gain. More significant results were measured on weight gain during the first compared with the second year of life. A weak evidence base made elaboration of the relationship between intervention effectiveness and content challenging. Home-delivered interventions may be more relevant for parents. Contextual factors, such as social norms, beliefs and professional identity should be considered during intervention development. Stakeholder involvement can be key to increase intervention acceptability and feasibility.
Conclusions
The field of RIWG prevention is new and evolving, but more research is needed before further conclusions about intervention effectiveness and intervention content can be drawn. Future interventions should take parents, health professionals and other contextual needs into account to improve chances of success. More research on long-term effects on overweight and obesity is needed.
Introduction
Rapid infant weight gain (RIWG), frequently defined in the literature as an increase in >0.67 in weight-for-age z-scores (WAZ) between two time points during the first 2 years of life,1 is associated with an increased risk of childhood overweight and obesity (COO)1,2 and of having a higher body-fat percentage, greater waist circumference and lower insulin sensitivity in early adulthood.3 COO is an important public health concern as it may have a great impact on the physical and psychosocial health of individuals,4 and early years prevention is needed as weight problems often persist into adulthood.5 Development of effective early years prevention strategies is desirable,6 and preventing RIWG in the first place can be a promising strategy due to its strong and consistent association with COO.1,2
Early years COO prevention undertaken via prevention of RIWG has received increasing interest over the last decade. Risk factors related to infant feeding have received particular attention,7–9 with a higher protein intake during infancy being causally related to both RIWG and COO.10,11 Increased risk of RIWG has also been associated with a range of other factors such as low birth weight,8 maternal smoking during pregnancy,12 gestational diabetes,12 infant day care attendance13 and low socioeconomic position (SEP).12,14 Despite growing interest for early years COO prevention, there is no published systematic review on RIWG prevention. Although finding some intervention effects of COO prevention for school-aged children,6 previous reviews considering evidence on COO prevention initiated at earlier ages have mainly identified small or no effect sizes,6,15 and there is little understanding as to why this is. Furthermore, an umbrella review on childhood obesity prevention has argued that most systematic reviews failed to provide clear recommendations for policymakers,16 making it difficult for decision makers and practitioners to know which interventions to implement.17 Thus, a comprehensive review of the existing RIWG evidence is necessary to identify and understand effective strategies.
We systematically reviewed evidence relating to RIWG prevention with the three following aims: (i) to explore intervention effectiveness, (ii) to understand deliverers’ and recipients’ experiences of intervention involvement and (iii) to identify key intervention components and processes. Results from this systematic review will potentially enhance understanding of RIWG prevention activities, as well as support intervention developers, policymakers and other relevant professionals in identifying effective RIWG prevention strategies that can strengthen early life COO prevention.
Methods
The protocol for this review was registered in the PROSPERO database of systematic reviews (https://www.crd.york.ac.uk/prospero/, ID: CRD42018076214). Quantitative and qualitative evidence was included in the review to address both intervention effectiveness and user experiences of involvement. The study is reported in accordance with PRISMA and ENTREQ guidelines.18,19 The review included published and unpublished quantitative and qualitative studies reporting on all types of interventions preventing RIWG in healthy term infants aged 0–2 years in high-income countries. The restriction in age corresponds to the ages covered in the definition of RIWG presented in the introduction. Studies written in English, Spanish or Nordic languages were included. No restrictions were put on publication year.
Eligibility criteria for quantitative studies
Quantitative studies using differences in infant weight gain between two time points as primary or secondary outcomes were eligible for inclusion. Preferably, RIWG was defined as an increase in more than 0.67 standard deviations in WAZ measured between two time points during the first 2 years of life,1 but similar definitions using WAZ to capture rapid or excessive weight gain were also included. The review included primary experimental studies with randomized, non-randomized, quasi-experimental designs, before-and-after and observational studies reporting on relevant interventions. Eligible studies had to include a control group receiving standard care if appropriate in terms of the study design.
Eligibility criteria for qualitative studies
Eligible qualitative studies included information on intervention deliverers’ or recipients’ experiences with involvement in interventions that aimed to prevent rapid or excessive weight gain during infancy, or information on intervention development, implementation or evaluation processes of such interventions. All types of qualitative study designs were included.
Search strategy
An initial search in PubMed, MeSH database and CINAHL enabled identification of relevant index terms and text words used to develop the final search strategy that consisted of three blocks: (i) study population (Infants), (ii) the phenomenon of interest (RIWG) and (iii) study designs (Quantitative or qualitative) (Supplementary table S1). PubMed, EMBASE, CINAHL, PsycINFO, The Cochrane Library, Web of Science and Scopus were searched using this strategy. Reference lists of all included studies were searched for additional studies. Qualitative search filters were used to identify qualitative studies.20 Searches for unpublished studies were conducted in http://www.opengrey.eu/, http://www.greylit.org/, https://clinicaltrials.gov/, https://www.isrctn.com/ and Research Gate using central keywords. A search for additional information related to relevant trials were conducted using http://www.google.com/. The searches for quantitative and qualitative evidence were conducted on the 31 October 2017 and the 13 February 2018, respectively. These searches were rerun on 31 May 2018 to identify any newly published research.
Study selection, quality appraisal and data extraction
The processes of study selection, critical appraisal and data extraction were conducted and crosschecked by two reviewers working in duplicate and independently. Bibliographic data from each database were imported into Excel, where duplicates were identified using the filter function. Titles and abstracts were initially screened based on relevance. Relevant records were then screened based on full-text where eligibility criteria decided final in- or exclusion. A third reviewer was included to solve disagreements between reviewers. Based on predefined data extraction forms, data on intervention characteristics, settings, outcomes denoting infant weight gain and adverse outcomes were extracted from quantitative papers, and entire result sections were extracted from qualitative papers. Data on process outcomes and informal evidence were extracted from all papers when identified. Quantitative and qualitative data extraction was performed using Excel and NVivo 10, respectively. Quality appraisal of quantitative studies was conducted parallel to data extraction using Cochrane risk of bias tool for randomized controlled trial (RCTs)21 or the ROBINS-I (Risk of Bias in Non-randomized Studies-of Interventions),22 depending on study design. Assessment criteria applied by Rees et al. were used to evaluate quality reliability, trustworthiness and usefulness of qualitative study findings,23 as these are suitable for appraisal of qualitative evidence comprising evaluations of intervention processes.23,24 Use of qualitative study findings in review analyses was weighted based on appraisal. No study was excluded based on poor quality.
Synthesis of included studies
Quantitative evidence on intervention effectiveness was presented in a narrative synthesis that included information on study quality, outcome measures, timing of measurements and effects. Qualitative data on end-users’ and intervention deliverers’ experiences of intervention involvement were analyzed through thematic synthesis as described by Thomas and Harden.25 This was conducted due to a need for translation of concepts across occasionally thin descriptions.19,25 The process of analysis was carried out in NVivo 10 in three steps: (i) identification of initial codes, (ii) development of descriptive themes and (iii) development of analytical themes. Identification of 37 initial codes led to the development of five descriptive themes and further elaboration enabled identification of three analytical themes (Supplementary figure S1). An intervention component analysis (ICA) integrated evidence from all included studies to describe and analyze key intervention features and implementation processes.17 Intervention features and processes were in each study identified through line-by-line coding and presented in a table, where each intervention or intervention arms were presented as individual cases. Thematic synthesis findings guided identification of relevant features and processes, but additional features were inductively identified in the coding process. Further elaboration of feature and process significance were conducted by integrating informal evidence, defined as authors’ accounts and reflections on intervention content, components and processes found in ‘Discussion’ section.17
Results
Search results
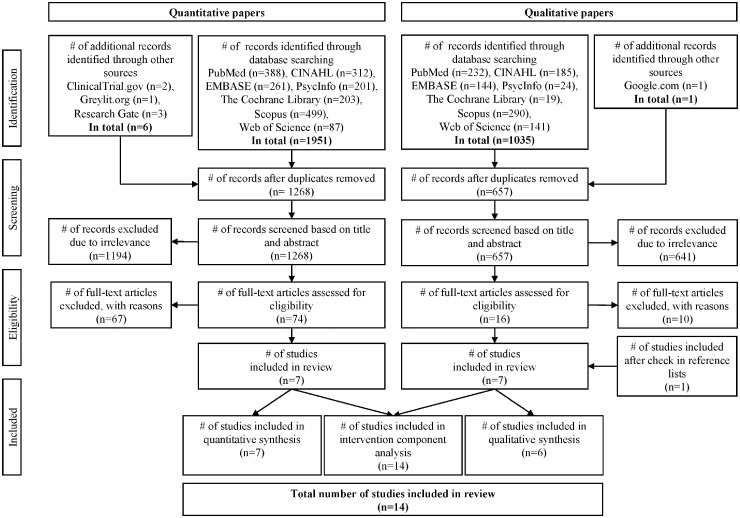
In total, 1957 quantitative studies of which 689 were duplicates and 1036 qualitative studies of which 379 were duplicates were retrieved in the literature search. 67 quantitative studies including three RCTs were excluded after full-text screening for not comprising an intervention (n = 47), not having published results at the time (n = 7), being trial duplicates (n = 6), not applying eligible outcomes (n = 6) and being conducted in a lower middle-income country (n = 1). Ten qualitative studies were excluded for not being related to any relevant intervention (n = 5), not including qualitative data (n = 4) and being a duplicate of included work (n = 1). Seven quantitative and seven qualitative studies, all published in English, were deemed eligible for inclusion (figure 1). Study characteristics are presented in table 1.
Figure 1.
PRISMA flow diagram illustrating the study selection process
Table 1.
Characteristics of included papers
| Authors, year, country, trial name, study design | No. of participants (% of original sample) | Study population, setting and intervention duration | Outcome definition and timing | Intervention content and delivery agent | Risk of biasa |
|---|---|---|---|---|---|
| Narrative syntdesis and ICA | |||||
| Daniels et al., 2012, Australia, The NOURISH RCT, RCT26 | N = 541 (77.5%) | First-time, generally affluent mothers with mean age of 30 years. Healthcare. 3 months | WAZ change and >0.67 change in WAZ (binary 1/0) between 0 and 14 months | Behaviourally focused intervention promoting healthy feeding strategies when introducing solids foods. Delivered by dietitians and psychologists | Low to moderate risk |
| Edmunds et al. 2014, The USA, WIC, Observational study30 | N = 157.590 (−) | Low-income mothers with mean age of 26.6 years. Healthcare and community. 5 years | >0.67 change in WAZ (binary 1/0) between 0 and 12 months | Behaviourally focused and community-based intervention providing nutritious supplemental foods, breastfeeding support, nutrition education, and medical and social referrals. Delivered by health professionals | Moderate risk |
| Karanja et al. 2010, The USA, The TOTS Trial, Two-armed separate sample pretest–posttest study31 | N = 177 (86.3%) | Mothers from a native population with a mean age of 25 years. Home and community. No information on duration | WAZ change between 0 and 24 months | Behaviourally focused and community-based intervention promoting breastfeeding and reducing sugar-sweetened beverages. Delivered by community workers | Moderate to high risk |
| Koletzko et al. 2009, Belgium, Germany, Italy, Poland, and Spain, The CHOP Study, RCT11 | N = 635 (55.8%) | General population of mothers with a mean age of 30 years. Home. 12 months | WAZ change between 0 and 3, 6, 12 and 24 months | Non-behaviourally focused intervention providing low or high protein content formulas. Delivered by researchers | Low risk |
| Lakshman et al. 2018, The UK, The Baby Milk Trial, RCT27 | N = 586 (87.6%) | General healthy formula feeding mothers. Home and healthcare. 6 months | WAZ and >0.67 change in WAZ (binary 1/0) between 0 and 6 and 12 months | Behaviourally focused intervention reducing formula-milk intake, promoting responsive feeding, and monitor growth. Delivered by health professionals | Low risk |
| Paul et al. 2011, The USA, The SLIMTIME Pilot Study, RCT28 | N = 110 (68.8%) | First-time, generally affluent mothers with mean age of 27 years. Home. 6 months | CWG between 0 and 12 months | Behaviourally focused intervention promoting healthy practices in terms of (i) infant sleep and/or (ii) introduction to solid foods. Delivered by health professionals | Moderate to high risk |
| Savage et al. 2016, The USA, The INSIGHT Trial, RCT29 | N = 250 (85.9%) | First-time, generally affluent mothers with mean age of 29 years. Home. 10 months | CWG between 0 and 6 months | Behaviourally focused intervention promoting responsive parenting focusing on infant emotional regulation, feeding, active social play, sleep and growth chart education. Delivered by health professionals | Low to moderate risk |
| Authors, year of publication, country | Relation to included trial | Study aim | Informants and data collection | Method of analysis | Weighting of study findingsa |
|---|---|---|---|---|---|
| Thematic synthesis and ICA | |||||
| Guell et al. 2018, The UK33 | The Baby Milk Trial | To explain some of the underlying mechanisms that might have been at play when implementing and participating in The Baby Milk Trial and shaped its outcome | 10 intervention and 9 control mothers and 3 health professionals contributed in 22 individual interviews | Thematic analysis | High |
| Lakshman et al. 2012, The UK34 | The Baby Milk Trial | To explore the views of healthcare professionals and bottle-feeding mothers on: (i) the Programme for Healthy Growth and Nutrition during infancy; (ii) the trial design for the planned Baby Milk trial and (iii) two draft leaflets | 10 mothers contributed in 3 focus groups discussions and 8 health professionals and one mother contributed in 9 individual interviews | Hierarchical thematic framework | Moderate to high |
| Redsell et al. 2010, The UK39 | No | To explore UK parents’ beliefs on infant’s size, growth and feeding behaviour and parental receptiveness to early intervention aimed at reducing the risk of childhood obesity | 38 parents contributed in six focus groups | Thematic analysis | Moderate to high |
| Redsell et al. 2017, The UK38 | No | To assess the feasibility and acceptability of using digital technology for Proactive Assessment of Obesity Risk during Infancy with the UK health visitors and parents | 12 parents and 15 health professionals contributed in 27 individual interviews | Thematic content analysis | Moderate to high |
| Thébaud 2015, Australia36 | The NOURISH RCT | To develop and apply an evaluation framework based on pre-existing effect and process data collected as part of the NOURISH RCT, an obesity prevention research programme starting early in infancy | 344 mothers responded to questionnaires that included open-ended questions and health professionals ratings of 293 intervention sessions | Thematic content analysis | Low to moderate |
| Valencia et al. 2016, The USA37 | Indirectly (WIC population) | To conduct a formative assessment among the WIC population in Southern Arizona, a group with a high percentage of Latino families, to evaluate mothers’ perceptions of infants’ growth/weight change in early life | 34 mothers and 19 caregivers contributed in 7 focus groups and 6 individual interviews were conducted with health professionals | Grounded theory | Moderate |
| Authors, year of publication, country | Related to included trial | Study aim | Informants and data collection | Method of analysis | Weighting of study findings a |
| ICA only | |||||
| Lakshman et al. 2014, The UK35 | The Baby Milk Trial | To describe the experience of using the 2008 Medical Research Council’s framework to develop and evaluate a theory-based, behavioural infant feeding intervention aimed at preventing childhood obesity, including benefits and challenges of using this framework | Different health professionals and stakeholder-mothers were interviewed using both individual and focus group interviews to inform intervention development | Not specified in paper | Low |
Based on quality appraisal.
WAZ, weight-for-age z-scores; WIC, The Special Supplemental Nutrition Program for Women, Infants and Children; CWG, conditional weight gain.
Narrative synthesis of quantitative evidence
Four individual behaviour change interventions26–29 and three non-behaviourally focused or mixed interventions11,30,31 were identified. Three different outcomes denoting changes in infant weight gain were identified; mean change in WAZ,11,26,27,31 an increase in WAZ of >0.6726,27,30 and conditional weight gain (CWG) scores,28,29 as explained by Griffiths et al.32 All studies, except for The TOTS Trial,31 reported positive intervention effects on at least one outcome. Change in infant weight gain was most frequently measured around the age of 0 and 12 months.11,26–28,30 Two studies reported changes between 0 and 24 months11,31 and three studies between 0 and 6 months.11,27,29 All studies measuring changes between 0 and 6 months reported significant intervention effects,11,27,29 while significant effects were reported in four of five studies measuring changes between 0 and 12 months11,26,28,30 (table 2).
Table 2.
Intervention effectiveness across infant weight gain outcomes
| Mean change in WAZ | |
|---|---|
| Daniels et al. 2012, The NOURISH RCT26 | Between 0 and 12 months of age: ↓ mean change in intervention (0.06 ± 1.06) compared with control infants (0.22 ± 1.06), P=0.05 |
| Karanja et al. 2010, The TOTS Trial31 | Between 0 and 24 months of age: No significant intervention effects when comparing Tribe B and C receiving community and family intervention (0.89 ± 1.46) to Tribe A receiving community intervention only (1.57 ± 1.64), P=0.17 |
| Koletzko et al. 2009, The CHOP Study11 | Between 0 and 6 months of age: ↓ mean change in lower compared with higher protein group (P <0.01, no exact effect estimate provided) |
| Between 0 and 12 months of age: ↓ mean change in lower compared with higher protein group (P <0.01, no exact effect estimate provided) | |
| Between 0 and 24 months of age: No intervention effects (0.12, 95% CI [−0.11 to 0.25], P=0.072) | |
| Lakshman et al. 2018, The Baby Milk Trial27 | Between 0 and 6 months of age: ↓ mean change in intervention compared with control infants (−0.08 95% CI [−0.17 to −0.004]) |
| Between 0 and 12 months of age: No intervention effects (−0.04 95% CI [−0.14 to 0.07]) | |
| Risk of >0.67 SD WAZ | |
| Daniels et al. 2012, The NOURISH RCT26 | Between 0 and 12 months of age: ↑ risk in control compared with intervention infants [Odds ratio (OR) 1.6, 95% CI [1.1–2.4], P=0.008] |
| Edmunds et al. 2014, WIC30 | Between 0 and 12 months of age: ↓ risks in infants enrolled to WIC during first trimester (OR 0.76, 95% CI [0.74–0.79]), second trimester (OR 0.81, 95% CI [0.78–0.84]) and third trimester (OR 0.85, 95% CI [0.82–0.89]) compared with infants enrolled postpartum |
| Lakshman et al. 2018, The Baby Milk Trial27 | Between 0 and 6 months of age: No intervention effects (OR 0.74 95% CI [0.51–1.07]) |
| Between 0 and 12 months of age: No intervention effects (OR 0.84 95% CI [0.59–1.17]) | |
| CWG scores | |
| Paul et al. 2011, The SLIMTIME Pilot Study28 | Between 0 and 12 months of age: ↓ scores in intervention infants receiving sleep/soothe component (−0.394) compared with controls (0.08), P=0.02. No intervention effects of solid food component alone or both components combined compared with controls |
| Savage et al. 2016, The INSIGHT Trial29 | Between 0 and 6 months of age: ↓ scores in intervention infants (−0.18 95% CI [−0.36 to 0]) compared with controls (0.18 95% CI [0.02–0.34]), P = 0.004 |
None of the studies that measured changes between 0 and 24 months reported significant intervention effects. In the three-armed SLIMTIME Pilot Study, significant intervention effects were only observed for the sleep/soothe intervention group when compared with controls.28 The three out of seven studies reporting on possible adverse outcomes did not observe any adverse effects such as insufficient weight gain and downward centile crossing26–28 (Supplementary table S2). The risk of bias in the included studies varied from low to moderate/high across studies (table 1). Inadequate confounder control, deviations from intended intervention, bias in the selection of participants and in relation to missing data were the most common reasons for lower study quality (Supplementary table S3).
Thematic synthesis of qualitative evidence
Five qualitative studies were associated with three of the included intervention studies33–37 (table 1). One of these was a doctoral dissertation that included a process evaluation of The NOURISH RCT.36 Six studies were included in the thematic synthesis as they contained relevant data on end-users’ and intervention deliverers’ experiences in relation to intervention involvement.33,34,36–39 One study contained process-related quantitative data only and was thus not included in the thematic synthesis.35 Study quality varied from low/moderate to high across studies and study findings were weighted accordingly in the synthesis (table 1). Most studies provided appropriate information on sampling strategy, study participants and method of analysis. However, common limitations were lack of transparency in how data supported study findings, lack of data collection tool piloting and lack of both breadth and depth in the presentation of study findings (Supplementary table S3).
Factors affecting parental acceptance and involvement
General misconceptions on infant feeding and growth were observed across studies. Common misconceptions include: all infancy weight gain equals health33,34,37,39 and infants cannot be overfed or obese.34,39 These beliefs, together with the social environment (family, friends and health professionals), influence which parental practices are socially accepted and performed.37,39 Performance of parental practices that are less socially accepted or not seen as medical gold standard, e.g. bottle-feeding, could lead to parents being judged or stigmatized by their social environment.33
… it’s like other parents looking down on you, that are breastfeeding, I found that that was a major thing. If I went to any baby groups, I’d try and make sure that she’d already had a bottle. (33, p. 4, Bottle-feeding mother)
As such, it can be challenging for parents to accept and comply with RIWG interventions that promote practices that conflict with social norms, e.g. not always using feeding as the first response to infant crying, reducing formula-milk intake or preventing excess infant weight gain in general. Conversely, feeding formula can reduce parental anxiety, as parents are able to control the amount of formula given and, thus, better distinguish reasons for infant crying.39
Factors affecting the intervention deliverer and recipient interaction
New parents’ ability to participate in RIWG prevention can be reduced due to multiple commitments to family, work and other life events.36 Frustration of receiving conflicting and non-individualized information, guidance and support from different health professionals were also reported.33,34 This can indicate that parents value flexible and individually tailored interventions involving consistent messaging. Home visits can be an ideal delivery form, but it might be important for intervention acceptance that delivery agents are already familiar to the families.38,39
So I think that then when I said someone else would come in after me, some families were not keen to take part. (38, p. 8, Health professional)
Furthermore, parents reported feeling guilty about bottle-feeding,33,34 which may be why parents involved in The Baby Milk Trial especially valued the non-judgemental support given by health professionals involved in the trial.33
Factors affecting health professionals’ acceptance and involvement
Health professionals often have time constraints and high workloads, which can challenge their opportunities of delivering RIWG interventions as intended.33,37,38 Effort should also be put on matching intervention activities with health professionals’ identities and current practices, as failing to do to can result in compromised fidelity.
You said to discuss one topic, we ended up discussing them all. Because all of those topics are covered in health visiting anyway, to me it didn’t feel right that we talked about diet without exercise and feeding cues. (38, p. 8, Health professional)
Intervention delivery may also be complicated by health professionals’ concerns regarding unintended consequences like introducing obesity risk communication too early and starving babies.38 Early life obesity prevention is also perceived as a sensitive topic, thus intervention delivery can be challenging.
So yes, I feel that I would need more training, because this is such sensitive issues. How do you gently put it to them that they are overweight?(…). (37, p. 530, Health professional)
Thus, some health professionals may need additional training and support to deliver early life obesity prevention. In addition, this may apply for health professionals’ use of growth charts, as they may be underutilized for checking upward percentile crossing and excessive weight gain.34,37 Some healthcare settings also lacked specific guidelines for carrying out early overweight risk identification,37 which indicate RIWG prevention being of low priority on higher organizational and political levels.
Intervention component analysis
All included studies were used to identify features and processes with importance for intervention success. Existence or absence of features was mapped across studies and intervention arms (Supplementary table S4). The following sections present further elaboration of relevant features and processes.
Intervention delivery
All studies reporting early effect-measures (≤6 months) presented positive intervention effects. Fewer studies reporting longer-term effect measures (between 0–12 and 0–24 months) presented positive intervention results. Infancy is characterized by rapid developmental processes. As such, early initiation of prevention activities can be important for creating lasting changes in parental practices that target RIWG prevention before other practices are strongly embedded into everyday life.29,33 In line with this, early enrolment to The Special Supplemental Nutrition Program for Women, Infants and Children (WIC) was associated with lower risk of RIWG compared with infants of parents enrolled postpartum.30 Furthermore, a long time-lag between recruitment and initiation can give parents time to re-evaluate participation during the first months as new parents. This was suggested as an explanation for high attrition rates observed in The NOURISH RCT.36 Conversely, early initiation after recruitment may have secured the low attrition rates observed in The INSIGHT Trial.29 Moreover, few formal intervention contacts could have led to the high attrition rates observed in The CHOP Study.11
All interventions were delivered individually at home, except from The NOURISH RCT, which was delivered through group sessions in a healthcare setting.26 The high attrition rates observed in this trial may be related to group delivery, as it restricts possibilities of personal tailoring compared with individually delivered interventions, which were identified as important by parents in thematic synthesis findings. Group delivery may also challenge parents need for flexibility, as they are required to travel and meet at a certain time and place.
Intervention content
Most interventions were multifaceted. Providing responsive parenting training was a recurrent component.26–29 Increasing parental responsiveness in feeding situations may be important, as parents, supported by peers and grandparents, could overattribute hunger as explanation for crying.39 Thus, some parents need to strengthen their ability to explore alternative explanations for infant distress as a means to prevent overfeeding and excessive weight gain. In two effective interventions, growth charts were used to communicate early life obesity risks.27,29 This strategy might be effective, as some parents have poor abilities to, and few concerns about, recognizing and acknowledging their own child’s obesity risk.39 Such risk communication should be combined with culturally relevant education and support on infant feeding and growth to have the most impact.37 However, thematic synthesis findings indicate that some health professionals would need additional training on how to use growth charts for such purposes. The variability in intervention components applied and how these are combined weaken the evidence base on effective component combinations, although most studies reported some positive intervention effects.
Intervention development
Higher attrition rates were observed in less educated, younger and single parents in several trials.11,26,28,29 These are population groups associated with the highest prevalence of RIWG,40 and attrition in high-risk groups contributes to existing uncertainties about how RIWG interventions actually work for groups with the greatest need for these initiatives. Low risk perception, lack of subject prioritization, lack of time and resources needed to commit to interventions, and high expectations of negative experiences of participating have been suggested as reasons why there is low interest in intervention involvement in these groups.36
Increasing interest may be achieved by involving deliverers and recipients in development processes. This was performed in The Baby Milk Trial,34,35 which resulted in trial communication messages focusing on healthy growth instead of obesity prevention, in addition to emphasis on delivery through a client-centred and non-judgemental communication style,35 and no socioeconomic differences were observed in trial attrition rates.27 Lack of stakeholder involvement may lead to development of interventions that mainly reflect researchers’ perspectives, which can appear unfamiliar and meaningless for groups with other life conditions.36 Consultancy work was also conducted in the formative phase in The TOTS Trial,31 which could have contributed to the high participation rates.
Intervention contextual factors
Having health professionals delivering interventions may itself initiate complex processes due to personal and relational factors. Accordingly, it can be important to consider the value parents place upon their health professional relation when designing interventions.39 Relational processes can influence intervention effectiveness and implementation, but they may be difficult to disentangle. For instance, some mothers in The Baby Milk Trial were possibly reluctant to tell health professionals that they bottle-fed due to worries of being judged, which could have challenged identification and recruitment of bottle-feeding mothers.33 Identification and recruitment of eligible parents may also be compromised by health professionals’ own evaluation of parents’ suitability and eligibility, as they may choose not to contact eligible parents if they judge them unsuitable for inclusion.38
Only The Baby Milk Trial was explicitly informed by theory. While informed by social cognitive theory, the trial failed to produce longer-term effects,27 and the authors suggest that their use of psychologically oriented theory could have been inefficient for addressing problem complexity.33 Thus, application of theories with broader foci, such as socio-ecological models,41 may be needed to address the complex nature of RIWG. In line with socio-ecological thinking, Guell et al. suggest that changes should be made on higher level determinants defining social norms to help parents overcome stress of going against socially accepted practices when preventing RIWG.33 Creating supportive environments on several levels can be key to promote intervention effects. This is supported by informal evidence suggesting that future interventions should emphasize building constructive and enduring partnerships and collaborations between healthcare sectors, professionals and researchers.31,38
Discussion
Key findings
The application of three different definitions of RIWG and several different timings of measurement challenged elaboration of intervention effectiveness across studies. Most intervention studies reported small but significant effects. All three studies measuring weight gain between 0 and 6 months of age reported at least one significant effect measure, and all but one study with similar measures between 0 and 12 months reported positive effects. Notably, no intervention effects were observed in the two studies measuring weight gain between 0 and 24 months.11,31 This may indicate that included intervention strategies target mechanisms with importance for infant weight gain during the first, but not the second year of life. Several of these strategies comprise early infant feeding factors, which may be less relevant for weight gain when infants grow older.
It is, however, unclear if and how short-term effects on weight gain affect later risk of developing COO. Long-term effects on COO risk have been explored for The CHOP Study10 and The NOURISH RCT,42,43 where only The CHOP Study reported a significantly lower risk of obesity. Interestingly, this study reported significant changes in infant weight gain between 0–6 and 0–12 months, but not between 0 and 24 months,11 followed by significant lower mean BMI and lower risks of obesity at age 6 years.10 These findings could indicate that provision of formula with reduced protein content, a structural rather than a behavioural strategy, is effective in preventing both increased weight gain in early infancy and obesity in childhood.
Findings from synthesizing qualitative evidence showed that parents ideally request tailored, but consistent, support, information and guidance delivered flexibly in a non-judgementally manner by known relations. Findings from the ICA support the use of home delivery to meet these parental needs. A preceding concept paper suggests that home visiting structures can be ideal for obesity prevention delivery due to their potential of being cost-effective and sustainable, as well as reaching low-income infants and families with high-risk of COO.44 These groups can be hard to reach and retain in intervention studies, as shown by social differences in attrition rates across studies delivered at home and in healthcare settings in the current review. Exploration and integration of recipient views and requests on intervention delivery resulted in emphasis on a non-judgemental communication style in The Baby Milk Trial. This trial showed no social differences in attrition rates, which could suggest that broad involvement of participants in intervention development processes can be essential for keeping different types of participants interested in continued intervention engagement.
The effects of an intervention can potentially be moderated by different contextual factors.45 The great variability in components applied and combinations thereof made it impossible to draw conclusions on how specific intervention features and content were related to effectiveness. However, review results identified some social and contextual factors that could influence intervention effectiveness and implementation processes. Some beliefs and social norms tended to support infant weight gain in general, and a professional focus on promoting sufficient weight gain may overshadow any importance of preventing RIWG. This could work against professional’s and parental acceptability of delivering and receiving RIWG prevention activities. In addition, early life obesity prevention was evaluated as a sensitive topic by some health professionals and this can complicate RIWG prevention delivery. Some health professionals may be anxious about bringing up the topic with parents and thus some may need additional training and support prior to doing so. These challenges indicate that using health professionals as intervention deliverers adds an additional dimension of complexity that should be considered when planning and evaluating such interventions. In general, consideration of social, institutional and community factors should identify barriers that need to be addressed to create environments supportive of RIWG prevention activities. This is essential for intervention effectiveness.
Strength and limitations
Some limitations of the review should be acknowledged. It is possible that relevant results have been missed despite the comprehensiveness of the search. No indexed keywords existed for RIWG and using only text words to identify this phenomenon may have compromised the precision of the search. However, using a three-stepped search strategy involving identification of words used in current literature led to identification of a range of relevant text words. In addition, a search of grey literature enabled identification of ongoing trials, and relevant trials were tracked during the review process, so the review included the latest research. Nevertheless, the coverage of the search for grey literature may have been insufficient, as few relevant citations were identified and only one of these was eligible for inclusion in the review.36
The risk of bias varied across intervention studies and were generally higher in community-based interventions,30,31 which could reflect a challenge of reducing bias when intervening in more complex settings. Thus, the evidence on the effect of community-based interventions is weak compared with the evidence on behaviourally focused interventions. Due to the nature of the interventions, The CHOP Study was the only double-blinded intervention,11 however, outcome assessors were blinded in most trials. Attrition bias may compromise the ability to generalize review results onto groups with low SEP. Most interventions were carried out in the USA and the UK, and thus generalizability may be restricted to similar contexts. Application of these review findings should be refined and adjusted to the context in which interventions are delivered to enhance the probability of intervention success.45,46
The quality of the qualitative evidence was generally high, but some studies included data of low quality, such as data collected from open-ended questionnaires36 or data with less transparent audit trails.35 No studies were excluded due to poor quality to consolidate as much knowledge as possible on this new and evolving field. Nevertheless, the qualitative evidence was analyzed with regard to the critical appraisal to enhance the validity of the review findings. Furthermore, validated appraisal tools do not exist for informal evidence which limits our ability to judge the validity of such information. The lack of formal process evaluations of included trials may, however, justify the use of informal evidence, as this type of information may then represent current best evidence.17
Implications
Several context and process factors were identified as potentially influential on intervention success, such as the significance of early intervention initiation after recruitment for preventing attrition. However, the low number of relevant interventions and the heterogeneity between them leaves uncertainties on how effectiveness relates to intervention content, components and timing. Most included interventions were initiated after birth, but earlier initiation during pregnancy could have additional value as it widens the window for intervening. Prenatal provision of support and guidance can be important for reducing RIWG risk, as observed in the study of timing of enrolment to WIC.30 Here, infants of mothers enrolled prenatally to WIC, compared with postnatally, were associated with lower RIWG risk. A previous review identified anticipatory guidance as an important strategy for amending early life parental behaviours preventive of COO, such as breastfeeding and timing of introduction to solid foods.47 More research on timing for intervention initiation and intervention content and components are needed to explore how these findings relate to RIWG prevention. An important focus for further research is also to identify the long-term effects of RIWG prevention on COO to clarify the value of these prevention strategies as means of early life obesity prevention. Follow-up on recent and on-going trials will hopefully provide such long-term results.
Review findings also indicate a need for understanding the context of intervention delivery and its actors. Certain beliefs and social norms regarding infant weight gain can result in low levels of readiness and acceptability of RIWG prevention activities in both deliverers and recipients. Increasing readiness and acceptability in these key stakeholders can be an important next step to accelerate early life obesity prevention through a focus on preventing RIWG. More research is needed on exploring professionals’ needs in terms of additional education, training and support, so the right support can be provided. Furthermore, parental, professionals and organizational values and views should be considered during intervention development processes. This can be accomplished through stakeholder involvement to support development of RIWG prevention activities that are meaningful and feasible on multiple levels. Most included studies embraced psychologically oriented behaviour change theories, thus potentially ignoring the importance of environmental factors. More substantive use of ecological theories during these processes may potentially support identification of important contextual and environmental factors. Applying an ecological lens on RIWG as a problem also implies a focus on undertaking non-agentic and environmental level changes that support lower-level changes.41 As such, more RIWG prevention research should emphasize non-behavioural interventions or public health policy changes.
Conclusion
Prevention of RIWG as a part of early life obesity prevention is a new and evolving research field. The existing evidence base on RIWG prevention is generally weak, though most interventions produced small, but significant changes in infant weight gain. More interventions reported significant results on change in infant weight gain during the first year, compared with the second year of life. Future intervention programmes may advantageously offer parents non-judgemental support delivered in a flexible manner by trusted relations, be initiated quickly after recruitment, take into account the norms, values and beliefs operating in the delivery context, and provide a sufficient amount of resources to intervention deliverers, such as time, training and support. Effort should be spent on reaching and sustaining participation of groups in low resource settings. More knowledge on how RIWG prevention affects long-term COO risk is also needed.
Supplementary Material
Acknowledgements
The authors would like to thank Mette Buje Grundsøe, MLISc, Aalborg University Library, for specialized knowledge and support regarding the systematic searches for this review.
Funding
The project is funded by Aalborg University, Denmark. The work was undertaken with the support of The Centre for the Development and Evaluation of Complex Interventions for Public Health Improvement (DECIPHer), a UKCRC Public Health Research Centre of Excellence. Joint funding (MR/KO232331/1) from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, the Welsh Government and the Wellcome Trust, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged.
Conflicts of interest: None declared.
Key points
Prevention of rapid infant weight gain as a means for early life obesity prevention is a new and evolving field.
Intervention strategies tend to be more effective on infant weight gain during the first year, compared with the second year of life.
Social norms and beliefs about infant weight gain can challenge intervention acceptance.
Parents request tailoring, flexibility and consistency in intervention activities, thus home delivery can be key.
Health professionals’ identity and everyday practices should be considered during intervention development if they are used as intervention deliverers.
References
- 1. Monteiro POA, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev 2005;6:143–54. [DOI] [PubMed] [Google Scholar]
- 2. Zheng M, Lamb KE, Grimes C, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev 2018;19:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leunissen RWJ, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009;301:2234–42. [DOI] [PubMed] [Google Scholar]
- 4. Lobstein T, Baur L, Uauy R, Force I. Obesity in children and young people: a crisis in public health. Obes Rev 2004;5(Suppl):4–104. [DOI] [PubMed] [Google Scholar]
- 5. Singh AS, Mulder C, Twisk JWR, et al. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9:474–88. [DOI] [PubMed] [Google Scholar]
- 6. Waters E, de Silva-Sanigorski A, Burford BJ, et al. Interventions for preventing obesity in children. Cochrane database. Syst Rev 2011;7:CD001871. [DOI] [PubMed] [Google Scholar]
- 7. Wood CT, Skinner AC, Yin HS, et al. Bottle size and weight gain in formula-fed infants. Pediatrics 2016;138:e20154538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mihrshahi S, Battistutta D, Magarey A, Daniels LA. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr 2011;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Appleton J, Russell CG, Laws R, et al. Infant formula feeding practices associated with rapid weight gain: a systematic review. Matern Child Nutr 2018;14:e12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weber M, Grote V, Closa-Monasterolo R, et al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr 2014;99:1041–51. [DOI] [PubMed] [Google Scholar]
- 11. Koletzko B, Kries V, Closa R, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Int J Obes Relat Metab Disord 2009;89:1836–45. [DOI] [PubMed] [Google Scholar]
- 12. Reeske A, Spallek J, Bammann K, et al. Migrant background and weight gain in early infancy: results from the German study sample of the IDEFICS study. PLoS One 2013;8:e60648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oyama M, Nakamura K, Tsuchiya Y, Yamamoto M. Unhealthy maternal lifestyle leads to rapid infant weight gain: prevention of future chronic diseases. Tohoku J Exp Med 2009;217:67–72. [DOI] [PubMed] [Google Scholar]
- 14. Wijlaars L, Johnson L, van Jaarsveld CHM, Wardle J. Socioeconomic status and weight gain in early infancy. Int J Obes (Lond) 2011;35:963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Redsell SA, Edmonds B, Swift JA, et al. Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Matern Child Nutr 2016;12:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cauchi D, Glonti K, Petticrew M, Knai C. Environmental components of childhood obesity prevention interventions: an overview of systematic reviews. Obes Rev 2016;17:1116–30. [DOI] [PubMed] [Google Scholar]
- 17. Sutcliffe K, Thomas J, Stokes G, et al. Intervention Component Analysis (ICA): a pragmatic approach for identifying the critical features of complex interventions. Syst Rev 2015;4:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 19. Tong A, Flemming K, McInnes E, et al. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol 2012;12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glanville J, Lefebvre C, Wright K. Filters to Identify Qualitative Research [Internet]. York, UK: The InterTASC Information Specialists’ Sub-Group, 2018.
- 21. Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available at: http://handbook.cochrane.org. [Google Scholar]
- 22. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rees R, Oliver K, Woodman J, Thomas J. The views of young children in the UK about obesity, body size, shape and weight: a systematic review. BMC Public Health 2011;11:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rees R, Caird J, Dickson K, et al. ‘It’s on your conscience all the time’: a systematic review of qualitative studies examining views on obesity among young people aged 12–18 years in the UK. BMJ Open 2014;4:e004404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol 2008;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daniels LA, Mallan KM, Battistutta D, et al. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. Int J Obes 2012;36:1292–8. [DOI] [PubMed] [Google Scholar]
- 27. Lakshman R, Sharp SJ, Whittle F, et al. Randomised controlled trial of a theory-based behavioural intervention to reduce formula milk intake. Arch Dis Child 2018;103:1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paul IM, Savage JS, Anzman SL, et al. Preventing obesity during infancy: a pilot study. Obesity 2011;19:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savage J, Birch L, Marini M, et al. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatr 2016;170:742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edmunds LS, Sekhobo JP, Dennison BA, et al. Association of prenatal participation in a public health nutrition program with healthy infant weight gain. Am J Public Health 2014;104(Suppl 1):S35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karanja N, Lutz T, Ritenbaugh C, et al. The TOTS community intervention to prevent overweight in American Indian toddlers beginning at birth: a feasibility and efficacy study. J Community Health 2010;35:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griffiths LJ, Smeeth L, Hawkins SS, et al. Effects of infant feeding practice on weight gain from birth to 3 years. Arch Dis Child 2009;94:577–82. [DOI] [PubMed] [Google Scholar]
- 33. Guell C, Whittle F, Ong KK, Lakshman R. Toward understanding how social factors shaped a behavioral intervention on healthier infant formula-feeding. Qual Health Res 2018;28:1320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lakshman R, Landsbaugh JR, Schiff A, et al. Developing a programme for healthy growth and nutrition during infancy: understanding user perspectives. Child Care Health Dev 2012;38:675–82. [DOI] [PubMed] [Google Scholar]
- 35. Lakshman R, Griffin S, Hardeman W, et al. Using the Medical Research Council framework for the development and evaluation of complex interventions in a theory-based infant feeding intervention to prevent childhood obesity: the baby milk intervention and trial. J Obes 2014;2014:646504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thebaud V. Effect and Process Evaluations of an Early Childhood Obesity Prevention Intervention. Queensland University of Technology, Australia, 2015. [Google Scholar]
- 37. Valencia AC, Thomson CA, Duncan B, Arthur A. Evaluating Latino WIC mothers’ perceptions of infant’s healthy growth: a formative assessment. Matern Child Health J 2016;20:525–33. [DOI] [PubMed] [Google Scholar]
- 38. Redsell SA, Rose J, Weng S, et al. Digital technology to facilitate Proactive Assessment of Obesity Risk during Infancy (ProAsk): a feasibility study. BMJ Open 2017;7:e017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Redsell S, Atkinson P, Nathan D, et al. Parents’ beliefs about appropriate infant size, growth and feeding behaviour: implications for the prevention of childhood obesity. BMC Public Health 2010;10:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boone-Heinonen J, Messer L, Andrade K, Takemoto E. Connecting the dots in childhood obesity disparities: a review of growth patterns from birth to pre-adolescence. Curr Epidemiol Rep 2016;3:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.