Abstract
Male mice emit ultrasonic vocalizations (USVs) in response to the presence of female mice and their urine. Male USVs attract females, enhancing female reproductive functions, and are thus considered as the courtship song. Previous studies have shown that female mice exhibit disassortative social preferences for male USVs. However, it remains unclear what acoustic features female mice use for the development of these preferences. To address this, we examined social preferences of female C57BL/6 and BALB/c mice using the three-chamber preference test using recorded male USVs. To dissociate the peak frequencies of these USVs from their syllable structure, we digitally manipulated the peak frequencies accordingly. We found that female mice preferred USVs that were dissimilar to those of their own strain. We also observed that, while female C57BL/6 mice were sensitive to changes in the syllable structure and the peak frequency, female BALB/c mice were sensitive to differences in the syllable structure. Our results demonstrate that female C57BL/6 and BALB/c mice differently use the acoustic features such as the peak frequency and the syllable structure for exhibiting disassortative social preferences.
Keywords: BALB/c mice, behavior, C57BL/6 mice, social preference, ultrasonic vocalization
Introduction
Male mice emit ultrasonic vocalizations (USVs) in response to the presence of female mice and their urine [13, 18]. Male USVs attract female mice [2, 5, 11, 15] and enhance female reproductive functions [2]. Thus, the USVs of male mice are thought to serve as a courtship song. Previous studies have shown that female wild-derived mice showed preferences towards the USVs of novel mice over those of familiar mice or siblings [9], and that female laboratory mice prefer USVs that are from a different strain in the preference test [1]. These findings suggest that female mice exhibit disassortative social preferences for male USVs.
Acoustic features of USVs such as average peak frequency and the composition of syllables are variable between several strains of laboratory mice. We have shown that the strain-specific features of male USVs are not changed by cross-fostering procedures, suggesting that they are genetically controlled [8]. A recent study has shown that each individual in the F1 generation of wild-caught mice has distinct acoustic features [6]. These observations suggest that USVs could serve as an individual signature, raising the possibility that female mice may use information conveyed via USVs for the development of disassortative social preferences.
However, it remains unclear what acoustic features of male USVs female mice use for exhibiting social preferences. To address this, we performed the three-chamber preference test using playback sounds of male USVs, focusing on two acoustic features: the peak frequency and the syllable structure. We used two laboratory mouse strains, C57BL/6 and BALB/c, the USVs of which are known to have distinct acoustic characteristics [8]. While male C57BL/6 mice frequently use “jump” and “upward” syllables with a peak frequency of ~74 kHz, male BALB/c mice frequently use “chevron” and “harmonics” syllables with a peak frequency of ~60 kHz. In order to dissociate the peak frequencies of the USVs from their syllable structure, we digitally manipulated the former without changing the latter.
Materials and Methods
Animals
We used 47 C57BL/6JJcl and 43 BALB/cAJcl female mice (originally purchased from CLEA Japan, Inc., Tokyo, Japan, and bred in the laboratory), which will be referred to as C57BL/6 and BALB/c hereafter, respectively. Each female mouse was tested once. Groups of two to five animals were housed together (cage size, 25 × 18 × 13 cm; temperature, 23°C; humidity, 50%; 12:12 light/dark cycle; lights on at 06:00 am). Food and water were available ad libitum. All procedures were performed in accordance with the guidelines of “Policies Governing the Use of Live Vertebrate Animals” of Azabu University, and approved by The Ethical Committee for Vertebrate Experiments of Azabu University (ID #160303-6). All females were sexually naïve. The estrous stage of female mice was monitored daily by vaginal lavage. Diestrus female mice were used in the behavioral experiments, because our previous study showed that female mice exhibited social preferences about male USVs during diestrus stages [1].
Preparation of USVs for playback
One male C57BL/6 mouse and one male BALB/c mouse were used for recording USVs. To promote vocalizations, we used one female C57BL/6 mouse and one female BALB/c mouse, which were ovariectomized and devocalized [1, 11, 12], and allowed at least two weeks for recovery. Sexual receptivity was induced by injecting 17-β estradiol in corn oil (0.05 ml at a concentration of 0.4 mg/ml) 48 and 24 h prior to the USV recording session, and progesterone in corn oil (0.03 ml at a concentration of 10 mg/ml) four hours before the USV recording session. During USV recording sessions, either the C57BL/6 or the BALB/c male mouse was paired with the female mouse of the same strain in a sound-attenuated chamber. A microphone (CM16/CMPA, Avisoft Bioacoustics, Glienicke/Nordbahn, Germany) was placed next to the mesh hole (diameter, 6 cm) in the wall of the chamber. The USVs were digitally converted, filtered using a 20–145.8 kHz band-pass filter, and recorded at a sampling rate of 300 kHz (UltraSoundGate 416-200, Avisoft Bioacoustics). A fragment of 20 s was extracted from the original USV sound file such that a fragment contained typical features of the USVs of each strain in terms of the syllable composition (Adobe Audition 3, Adobe, San Jose, CA, USA). Modified USVs were created by digitally changing the peak frequencies of C57BL/6 and BALB/c USVs (Adobe Audition 3). Ambient noises were digitally reduced. The acoustic features of four types of USVs were as follows (Avisoft-SASLab Pro, Avisoft Bioacoustics; peak frequencies and amplitudes of syllables are shown as mean ± SD; the peak amplitude is expressed in decibels relative to full scale (dBFS)): original C57BL/6 USVs (termed B6-74; 123 syllables; peak frequency, 73.3 ± 7.3 kHz; peak amplitude, −28.5 ± 8.3 dBFS), modified C57BL/6 USVs (termed B6-60; 123 syllables; peak frequency, 59.6 ± 4.7 kHz; peak amplitude, −29.0 ± 8.5 dBFS), original BALB/c USVs (termed BC-60; 108 syllables; peak frequency, 61.2 ± 7.2 kHz; peak amplitude, −29.2 ± 10.6 dBFS), and modified BALB/c USVs (termed BC-74; 108 syllables; peak frequency, 75.0 ± 8.2 kHz; peak amplitude, −29.5 ± 11.3 dBFS). Spectrograms of four types of USVs were computed by the fast Fourier transform algorithm (Fig. 1; Matlab, Mathworks). We performed the preference tests between B6-60 and B6-74, BC-60 and BC-74, B6-74 and BC-74, and B6-60 and BC-74. The first two tests were designed to examine the effects of the peak frequencies for both C57BL/6 and BALB/c strains. The third test was designed to examine the effects of the syllable structure independently of the peak frequency. The fourth test was designed to examine the effects of the syllable structure when both C57BL/6 and BALB/c USVs were digitally manipulated.
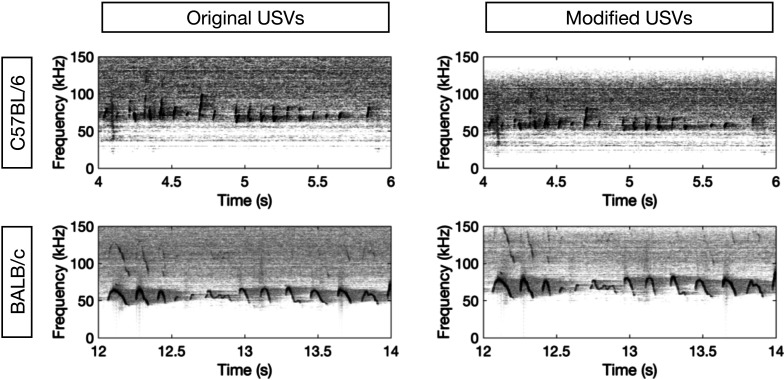
Fig. 1.
Spectrograms for four types of male USVs. Spectrograms of 2-s fragments of four types of male USVs are shown (the horizontal axis indicates time, the vertical axis indicates frequency, darkness represents amplitude). The upper row shows C57BL/6 USVs and the lower row shows BALB/c USVs. The left column shows the original USVs and the right column shows the modified USVs.
Behavioral apparatus
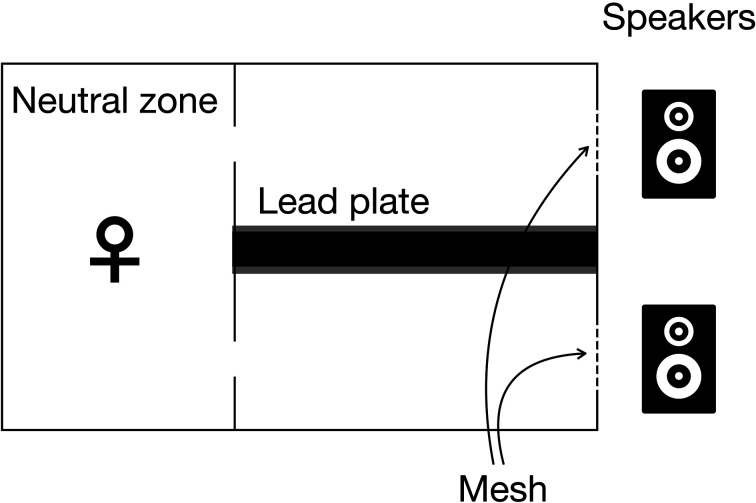
The behavioral experiments were performed in an acrylic apparatus (35 × 20 × 20 cm; Fig. 2), which consisted of three zones. The largest zone (15 × 20 × 20 cm; designated as ‘neutral zone’) was connected to two smaller zones (20 × 10 × 20 cm each; designated as ‘speaker zone’). Speakers (nc-Si emitters [17]; Kato Acoustics Consulting Office, Yokohama, Japan) were placed behind copper meshes (hole diameter, 6 cm) that were located at the end of the speaker zones. Lead plates were attached to the wall between two speaker zones such that the USVs played back in one zone were largely attenuated in the other zone.
Fig. 2.
Schematic illustration of the behavioral apparatus.
Behavioral paradigm
The behavioral experiments were performed in the above described, sound-attenuated box at 5:00 pm or later as previously described [1]. The female mouse was first habituated to the neutral zone for 15 min with the entrances to the speaker zones closed by means of acrylic plates. The female mouse was habituated further for 30 min with the entrances open, during which it was exposed to a cotton ball containing bacterial recombinant exocrine gland-secreting peptide 1 (ESP1; 20 µg) in Tris buffer. ESP1 is a male-specific pheromone that is released into tear fluids [4]. In our previous study, we found that preferences of female mice for the USVs from a different strain were only observed when these mice were concomitantly exposed to ESP1 [1]. Behavioral experiments were initiated when the female mouse was located in the neutral zone, after having explored both speaker zones and both meshes. Two recorded USVs were played back simultaneously during behavioral experiments. Sound levels of the speakers were calibrated by using the same USVs file and the same microphone before each experiment such that the speakers reproduced the acoustic features of the recorded USVs sound. The USV played back from each speaker was counterbalanced across animals. The experiments were monitored by a video camera positioned at the top of the apparatus. The obtained videos were analyzed offline, measuring the number of entries into the speaker zones and the time spent exploring the mesh in front of the speaker. The time exploring the mesh was termed as song searching time.
Statistics
All statistical analyses were performed using GraphPad Prism 8. Potential differences between groups were assessed using Wilcoxon signed rank test. The significance level was set to P˂0.05.
Results
While male C57BL/6 mice emit USVs with a peak frequency of ~74 kHz, male BALB/c mice emit USVs with a peak frequency of ~60 kHz. To determine which acoustic feature of the USVs influences social preferences shown by female mice, we performed a preference test between two USVs. We used the originally recorded USVs as well as USVs the peak frequencies of which were digitally manipulated.
Social preferences of female C57BL/6 mice
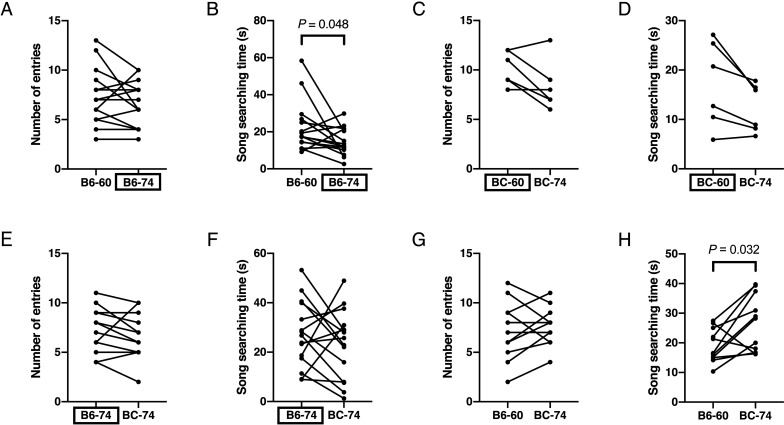
First, we investigated social preferences of female C57BL/6 mice, presenting the USVs from a single mouse at two different peak frequencies (60- and 74-kHz). We found that female C57BL/6 mice showed longer song searching times for B6-60 as compared with B6-74 (P=0.048, Wilcoxon signed rank test; n=15), but no change in the number of entries (Figs. 3A and B). When BC-60 and BC-74 were played, we did not observe any differences in the number of entries or the song searching time (n=6; Figs. 3C and D).
Fig. 3.
USV-associated social preferences of female C57BL/6 mice. Vertical axes indicate number of entries to speaker zones (A, C, E, G) and song searching time (B, D, F, H). Mice were given the choice between B6-60 and B6-74 (A, B), BC-60 and BC-74 (C, D), B6-74 and BC-74 (E, F), and B6-60 and BC-74 (G, H). Boxed labels in horizontal axes indicate the original USVs.
Presenting female C57BL/6 mice with B6-74 and BC-74, we did not observe any differences in the number of entries or the song searching time (n=15; Figs. 3E and F).
Next, the peak frequencies of both USVs were manipulated, so that female C57BL/6 mice were presented with B6-60 and BC-74. Our results demonstrate that these mice showed significantly longer song searching time for BC-74 as compared with B6-60 (P=0.032, Wilcoxon signed rank test, n=11), but no change in the number of entries (Figs. 3G and H).
Social preferences of female BALB/c mice
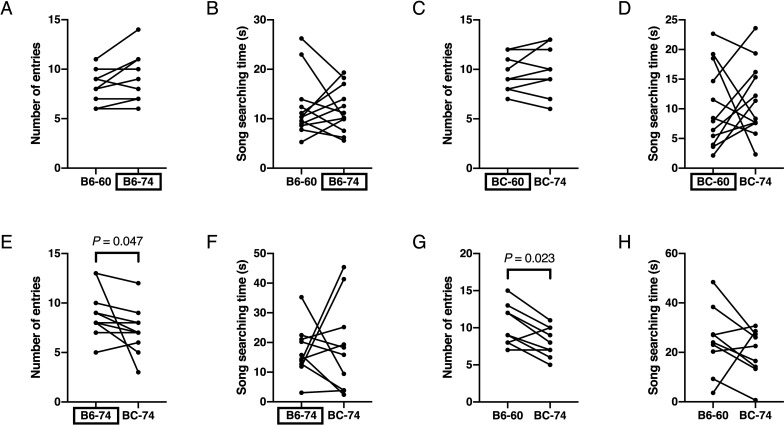
Next, we examined USV-associated social preferences of female BALB/c mice in the same manner as described above. When B6-60 and B6-74 were played, we did not observe any differences in the number of entries or the song searching time (n=12; Figs. 4A and B). Similarly, in response to BC-60 and BC-74, female BALB/c mice did not show any differences in the number of entries or song searching time (n=12; Figs. 4C and D).
Fig. 4.
USV-associated social preferences of female BALB/c mice. Vertical axes indicate number of entries to speaker zones (A, C, E, G) and song searching time (B, D, F, H). Mice were given the choice between B6-60 and B6-74 (A, B), BC-60 and BC-74 (C, D), B6-74 and BC-74 (E, F), and B6-60 and BC-74 (G, H). Boxed labels in horizontal axes indicate the original USVs.
There were also no differences in the song searching time upon playback of B6-74 and BC-74; nevertheless, female BALB/c mice entered the area associated with B6-74 more frequently as compared to that associated with BC-74 (P=0.047, Wilcoxon signed rank test, n=10; Figs. 4E and F).
When mice were given the choice between B6-60 and BC-74, we found that they entered the area associated with B6-60 more frequently as opposed to BC-74 (P=0.023, Wilcoxon signed rank test; n=9), but did not show any differences in song searching time (Figs. 4G and H).
Discussion
In the present study we investigated what acoustic features of the USVs of male mice affect social preferences of female mice. We found that female mice preferred USVs that were dissimilar to the USVs of their own strain. We also found that, while female C57BL/6 mice were sensitive to changes in the syllable structure and the peak frequency, female BALB/c mice were sensitive to differences in the syllable structure. These results suggest that different strains of female mice differently use acoustic features for exhibiting social preferences.
Female C57BL/6 mice displayed significantly longer song searching times for B6-60 than for B6-74. These observations indicate that, when familiar syllables were used for a choice, these mice prefer the USVs with unfamiliar peak frequencies, providing supporting evidence for disassortative social preferences. When these mice were given the choice between BC-60 and BC-74, they did not exhibit social preferences, raising the possibility that these mice were not sensitive to the peak frequencies of unfamiliar syllables. When female mice were given a choice between B6-60 and BC-74, these mice displayed significantly longer song searching time for BC-74 than for B6-60, suggesting that the syllable structure also affects the social preferences of these mice. When these mice were given a choice between B6-74 and BC-74, they did not display social preferences, which seemed to violate transitivity of preferences shown in the other three tests. Although the underlying mechanisms are not clear, our results suggest that social preferences for male USVs are determined based on several acoustic features in a non-transitive manner.
Female BALB/c mice, on the other hand, did not show significantly different song searching times in all experiments. Instead, they entered the chamber associated with C57BL/6 vocalizations more frequently than that associated with BALB/c vocalizations, when a choice was given between B6-74 and BC-74 or between B6-60 and BC-74. These results suggest that female BALB/c mice prefer the syllable structure of C57BL/6 USVs over that of BALB/c USVs regardless of the peak frequency.
The present study indicates that female mice of both C57BL/6 and BALB/c strains exhibit social preferences in a disassortative manner. Disassortative mating refers to the type of mating in which one animal mates with another animal that is not similar to itself, which is thought to be helpful in facilitating genetic diversity. It is suggested that mice use olfactory information for disassortative mating to avoid inbreeding [19]. Additionally, consistent with our results, recent studies have shown that female mice are also able to use auditory information for disassortative preferences [1, 9]. By contrast, a previous study using F1 offspring of wild-caught mice showed that female Mus musculus musculus did not differentiate between the USVs of their own subspecies and those of another subspecies, Mus musculus domesticus [10], which seems inconsistent with the disassortative preference hypothesis. The discrepancy between findings may be explained by differences in the recording of USVs. Musolf et al. discussed that, since they used pools of male USVs derived from several male mice [10], the individual differences in the USVs might mask the difference in the USVs of two subspecies. Because we used the USVs from only one individual mouse per strain, it was possible that female mice were able to easily discriminate the USVs of their own strain from those of the other strain, making the assessment of disassortative social preferences more feasible.
Recent studies have started to elucidate how social preferences of male USVs by female mice are shaped. Our previous study has shown that disassortative preferences by female mice can be reversed by cross-fostering procedures [1], suggesting that social preferences of female mice are flexible rather than strictly inherent. Since, in the present study, female mice were housed together with their fathers, weaned at postnatal day (PD) 28, and housed in same-sex groups thereafter in our previous study, female mice were not exposed to male USVs after weaning. This implicates that female mice acquire social preferences associated with male USVs before PD28, possibly through hearing the USVs of their fathers. Interestingly, previous studies reported that exposure to music between PD10 and PD20 [7], or PD15 and PD24 [20], changed acoustic preferences of mice during adulthood in a way that they tolerated that particular music. Consequently, there is a critical period before the time of weaning that affects auditory preferences. It is tempting to speculate that female mice are imprinted with the USVs of males that they are surrounded by between PD15 and PD24, and that, during adulthood, they prefer dissimilar USVs to those which they have heard prior to weaning.
We also demonstrate that the acoustic features that influence social preferences of female mice are different between C57BL/6 and BALB/c strains. While social preferences of female C57BL/6 mice were influenced by the syllable structure and the peak frequency of the USVs, those of female BALB/c mice were influenced to a greater extent by the syllable structure of the USVs. Currently, the underlying mechanisms are not clear. One possibility is that the auditory sensitivity could be different between C57BL/6 and BALB/c offspring. Although many mouse strains such as NMRI, C57BL/6 and BALB/c are known to suffer from aging-associated hearing loss [3, 14], there is little evidence that the auditory sensitivity is different between C57BL/6 and BALB/c offspring, rendering this possibility unlikely. It may also be conceivable that the acoustic preferences of the offspring are shaped differently based on the differences in the C57BL/6 and BALB/c USVs. Male C57BL/6 mice frequently use “jump” and “upward” syllables, both of which are of short duration and often contain sudden, sometimes discontinuous, changes in the frequency. These characteristics may allow for C57BL/6 offspring to focus on the frequency of the USVs, making these mice more sensitive to the peak frequency in the adulthood. Future studies, in which the syllable composition of the USVs to which the offspring are exposed is strictly controlled, may clarify this issue.
In the present study, while female C57BL/6 mice exhibited social preferences by differences in song searching behavior, female BALB/c mice exhibited social preferences by the number of entries to the speaker zones associated with different USVs. This suggests that, although female BALB/c mice were more attracted to one of the two zones, they appeared to hesitate in going to the end of the respective zone and to explore the mesh. BALB/c mice are known to have lower sociability than C57BL/6 mice [16]. This may result in lower motivation of approaching the source of the auditory stimuli, which could explain the lack of song searching behavior in female BALB/c mice.
There are at least three limitations in this study. The primary limitation was that male USVs for the playbacks were recorded from two individual mice, one male per strain. This indicates that our results might not be due to strain differences in the USVs, but to individual differences in the USVs. However, we believe that our results are due to strain differences in the USVs, because we extracted a fragment of male USVs such that a fragment contained typical features of the USVs of each strain in terms of the syllable composition. The second limitation was that, since we did not manipulate the syllable structure of the USVs, it is not possible to determine whether the order of syllables is important, or whether a particular syllable plays a critical role for social preferences. The third limitation was that we did not perform the choice between B6-60 and BC-60. Together with the results of the choice between B6-74 and BC-74, the results of this test would give us a clue on peak-frequency-preferences of the syllable structure. Since female BALB/c mice preferred B6-74 over BC-74, we further examined if these mice preferred B6-60 over BC-74 even if the peak frequency of B6-60 was similar to that of the USVs of their own strain. Accordingly, the choice between B6-60 and BC-74 was prioritized compared to the choice between B6-60 and BC-60 in the present study.
In conclusion, we found that female mice exhibit disassortative social preferences in response to male USVs, and that the acoustic features that affect these social preferences are different between C57BL/6 and BALB/c strains. Together, these observations indicate that female mice of different strains differently use acoustic features for exhibiting disassortative social preferences.
Conflict of Interest
The authors have no competing interests to declare.
Acknowledgments
The authors thank the members of the Companion Animals Research laboratory for help. This work was supported by JSPS KAKENHI grant number 17K19408, 15K14881, 25118007 (TK), 13J08901 (AA), and ERATO Touhara Chemosensory Signal Project, JST grant number JPMJER1202 (TO).
References
- 1.Asaba A., Okabe S., Nagasawa M., Kato M., Koshida N., Osakada T., Mogi K., Kikusui T.2014. Developmental social environment imprints female preference for male song in mice. PLoS One 9: e87186. doi: 10.1371/journal.pone.0087186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asaba A., Osakada T., Touhara K., Kato M., Mogi K., Kikusui T.2017. Male mice ultrasonic vocalizations enhance female sexual approach and hypothalamic kisspeptin neuron activity. Horm. Behav. 94: 53–60. doi: 10.1016/j.yhbeh.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 3.Ehret G.1974. Age-dependent hearing loss in normal hearing mice. Naturwissenschaften 61: 506–507. doi: 10.1007/BF00622976 [DOI] [PubMed] [Google Scholar]
- 4.Haga S., Hattori T., Sato T., Sato K., Matsuda S., Kobayakawa R., Sakano H., Yoshihara Y., Kikusui T., Touhara K.2010. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 466: 118–122. doi: 10.1038/nature09142 [DOI] [PubMed] [Google Scholar]
- 5.Hammerschmidt K., Radyushkin K., Ehrenreich H., Fischer J.2009. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol. Lett. 5: 589–592. doi: 10.1098/rsbl.2009.0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann F., Musolf K., Penn D.J.2012. Spectrographic analyses reveal signals of individuality and kinship in the ultrasonic courtship vocalizations of wild house mice. Physiol. Behav. 105: 766–771. doi: 10.1016/j.physbeh.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Jouhaneau J., Bagady A.1984. Effect of early auditory stimulation on the choice of acoustical environment by adult Swiss albino mice (Mus musculus). J. Comp. Psychol. 98: 318–326. doi: 10.1037/0735-7036.98.3.318 [DOI] [PubMed] [Google Scholar]
- 8.Kikusui T., Nakanishi K., Nakagawa R., Nagasawa M., Mogi K., Okanoya K.2011. Cross fostering experiments suggest that mice songs are innate. PLoS One 6: e17721. doi: 10.1371/journal.pone.0017721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musolf K., Hoffmann F., Penn D.J.2010. Ultrasonic courtship vocalizations in wild house mice, Mus musculus musculus. Anim. Behav. 79: 757–764. doi: 10.1016/j.anbehav.2009.12.034 [DOI] [Google Scholar]
- 10.Musolf K., Meindl S., Larsen A.L., Kalcounis-Rueppell M.C., Penn D.J.2015. Ultrasonic vocalizations of male mice differ among species and females show assortative preferences for male calls. PLoS One 10: e0134123. doi: 10.1371/journal.pone.0134123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomoto K., Ikumi M., Otsuka M., Asaba A., Kato M., Koshida N., Mogi K., Kikusui T.2018. Female mice exhibit both sexual and social partner preferences for vocalizing males. Integr. Zool. 13: 735–744. doi: 10.1111/1749-4877.12357 [DOI] [PubMed] [Google Scholar]
- 12.Nunez A.A., Pomerantz S.M., Bean N.J., Youngstrom T.G.1985. Effects of laryngeal denervation on ultrasound production and male sexual behavior in rodents. Physiol. Behav. 34: 901–905. doi: 10.1016/0031-9384(85)90011-3 [DOI] [PubMed] [Google Scholar]
- 13.Nyby J., Wysocki C.J., Whitney G., Dizinno G.1977. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus). Anim. Behav. 25: 333–341. doi: 10.1016/0003-3472(77)90009-4 [DOI] [PubMed] [Google Scholar]
- 14.Ohlemiller K.K., Jones S.M., Johnson K.R.2016. Application of mouse models to research in hearing and balance. J. Assoc. Res. Otolaryngol. 17: 493–523. doi: 10.1007/s10162-016-0589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pomerantz S.M., Nunez A.A., Bean N.J.1983. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol. Behav. 31: 91–96. doi: 10.1016/0031-9384(83)90101-4 [DOI] [PubMed] [Google Scholar]
- 16.Sankoorikal G.M.V., Kaercher K.A., Boon C.J., Lee J.K., Brodkin E.S.2006. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol. Psychiatry 59: 415–423. doi: 10.1016/j.biopsych.2005.07.026 [DOI] [PubMed] [Google Scholar]
- 17.Uematsu A., Kikusui T., Kihara T., Harada T., Kato M., Nakano K., Murakami O., Koshida N., Takeuchi Y., Mori Y.2007. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Res. 1163: 91–99. doi: 10.1016/j.brainres.2007.05.056 [DOI] [PubMed] [Google Scholar]
- 18.Whitney G., Coble J.R., Stockton M.D., Tilson E.F.1973. Ultrasonic emissions: do they facilitate courtship of mice. J. Comp. Physiol. Psychol. 84: 445–452. doi: 10.1037/h0034899 [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki K., Beauchamp G.K., Kupniewski D., Bard J., Thomas L., Boyse E.A.1988. Familial imprinting determines H-2 selective mating preferences. Science 240: 1331–1332. doi: 10.1126/science.3375818 [DOI] [PubMed] [Google Scholar]
- 20.Yang E.J., Lin E.W., Hensch T.K.2012. Critical period for acoustic preference in mice. Proc. Natl. Acad. Sci. USA 109:(Suppl 2): 17213–17220. doi: 10.1073/pnas.1200705109 [DOI] [PMC free article] [PubMed] [Google Scholar]