Abstract
To effectively use a common marmoset (Callithrix jacchus) as an experimental animal species, it is critical to establish a normal characteristics and morphology of the organs of the common marmoset. Although gross morphology of the common marmoset heart is reportedly the same as that of humans, little information is available regarding detailed morphology of the right atrium and the interatrial septum. Heart specimens were collected from three male and 10 female marmosets aged 9 to 65 months to determine the morphological features of the right atrium and the interatrial septum. Ten specimens were evaluated morphologically with a stereoscopic microscope in accordance with preparation and investigation methods designed to facilitate evaluation. Three specimens were histologically evaluated after being stained with hematoxylin-eosin, Elastica van Gieson and periodic acid Schiff. An annular ridge that is not present in the human heart was present in the right atrium and the interatrial septum of the common marmoset hearts. Tissue structure of the annular ridge was similar to atrial myocardial fibers. Furthermore, location of the coronary sinus ostium was different to that in humans. Present findings were used to create a schematic view of the annular ridge in the common marmoset heart. In the common marmoset heart, the annular ridge may function as a valve of the superior vena cava ostium, inferior vena cava ostium, and coronary sinus ostium. Present study provides morphological evidence that common marmosets have a valve-like structure in the right atrium.
Keywords: common marmoset, coronary sinus ostium, interatrial septum, right atrium
Introduction
To effectively use a common marmoset (Callithrix jacchus) as an experimental animal species, it is critical to establish normal characteristics and morphological data of organs of the common marmoset (henceforth referred to simply as the marmoset). The marmoset is increasingly being used in many fields of biomedical research, and is a New World primate that exhibits reactivity more similar to that of humans compared with rats. Average life span of the marmoset is approximately 12.4 years for males and 9.3 years for females [16]. The marmoset is fully mature by about 2 years of age and is considered aged by 8 years [1]. Information on pathology of healthy marmoset organs is available, and spontaneous lesions detected in the marmoset have been reported [3, 4, 10, 17, 22]. According to those reports, cardiovascular disease is relatively infrequent in the marmoset compared with other New World primates [14]. However, morbidity and mortality from cardiac disease is increased in marmosets aged 6 years and older in comparison with young marmosets [19].
A heart is one of the major organs. Effects of procedures such as drug administration on the heart are monitored not only for drug safety testing and cardiovascular research, but also in central nervous system pharmacology or dependency studies. Studies have been conducted to monitor the heart function of the marmoset. The waveform of the electrocardiogram of the healthy marmoset is similar to that of a healthy human [8]. Physiological and pathological blood pressure values of the marmoset have also been defined [15]. Blood pressure of the marmoset increases in line with age and body weight, as observed in humans, and cardiac diseases that alter blood pressure can be diagnosed at an early stage in marmosets [15]. Moreover, the mean morphometric values of the marmoset heart do not differ between males and females, and a relative weight of the marmoset heart is similar to that of humans [20]. Gross morphology of the marmoset heart has been reportedly the same as that of the heart of humans and domestic carnivorous animals [20].
Despite this preceding research on the marmoset heart, to our knowledge there has been little reported on the detailed anatomical morphology of the right atrium of the marmoset heart. A study by Senos et al. reported the morphometry of the ventricles but not of the atria [20]. However, we believe that a precise understanding of structures of the right atrium is crucial for the construction of the basic data of the heart. The right atrium is the primary lumen of the heart pump, and the main part of the cardiac conduction system is located in the right atrium. Furthermore, stretch stimulation of myocardial fibers in the atria causes production and secretion of atrial natriuretic peptide. In humans, an analysis of the morphology of the right atrium and the interatrial septum is important for the performance of cardiac surgery such as catheter ablation [6, 13]. Thus, it is critical to establish normal characteristics and morphological data of the right atrium and the interatrial septum. An objective of the present study was to provide useful information concerning the morphology of the right atrium and the interatrial septum of the marmoset.
The present study described the morphological features of the right atrium and the interatrial septum of the marmoset. Anatomical evaluations of the marmoset heart specimens with a stereoscopic microscope and histological investigations revealed the presence of an annular ridge and a different location of the coronary sinus ostium (CSO) compared with humans.
Materials and Methods
Ethics statement
The present study was conducted in accordance with the Act on Welfare and Management of Animals of the Japanese government. All experimental procedures conformed to “Regulation for Animal Experimental And Related Activities at Tohoku University”, and were reviewed by the Institutional Laboratory Animal Care and Use Committee of Tohoku University, and finally approved by the President of University (approval numbers: 20 Med-Anim-199, 21 Med-Anim-279).
Animals
Two marmosets were obtained from CLEA Japan (Tokyo, Japan); one was a 12-month-old male with a body weight of 319 g, and the other was a 9-month-old female with a body weight of 300 g. The two animals were caged singly at 27 ± 2°C in 50 ± 10% humidity under 12:12-h light/dark cycles at Tohoku University Graduate School of Medicine, Institute for Animal Experimentation, for 1 week of acclimation. Filtered drinking water was delivered and a total of 40–50 g per individual of commercial marmoset chow (CMS-1M, CLEA Japan) was given twice per day. Table 1 summarizes the age, sex, weight, and life history stage (maturation stage) [1] of the marmosets used in the present study. Table 1 also shows the size of the heart specimens, the presence or absence of lesions, the usage of each specimen, and identifies the heart specimen used in each figure.
Table 1. List of marmosets.
| ID | Sex | Obtaind as | Age (month) | Maturation stage | Body weight (g) | Heart weight (g) | Heart lesion | Purpose | Figure |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Living body | 9 | Puberty | 300 | 1.72 | none | Histology | Figs. 6A–D, F, G and Supplementary Fig. 3 |
| 2 | M | Living body | 12 | Puberty | 319 | 2.15 | none | Histology | |
| 3 | F | Fixed heart | 13 | Puberty | 220 | 1.40 | none | Gross anatomy | Fig. 5 |
| 4 | F | Fixed heart | 18 | Sexual maturity | 270 | 1.89 | none | Gross anatomy | |
| 5 | F | Fixed heart | 19 | Sexual maturity | 395 | 2.37 | none | Gross anatomy | Fig. 2 |
| 6 | F | Fixed heart | 19 | Sexual maturity | 345 | 2.05 | none | Gross anatomy | |
| 7 | F | Fixed heart | 23 | Adult | 315 | 2.00 | none | Gross anatomy | |
| 8 | F | Fixed heart | 23 | Adult | 245 | NA | none | Histology | Fig. 6E and Supplementary Fig. 2 |
| 9 | F | Fixed heart | 24 | Adult | 290 | 1.71 | none | Gross anatomy | |
| 10 | F | Fixed heart | 24 | Adult | 220 | 1.38 | none | Gross anatomy | |
| 11 | M | Fixed heart | 27 | Adult | 245 | 1.65 | none | Gross anatomy | |
| 12 | F | Fixed heart | 51 | Adult | 310 | 2.29 | none | Gross anatomy | |
| 13 | M | Fixed heart | 65 | Adult | 335 | 2.11 | none | Gross anatomy | Figs. 3 and 4 |
Characteristics of the marmoset hearts used in the present study. Two marmosets were obtained live, while 11 were obtained as heart specimens fixed in formalin. The marmosets were between the life history stages of puberty and adulthood. Aged marmosets were not used. No heart lesions were observed. Heart weights were measured after fixation. NA, not available.
Euthanasia of animals and adjustment of specimens
The marmosets were euthanized by exsanguination from the abdominal vena cava under anesthesia with ketamine hydrochloride (50 mg/kg, intramuscularly) and medetomidine hydrochloride (5.0 mg/kg, intramuscularly). After euthanasia, the hearts were dissected. Approximately 5–10 mm of the great vessels close to the base of the heart were preserved. The hearts were fixed in aqueous 10% buffered formalin solution after being cleaned with isotonic saline solution. These specimens were used for histological evaluation. In addition, 11 heart specimens fixed with aqueous 10% buffered formalin solution were supplied by CLEA Japan. Ten of 11 specimens (ID 3–7 and 9–13 in Table 1) were used for evaluation with a stereoscopic microscope, while one (ID 8 in Table 1) was used for histological evaluation. These specimens were collected from two males and nine females aged 13 to 65 months. Thirteen heart specimens were used in the present study, including two heart specimens (ID 1 and 2 in Table 1) obtained from euthanized animals. The heart specimens used in the present study were from marmosets aged 9 to 65 months. Therefore, these specimens were unlikely to have cardiac disease, as marmosets younger than 6 years reportedly have low morbidity and mortality from cardiac disease [19].
Preparation and investigation of the heart specimens
For evaluation with a stereoscopic microscope, the heart specimens were prepared and investigated using four methods shown in Fig. 1. Morphological evaluations were done at 7× to 20× magnification. In the first method, the specimens were prepared in accordance with the procedure (a) illustrated in Fig. 1. The right atrium was incised and everted to show the sinus venarum and the interatrial septum as viewed from the anterior aspect (Fig. 2). In the second method, the specimens were prepared in accordance with the procedure (b) illustrated in Fig. 1. The surface of the heart and the inside of the inferior vena cava (IVC), viewed from the inferior side (the diaphragm side) of the heart was investigated (Fig. 3). In the third method, the specimens were prepared in accordance with the procedure (c) illustrated in Fig. 1. The inner view of the right atrium and the interatrial septum as viewed from the anterior side of the heart was investigated (Fig. 4). In the fourth method, the specimens were prepared in accordance with the procedure (d) illustrated in Fig. 1. The incised edges were opened outwards to give an inner view of the right atrium. The sinus venarum and the interatrial septum viewed from the base of the heart were investigated (Fig. 5).
Fig. 1.
Schematic views of the anterior side of the marmoset heart showing the incision procedures for four evaluation methods. In the marmoset heart, the right atrium is an irregular elliptical shape situated in the right half of the anterior side of the heart. The SVC, the right atrium and the IVC are almost linearly aligned. The azygos vein is joined to the SVC in the vicinity of the serous pericardium inversion. (a) A method for preparation and investigation of the specimen shown as Fig. 2. An incision is made along the dashed line as detailed below. An incision is made on the SVC wall from the cut end of the SVC to the portion where the superior edge of the right auricle ridge line and the SVC are joined. The ridge line of the right auricle is incised to the portion where it contacts the annulus fibrosus. The annulus fibrosus and the IVC wall are incised to the cut end of the IVC. The incision edge is lifted, and the right atrium is everted to obtain the inner view of the right atrium. (b) A method for investigation of the specimen shown as Fig. 3. The specimen is investigated from the inferior side to obtain the view of the outside of the inferior surface of the heart and the inside of the IVC lumen. (c) A method for preparation and investigation of the specimen shown as Fig. 4. A longitudinal section is made in accordance with a certain plane. The morphology of the posterior cut surface of the specimen is investigated. The aortic valve, the pulmonary valve, and inner views of the right atrium, the interatrial septum, and the right ventricle are investigated. (d) A method for preparation and investigation of the specimen shown as Fig. 5. An incision is made along the dashed line, as detailed below. A straight incision is made from the cut end of the SVC through the right atrial wall to the cut end of the IVC, on the SVC wall, passing just beside the junction point of the azygos vein. The incision edges are opened outwards to obtain an inner view of the right atrium. The sinus venarum and the interatrial septum are investigated. AF, annulus fibrosus; Ao, aorta; AzO, azygos vein ostium; IVC, inferior vena cava; LA, left atrium; PT, pulmonary trunk; RA, right atrium; RAA, right auricle; RV, right ventricle; SVC, superior vena cava.
Fig. 2.
A photograph and a schematic view of a marmoset heart specimen showing the AR and the PR on the surface of the SV. Two cut surfaces of the cut ends of the CT (asterisks) are present at the left and right side of the AzO, in the vicinity of the SVC. The AR and the PR originate at the CT, and travel on the SV between the SVC ostium and the IVC ostium. In the vicinity of the IVC ostium, the distal edge of the AR is disconnected. Ao, Aorta; AR, anterior ridge; AzO, azygos vein ostium; CT, crista terminalis; FO, fossa ovalis; IVC, inferior vena cava; PR, posterior ridge; PM, pectinate muscle; RAA, right auricle; RV, right ventricle; SV, sinus venarum; SVC, superior vena cava. Scale bar, 2 mm.
Fig. 3.
A photograph and a schematic view of the inferior surface of a marmoset heart specimen. In the lumen of the IVC investigated from the inferior side of the heart, the distal AR and the distal PR form a pair, with pocket-like valve-like morphology at the IVC ostium. AR, anterior ridge; CS, coronary sinus; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PR, posterior ridge; RA, right atrium; RV, right ventricle. Scale bar, 2 mm.
Fig. 4.
A photograph and a schematic view of the posterior cut surface of a longitudinal section of a marmoset heart. In the vicinity of the IVC ostium, the distal AR and the distal PR are wide and form a pair, with pocket-like valve-like morphology. The distal AR terminates at the MS. The distal PR terminates at the limbus of the FO. AL, attachment line of the tricuspid valve; Ao, aorta; AR, anterior ridge; EV, Eustachian valve and Eustachian ridge. The EV is a part of the AR and is shown in yellow, as the EV in the AR travels almost the same area as the human EV. To make it easier to understand the comparison of the marmoset AR and the human EV, a part of the distal AR has been highlighted in yellow as the EV. CTI, cavotricuspid isthmus; FO, fossa ovalis; IVC, inferior vena cava; LA, left atrium; LSC, left semilunar cusp of the aortic valve; MS, membranous atrioventricular septum; PR, posterior ridge; PV, left semilunar cusp of the pulmonary valve; PSC, post semilunar cusp of the aortic valve; RV, right ventricle; SEP, septal cusp of the tricuspid valve; SV, sinus venarum. Scale bar, 2 mm.
Fig. 5.
A photograph and a schematic view of the inside of the right atrium of a marmoset heart specimen viewed from the base of heart. An inside view of the right atrium centered on the RAVO is shown. The AR and the PR constitute an annular ridge on the SV and the interatrial septum. In the vicinity of the IVC ostium, the CSO is present between the AR and the PR. Ao, aorta; AR, anterior ridge; CSO, coronary sinus ostium; EV, Eustachian valve and Eustachian ridge. The EV is a part of the AR and is shown in yellow, as the EV in the AR travels almost the same area as the human EV. To make it easier to understand the comparison of the marmoset AR and human EV, a part of distal AR has been highlighted in yellow as the EV. FO, fossa ovalis; IVC, inferior vena cava; LA, left atrium; PR, posterior ridge; PT, pulmonary trunk; RAA, right auricle; RAVO, right atrioventricular ostium; RV, right ventricle; SV, sinus venarum; SVC, superior vena cava. Scale bar, 2 mm.
Preparation of the specimens for tissue staining
Heart specimens fixed with aqueous 10% buffered formalin solution were embedded in paraffin and cut into thin sections of 3-µm thickness with a microtome (Supplementary Fig. 1), then stained with hematoxylin-eosin (HE), Elastica van Gieson (EVG) and periodic acid Schiff (PAS). The stained tissue specimens were evaluated under an optical microscope at 10× to 400× magnification and assessed histologically.
Results
Morphological features of the right atrium and the interatrial septum of 9- to 65-month-old healthy and not aging male and female marmosets were investigated. No lesion was detected in the heart specimens by gross observation or stereoscopic microscopic observation. Results did not differ with the age and sex of the animals. Typical samples were used for the resulting images.
Inside the right atrium, two ridges that originated at the crista terminalis traveled in arcs on the surface of the sinus venarum from the superior vena cava (SVC) ostium to the IVC ostium (Fig. 2). For convenience, we coined the terms “anterior ridge” (AR) for the left-sided ridge of the azygos vein ostium and “posterior ridge” (PR) for the right-sided ridge of the azygos vein ostium. In the proximal AR and the proximal PR, the bases of the ridges were slightly raised, traveled almost along the boundary of the SVC ostium and the sinus venarum and then approached each other. Thereafter, the ridges descended in parallel on the surface of the sinus venarum toward the IVC ostium. Heights of the AR and the PR were low at the proximal portions and gradually increased to form wide flat shapes distally. Free edges of the ridges faced the inside of the arcs they created.
The crista terminalis originated at the anteromedial wall of the right atrium and bent past the portion where the right atrial appendage joined to the SVC ostium (Fig. 2). Thereafter, the crista terminalis formed the boundary of the right atrial appendage and the sinus venarum, and descended along the AR. The crista terminalis ramified into several thin trabeculations before it reached the IVC, and terminated its orbit in the vicinity of the cavotricuspid isthmus. The interior of the right atrial appendage was composed of a thin wall and the pectinate muscles.
The IVC was protruding outside of the inferior surface of the specimen, and the distal AR and the distal PR formed a pair in the lumen of the IVC, with pocket-like valve-like morphology (Fig. 3). Inside the right atrium, the wide and flat distal AR and the distal PR overlapped each other in the vicinity of the IVC ostium (Fig. 4). Even from the opposite sides, the distal AR and the distal PR formed a pair, with pocket-like valve-like morphology.
Similarly to the Eustachian valve and the Eustachian ridge, the distal AR travelled on the posterior side of the right atrioventricular ostium, created a membranous arc facing the right atrioventricular ostium side, and terminated in the membranous atrioventricular septum (Figs. 4 and 5). The distal PR covered most of the surface of the fossa ovalis on the interatrial septum and terminated in the superior anterior aspect of the limbus of the fossa ovalis (Figs. 4 and 5). Between the distal AR and the attachment line of the tricuspid valve, the cavotricuspid isthmus was present as a concave quadrilateral-shaped area (Fig. 4).
When the inside of the right atrium was viewed from the base of the heart, the AR and the PR constituted an annular ridge on the surface of the sinus venarum and the interatrial septum (Fig. 5). The annular ridge depressed inside at the portion where the proximal AR and the proximal PR originated at the crista terminalis. In addition, the CSO was present between the distal AR and the distal PR in the vicinity of the IVC ostium (Fig. 5). The CSO was tightly sandwiched between the two ridges, and was not visible until the both incision edges were pulled outwards to widen the gap between the bases of the two ridges. This pulling also enabled investigation of the frontal aspect of the fossa ovalis. The endocardial fold of the coronary sinus valve (the Thebesian valve) was not discerned.
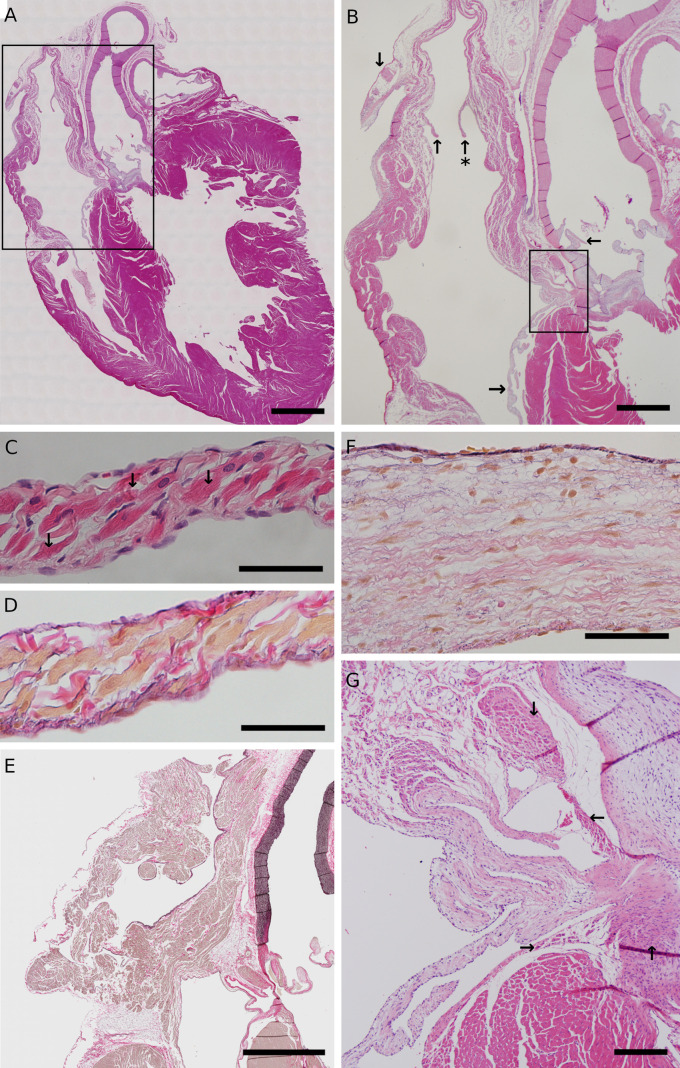
The tissue structure of the AR and the PR was different from that of general heart valves such as the atrioventricular valves and arterial valves. Histological evaluation of HE-stained and EVG-stained longitudinal sections of marmoset heart specimens showed that the AR and the PR were histologically similar to atrial myocardial fibers (Figs. 6A–E, Supplementary Figs. 1 and 2). In contrast, the tricuspid valve was mainly composed of collagen fibers, with elastic fibers present in layers under the endothelial cells (Figs. 6A, B and F).
Fig. 6.
Histological images of longitudinal sections of marmoset heart specimens. (A) HE-stained longitudinal section of a marmoset heart. (B) An enlarged histological image of the right atrium and the interatrial septum (square frame in A) stained with HE. The AR (upward arrow with an asterisk) and the PR (upward arrow) protrude toward the right atrium lumen, in the vicinity of the serous pericardium inversion (downward arrow) joined to the superior vena cava. The aortic valve (leftward arrow) and the right atrioventricular valve (rightward arrow) are shown. In the square frame, the atrioventricular node and the bundle of His are present. (C) An enlarged histological image of the AR (upward arrow with an asterisk in B) stained with HE. Histologically, the AR has atrial myocardial fiber-like structures with striations and intercalated discs (downward arrow), and its surface is covered with endothelial cells. (D) An enlarged histological image of the AR (upward arrow with an asterisk in B) stained by EVG. The AR is composed of atrial myocardial fibers stained yellow, while collagen fibers (red) and elastic fibers (black) are layered under the endothelium. (E) EVG-stained longitudinal section of the right atrium in the vicinity of the inferior vena cava ostium of a marmoset. (F) An enlarged histological image of the tricuspid valve (rightward arrow in B) stained by EVG. The tricuspid valve of the marmoset is composed of collagen fibers (red), and elastic fibers (black) are layered under the endothelium. The nuclei of fibroblasts are interspersed inside the valve, and the surface of the valve is covered with endothelial cells. (G) An enlarged histological image of the atrioventricular node and the bundle of His (square frame in B) stained with HE. The atrioventricular node (downward arrow) is present between the endocardium and the membranous atrioventricular septum, at the base of the interatrial septum. The myocardial fibers of the atrioventricular node have fewer myofibrils than normal myocardial fibers, and are buried in the connective tissue. The front end of the atrioventricular node contains myocardial fibers form a His bundle trunk (leftward arrow), which is a string-like bundle. The left branch of the His bundle (upward arrow) and the right branch of the His bundle (rightward arrow) are shown at the inferior border of the membranous atrioventricular septum. Scale bars, 2 mm (A), 1 mm (B), 50 µm (C), (D), 2 mm (E), 50 µm (F), 200 µm (G). AR, anterior ridge; EVG, Elastica van Gieson; HE, hematoxylin-eosin; PR, posterior ridge.
The atrioventricular node was present at the base of the interatrial septum. Histological evaluation of HE-stained, EVG-stained and PAS-stained longitudinal sections of the marmoset heart showed that the atrioventricular node was present between the endocardium and the membranous atrioventricular septum (Figs. 6A, B and G, Supplementary Fig. 3). The left and right branches of the His bundle were present at the inferior border of the membranous atrioventricular septum.
Our results are summarized in Fig. 7. The schematic view shows the inside morphology of the right atrium from the anterior side of the marmoset heart. The AR and the PR constituted an annular ridge on the sinus venarum and the interatrial septum, and the CSO was present between the two ridges. The annular ridge surrounded the three openings of the SVC ostium, the IVC ostium, and the CSO.
Fig. 7.

A schematic view of a marmoset heart showing the location of the AR, the PR, and the CSO. A schematic view shows the inside of the marmoset right atrium from the anterior side of the heart. Between the SVC ostium and the IVC ostium, the AR and the PR descend on the SV. The CSO is present between the two ridges in the vicinity of the IVC ostium. An annular ridge constituted by the AR and the PR surrounds the SVC ostium, the IVC ostium, and the CSO. Ao, aorta; AR, anterior ridge; AzO, azygos vein ostium; CSO, coronary sinus ostium; EV, Eustachian valve and Eustachian ridge. The EV is a part of the AR and is shown in yellow, as the EV in the AR travels almost the same area as the human EV. To make it easier to understand the comparison of the marmoset AR and human EV, a part of the distal AR has been highlighted in yellow as the EV. FO, fossa ovalis; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PR, posterior ridge; PT, pulmonary trunk; RAA, right auricle; RV, right ventricle; SV, sinus venarum; SVC, superior vena cava.
Discussion
The present study has shown two novel morphological features of the right atrium and the interatrial septum in the marmoset. First, there was an annular ridge composed of the AR and the PR, and second, the CSO was located between the AR and the PR. The results were similar for all evaluated specimens, and are summarized in Fig. 7. To our knowledge, this is the first study to reveal the presence of the AR and the PR in the marmoset heart, and to report that the CSO of the marmoset is not located in the cavotricuspid isthmus.
When the life history stage is classified in accordance with sexual maturation and aging, the marmosets used in the present study were in the life history stages of puberty (9–15 months), sexual maturity (15 months–2 years), and non-aged adulthood (2–5.5 years) [1]. The incidence of morbidity and mortality from cardiac disease is reportedly low in marmosets younger than 6 years [19]. Therefore, the presence of the annular ridges (the AR and the PR) and the location of the CSO seen in the present study are not pathogenic phenomena.
In contrast to humans and canines, the surface of the sinus venarum in the marmoset was not smooth walled [5, 6]. On the sinus venarum between the SVC ostium and the IVC ostium, the AR and the PR traveled in parallel (Fig. 2). The free edges of the AR and the PR faced the right atrioventricular ostium. The morphology of the ridges suggested that blood flowing into the right atrium from the SVC and the IVC is led into the right atrioventricular ostium by the ridges. The AR and the PR differed from the crista terminalis, as the proximal AR and the proximal PR were confirmed to originate at and descend along the crista terminalis.
The present results suggest that the proximal AR and the proximal PR function as the valve of the SVC ostium. In the vicinity of the SVC ostium, although the heights of the ridges were low, the ridges travelled along the boundary between the SVC ostium and the sinus venarum (Figs. 2 and 5). At the boundary, the bases of the ridges were slightly raised, and the form of the ridges appeared as if they were capable of imperfectly closing the SVC ostium when both ridges approached each other. Histologically, the proximal AR and the proximal PR were similar to atrial myocardial fibers (Figs. 6A–D). The morphology of the ridges suggest that the ridges open and close the SVC ostium in accordance with the systole and diastole of the heart. To our knowledge, this is the first study to report the presence of the valve of the SVC ostium in the marmoset heart.
The present results provide evidence that the distal AR and the distal PR function as the valve of the IVC ostium in the vicinity of the IVC ostium. When looking into the IVC lumen from the inferior side of the heart, the distal PR and the distal AR formed a pair, with pocket-like valve-like morphology that completely closed the IVC ostium (Fig. 3). This pocket-like valve-like morphology was also confirmed in the inside of the right atrium (Fig. 4). Histologically, the distal AR and the distal PR were similar to myocardial fibers (Fig. 6E and Supplementary Fig. 2). To our knowledge, this is the first study to report the presence of a pair of valve cusps of the IVC ostium in the marmoset heart. However, the Eustachian valve is reportedly present in approximately 70% of humans as a remnant of the right sinus venous valve in the vicinity of the IVC ostium [13].
The present results indicate that the distal AR of the marmoset corresponds to the human Eustachian valve and the human Eustachian ridge, as the course of the distal AR was similar to that of the Eustachian valve and the Eustachian ridge in humans (Figs. 4, 5 and 7). The human Eustachian valve is a triangular flap of fibrous or fibromuscular tissue [6] with an average height of 5 mm [13]. The Eustachian valve is derived from the combination of the right sinus venous valve and the sinus septum [21]. The present results showed that the distal AR created an arc facing the right atrioventricular ostium like the Eustachian valve, which is also called “the IVC valve” (Figs. 4, 5 and 7). The Eustachian ridge (tendon of Todaro) is an extension of the Eustachian valve that terminates in the membranous atrioventricular septum (central fibrous body) [13]. The Eustachian ridge is always present in humans. In the marmosets studied in the present study, the distal AR was always present and traveled through the interatrial septum to terminate in the membranous atrioventricular septum, similar to the human Eustachian ridge (Figs. 4, 5 and 7). However, boundary between the parts considered to be the Eustachian valve and the Eustachian ridge on the distal AR was not determined in the present study.
In contrast to the distal AR, the present results suggest that the distal PR is similar to the remnant of the left sinus venous valve, as the distal PR was confirmed to fuse to and terminate in the limbus of the fossa ovalis (Figs. 4, 5 and 7). Early in the human heart development, the left sinus venous valve is fused with the septum primum and becomes part of the interatrial septum [18]. However, if the left sinus venous valve fuses incompletely with the right aspects of the interatrial septum complex, remnants derived from the left sinus venous valve can present in adults as a thin fenestrated membrane or a trabecular membranous structure overlying the surface of the fossa ovalis [9, 18]. Klimek-Piotrowska et al. reported four variations of fossa ovalis anatomy in humans, one of which was a net-like formation within the fossa ovalis that occurs in 7.4% of humans [11]. In addition, the presence of remnants of the left sinus venous valve as a membranous or trabecular membranous structure over the fossa ovalis or the interatrial septum has been reported in 7.5% of humans [9].
The present results suggest that the PR originates from the left sinus venous valve, as the shape and location of the distal PR of the marmoset was similar to that of the left sinus venous valve in the 27-mm crown rump length germinal stage of a human fetus on scanning electron micrograph [21]. In the photograph taken from the right side of the right atrium, although the picture was taken before the foramen ovale was closed, the left sinus venous valve was present in the area of the limbus of the fossa ovalis [21]. In the present study, the distal PR was fused with the limbus of the fossa ovalis (Figs. 4, 5 and 7). The form of the left sinus valve in the photograph from the previous study [21] is very similar to the form of the PR in the present study; this strongly suggests that the PR originates from the left sinus venous valve.
The present results provide evidence that the phylogenetic development of the human heart differs from that of the marmoset heart in some ways. The location of the CSO differed between humans and marmosets. In the marmoset, the CSO was present between the distal AR and the distal PR in the vicinity of the IVC ostium (Fig. 5). In contrast, the CSO in humans is present in the cavotricuspid isthmus, between the Eustachian valve/ridge and the tricuspid valve [12]. Although the coronary sinus valve (Thebesian valve) is present at the edge of the CSO in 62–85% of human adults [13], the Thebesian valve was not identified in the marmosets studied in the present study (Fig. 5).
The morphology of the distal AR and the distal PR in the marmoset hearts suggests that the AR and the PR function as valve of the CSO. The distal AR and the distal PR were adjacent to both sides of the CSO, and the CSO was closed by being inserted among the two ridges (Fig. 5). The CSO seemed to be designed to open and close in accordance with the systole and diastole of the heart. In the marmoset hearts, the AR and the PR constituted an annular ridge that spanned the sinuses venarum and the interatrial septum (Fig. 5). When looking from the right atrioventricular ostium side, the annular ridge enclosed the three openings of the SVC ostium, the IVC ostium, and the CSO (Fig. 7). This morphology suggests that these ridges function as the valve of the marmoset right atrium.
We believe that the AR and the PR function to keep the marmoset heart pumping, and that the valve-like function of the ridges enables the venous blood to be sucked into the right atrium smoothly from the SVC and the IVC. Furthermore, we believe that the AR and the PR prevent backflow of blood from the right atrium into the veins. In particular, the venous return system at the IVC ostium may be useful for drawing venous blood from the lower body. At the CSO, the return of the blood from the heart wall may be facilitated by the presence of the valve, which may be useful for maintaining the blood circulation of the heart. Moreover, the blood volume inside the right atrium can be considered to remain almost constant due to the presence of the AR and the PR. The location and the shape of the ridges strongly suggests that the ridges act to relieve the pressure of venous blood flowing into the right atrium from the IVC. We believe that the ridges function to block the rapid inflow of venous blood into the right atrium and thus to prevent the rapid increase of blood volume therein to prevent the excessive increase of the atrial pressure. In other words, we consider that the ridges reduce the preload of the heart.
Although further research is needed to draw definitive conclusions, the present study suggests that in marmosets, the left sinus venous valve and the right sinus venous valve present in the fetal period may develop and become functional in adults as the AR and the PR. The limitation of our study is that we have not yet confirmed whether these valve-like ridges function in live marmoset hearts. Further research is needed to demonstrate that this valve-like morphology is necessary for the normal functioning of the marmoset heart.
In addition, the present results also indicate that the AR and the PR may be involved in the cardiac stimulation conduction system as stimulation pathways. The sinoatrial node is situated in the vicinity of the SVC ostium (the proximal crista terminalis) [7], and the atrioventricular node is present in the vicinity of the membranous atrioventricular septum [2]. In the present study, the proximal AR and the proximal PR in marmosets were confirmed to originate at the crista terminalis, and the distal AR eventually terminated in the membranous atrioventricular septum (Figs. 4 and 5). The histological evaluations showed that the locations of the atrioventricular node, His bundle trunk, and left and right branches of the His bundle were almost the same as in humans (Figs. 6A, G and Supplementary Fig. 3). However, the morphological findings alone are insufficient to understand the relationship between the AR and the PR and the cardiac conduction system; further studies based on cardiac physiological verification are required. However, we believe that conduction through three-dimensional pathways such as the AR and the PR is faster and more accurate.
In conclusion, the present study provides new and important morphological information about the right atrium and the interatrial septum and then helps establish the normal characteristics and morphological data of the marmoset heart. In contrast to the human heart, the marmoset heart has an annular ridge constituted by the AR and the PR, and the CSO is present between the AR and the PR (Supplementary Fig. 4). The annular ridge seems to function as a valve of the SVC ostium, the IVC ostium and the CSO, which suggests that the presence of the annular ridge is required for marmoset survival.
The differences in the heart morphology of the marmoset compared with humans must be considered when using marmosets as experimental animals and as human models. First, when manipulating the interior of the marmoset heart, it should be noted that the internal structure differs between marmosets and humans, and care must be taken to avoid damaging the annular ridge. Second, a marmoset heart with a dysfunctional annular ridge may not be normal. Third, when using the marmoset as a model animal for coronary circulation, it should be noted that the physiological hemodynamics of the marmoset heart may differ from that of the human heart. Thus, the normal characteristics and the morphological data of the marmoset heart are useful in enabling the effective use of the marmoset as an experimental or a model animal.
The present study provides the morphological evidence that some animals, like the marmoset, have a heart that contains a valve-like structure. So far, no study has been investigated the morphology of the right atrium and the interatrial septum of primates other than the marmoset from the same perspective as that used in the present study. From the perspectives of development and evolution, if primates closely related to the marmoset have similar heart structures to the marmoset, it may be possible to discuss the evolution of these animals morphologically.
Supplementary Material
Acknowledgments
We thank Dr. Tsunetoshi Itoh and Dr. Ryuji Suzuki for providing valuable suggestions and JAPAN CREA Co., Ltd. for providing the marmoset hearts. We thank Kelly Zammit, BVSc, and Hugh McGonigle, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
This work was partly supported by grants-in-aid awarded by the Ministry of Health, Labor and Welfare (H24-Shinko-Ippan-007), Research Program on Emerging and Reemerging Infection Diseases (2015–2017) from Japan Agency for Medical Research and Development (AMED), and by the Japan initiative for global Research network on Infectious Disease (J-GRID) (2015–2017) from Ministry of Education, Culture, Sports, Science and Technology in Japan and AMED.
References
- 1.Abbott D.H., Barnett D.K., Colman R.J., Yamamoto M.E., Schultz-Darken N.J.2003. Aspects of common marmoset basic biology and life history important for biomedical research. Comp. Med. 53: 339–350. [PubMed] [Google Scholar]
- 2.Anderson R.H., Ho S.Y.2002. The morphology of the specialized atrioventricular junctional area: the evolution of understanding. Pacing Clin. Electrophysiol. 25: 957–966. doi: 10.1046/j.1460-9592.2002.00957.x [DOI] [PubMed] [Google Scholar]
- 3.Chalmers D.T., Murgatroyd L.B., Wadsworth P.F.1983. A survey of the pathology of marmosets (Callithrix jacchus) derived from a marmoset breeding unit. Lab. Anim. 17: 270–279. doi: 10.1258/002367783781062217 [DOI] [PubMed] [Google Scholar]
- 4.David J.M., Dick E.J., Jr, Hubbard G.B.2009. Spontaneous pathology of the common marmoset (Callithrix jacchus) and tamarins (Saguinus oedipus, Saguinus mystax). J. Med. Primatol. 38: 347–359. doi: 10.1111/j.1600-0684.2009.00362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho S.Y., Anderson R.H., Sánchez-Quintana D.2002. Gross structure of the atriums: more than an anatomic curiosity? Pacing Clin. Electrophysiol. 25: 342–350. doi: 10.1046/j.1460-9592.2002.00342.x [DOI] [PubMed] [Google Scholar]
- 6.Ho S.Y., Sánchez-Quintana D.2009. The importance of atrial structure and fibers. Clin. Anat. 22: 52–63. doi: 10.1002/ca.20634 [DOI] [PubMed] [Google Scholar]
- 7.Ho S.Y., Sánchez-Quintana D.2016. Anatomy and pathology of the sinus node. J. Interv. Card. Electrophysiol. 46: 3–8. doi: 10.1007/s10840-015-0049-6 [DOI] [PubMed] [Google Scholar]
- 8.Horii I., Kito G., Hamada T., Jikuzono T., Kobayashi K., Hashimoto K.2002. Development of telemetry system in the common marmoset--cardiovascular effects of astemizole and nicardipine. J. Toxicol. Sci. 27: 123–130. doi: 10.2131/jts.27.123 [DOI] [PubMed] [Google Scholar]
- 9.Jansirani D.D., Deep S.S., Anandaraja S.2015. Anatomical study of Chiari network and the remnant of left venous valve in the interior of right atrium. Anat. Res. Int. 2015: 247680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaspareit J., Friderichs-Gromoll S., Buse E., Habermann G.2006. Background pathology of the common marmoset (Callithrix jacchus) in toxicological studies. Exp. Toxicol. Pathol. 57: 405–410. doi: 10.1016/j.etp.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Klimek-Piotrowska W., Hołda M.K., Koziej M., Piątek K., Hołda J.2016. Anatomy of the true interatrial septum for transseptal access to the left atrium. Ann. Anat. 205: 60–64. doi: 10.1016/j.aanat.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 12.Klimek-Piotrowska W., Hołda M.K., Koziej M., Hołda J., Piątek K., Tyrak K., Bolechała F.2016. Clinical anatomy of the cavotricuspid isthmus and terminal crest. PLoS One 11: e0163383. doi: 10.1371/journal.pone.0163383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucybała I., Ciuk K., Klimek-Piotrowska W.2018. Clinical anatomy of human heart atria and interatrial septum - anatomical basis for interventional cardiologists and electrocardiologists. Part 1: right atrium and interatrial septum. Kardiol. Pol. 76: 499–509. [DOI] [PubMed] [Google Scholar]
- 14.Ludlage E., Mansfield K.2003. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp. Med. 53: 369–382. [PubMed] [Google Scholar]
- 15.Mietsch M., Baldauf K., Reitemeier S., Suchowski M., Schoon H.A., Einspanier A.2016. Blood pressure as prognostic marker for body condition, cardiovascular, and metabolic diseases in the common marmoset (Callithrix jacchus). J. Med. Primatol. 45: 126–138. doi: 10.1111/jmp.12215 [DOI] [PubMed] [Google Scholar]
- 16.Nishijima K., Saitoh R., Tanaka S., Ohsato-Suzuki M., Ohno T., Kitajima S.2012. Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology 13: 439–443. doi: 10.1007/s10522-012-9388-1 [DOI] [PubMed] [Google Scholar]
- 17.Okazaki Y., Kurata Y., Makinodan F., Kidachi F., Yokoyama M., Wako Y., Yamagishi Y., Katsuta O., Takechi M., Tsuchitani M.1996. Spontaneous lesions detected in the common cotton-eared marmosets (Callithrix jacchus). J. Vet. Med. Sci. 58: 181–190. doi: 10.1292/jvms.58.181 [DOI] [PubMed] [Google Scholar]
- 18.Pinto N.M., Weinberg P.M., Rome J.J.2007. Membranous remnant of left venous valve of inferior vena cava: implications for device closure of atrial septal defects. Catheter. Cardiovasc. Interv. 69: 732–734. doi: 10.1002/ccd.21066 [DOI] [PubMed] [Google Scholar]
- 19.Ross C.N., Davis K., Dobek G., Tardif S.D.2012. Aging phenotypes of common marmosets (Callithrix jacchus). J. Aging Res. 2012: 567143. doi: 10.1155/2012/567143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senos R., Benedicto H.G., del Rio do Valle C.M., del Rio do Valle R., Nayudu P.L., Kfoury Junior J.R., Bombonato P.P.2014. Gross morphometry of the heart of the Common marmoset. Folia Morphol. (Warsz) 73: 37–41. doi: 10.5603/FM.2013.0064 [DOI] [PubMed] [Google Scholar]
- 21.Steding G., Xu J.W., Seidl W., Männer J., Xia H.1990. Developmental aspects of the sinus valves and the sinus venosus septum of the right atrium in human embryos. Anat. Embryol. (Berl.) 181: 469–475. doi: 10.1007/BF02433794 [DOI] [PubMed] [Google Scholar]
- 22.Tucker M.J.1984. A survey of the pathology of marmosets (Callithrix jacchus) under experiment. Lab. Anim. 18: 351–358. doi: 10.1258/002367784780865397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.