Abstract
Various cardiovascular diseases can be detected and diagnosed using echocardiography. The demand for cardiovascular system research using nonhuman primates is increasing, but echocardiographic references for nonhuman primates are limited. This report describes the first comparison of echocardiographic reference values in 247 normal cynomolgus monkeys (135 females, 112 males) over a wide age range. Echocardiography, electrocardiography, blood pressure and chest X-ray images were acquired under immobilization with intramuscular ketamine hydrochloride, then cardiac structure, function, and flow velocity were assessed. Cardiac hormone levels were also tested. We found that cardiac structures positively correlated with weight, that the size of these structures stabilized after reaching maturity and that cardiac output increased according to heart size. In contrast, fractional shortening of the left ventricle, ejection fraction and flow velocity showed no significant correlations with weight or age, and age and E wave correlated negatively. These findings appear sufficiently similar to those in humans to suggest that cynomolgus monkeys can serve as a suitable model of human cardiac disease. Our data should also prove useful for surveying cardiac dysfunction in monkeys.
Keywords: cardiac function, cardiovascular disease, cynomolgus monkey, echocardiography, elderly
Introduction
Cardiovascular disease (CVD) is a common cause of death in humans and other animals and rates of mortality due to CVD are increasing among humans. Modern medicine is rapidly developing but the various types of CVD are difficult to treat, and CVD negatively impacts the quality of life for patients. The prevalence of these issues will increase because societies around the world are rapidly aging and aging is associated with various types of CVD. Thus, social concerns about CVD have been raised, and improvements in the diagnosis and treatment of CVD are needed. Usually, CVD is diagnosed in humans and other animals using echocardiography, radiography, electrocardiography, and biochemical parameters including cardio-specific hormones [20, 21]. Echocardiography is the most common means of diagnosing CVD because it can detect abnormal myocardial motion and noninvasively and rapidly visualize blood flow in real time.
Nonhuman primates are optimal experimental animals because of their similarity to humans in terms of anatomy and physiology. In addition, their cardiovascular system resembles that of humans and their body size is appropriate for examinations; thus, experimental monkeys are necessary to develop cardiovascular medicines for humans [1, 29, 36]. In addition, general aging and growth patterns of monkeys, including cynomolgus monkeys (Macaca fascicularis) resemble those of humans, while the rate of aging in these monkeys is 3 times that in humans [26, 37]. The underlying diseases in monkeys need to be diagnosed and treated as soon as possible, because they are extremely valuable experimental animals.
Although echocardiographic parameters in nonhuman primates have been investigated [18, 29, 32, 34, 36], these studies investigated small cohorts with limited age ranges and primate species other than cynomolgus monkeys. The pathophysiological background of CVD can be spontaneous, autoimmune, heritable and age-related. Some cardiomyopathies associated with growth have been investigated in experimental animals at various stages of life [31]. Therefore, the reference age stages should be established to explain and aid in the treatment of various cardiomyopathies. The echocardiographic reference data derived from young to elderly monkeys would help to clarify the pathophysiology of various age-related CVD. Echocardiographic reference values do not appear to have been reported previously for elderly monkeys. We therefore analyzed echocardiographic data for a wide range of ages in monkeys, because age-related CVDs are reportedly increased specifically in the elderly stage [33, 36].
The medical research demand for cynomolgus monkeys is increasing, because they are smaller than other macaques, and because they have a nonseasonal breeding cycle, which means they can be bred more easily.
The Tsukuba Primate Research Center has the largest colony of cynomolgus monkeys maintained in a closed environment for biomedical research in Japan, at about 1,800. These monkeys are bred in the same environment and they are used for various investigations such as vaccinology, infectious diseases, gene therapy, intractable diseases, regenerative medicine, and aging. In fact, many advanced clinical studies have been implemented globally using this colony [1, 3, 38]. In addition, spontaneous CVD has frequently been found in this colony [17]. The establishment of survey techniques adjusted for cynomolgus monkey would be useful to detect spontaneous CVD monkeys, in addition to being highly useful in the development of cardiovascular medicine.
As noted above, echocardiography is a noninvasive approach that is commonly applied to evaluate cardiac structures and functions. Using echocardiography for annual medical checks might help to maintain the health of the colony because CVD can be quickly identified. However, echocardiographic reference data for cynomolgus monkeys remain limited. Thus, the present study aimed to establish echocardiographic reference values using 247 experimental cynomolgus monkeys with a broad age range. This is the first comparison of echocardiographic reference values between young and elderly monkeys that should support investigations into various cardiomyopathies and medical studies.
Materials and Methods
Animals
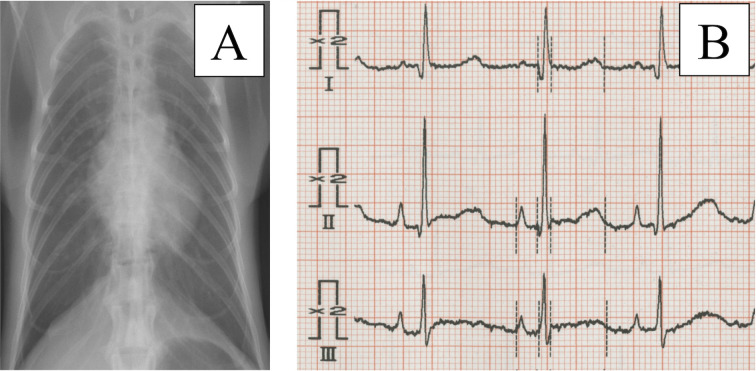
We clinically assessed 247 healthy cynomolgus monkeys (female, n=135; male, n=112) using echocardiography, electrocardiography, blood pressure (BP), chest X-rays and blood tests that included cardiac hormones (Tables 1, 2 and Fig. 1). For clinical evaluations, we used a 3-channel electrocardiograph (D300; Fukuda ME, Tokyo, Japan) to record standard 6-lead electrocardiograms (ECGs), and a sphygmomanometer (BP1000D; Fukuda ME) to record BP. Concentrations of the cardiac hormones human atrial natriuretic peptide (hANP) and human brain natriuretic peptide (hBNP) were examined using auto-enzyme immunoassay device (AIA-360 and AIA-900; Tosoh Corp., Tokyo, Japan). The monkeys were individually housed in stainless-steel cages and bred at the Tsukuba Primate Research Center. The housing conditions comprised: temperature, 23–27°C; humidity, 50–70%; 12 air changes/h; and 12/12-h light/dark cycles. Their daily diet consisted of 70 g of CMK-2 monkey chow (CLEA Japan, Inc., Tokyo, Japan) and 200 g of fruit unless otherwise indicated. All monkeys were annually assessed by standard blood tests, chest auscultation and standard physical examinations. This facility has kept around 1,800 monkeys constantly since 1978, with about 200 normal births obtained annually. Monkeys in this colony originated from Indonesia, Malaysia and the Philippines, with strains maintained by the “rotation line breeding system by different countries of origin” [10]. This study used only healthy monkeys showing no abnormalities on ECG, x-ray (Fig. 1) or blood tests, including cardiac hormones (Table 2), compared with our previous reports [21, 22].
Table 1. Mean weight and age of cynomolgus monkeys.
| n | Weight (kg) | Age (year) | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Immature | 90 | 3.24 ± 0.97 | 1.41–5.68 | 4.09 ± 1.46 | 0.0–6.4 |
| Mature | 114 | 4.60 ± 1.15 | 2.22–7.49 | 11.78 ± 3.66 | 6.5–19.3 |
| Elderly | 43 | 4.56 ± 1.41 | 2.00–8.85 | 23.72 ± 2.58 | 19.9–31.5 |
| Total | 247 | 4.10 ± 1.31 | 1.41–8.85 | 11.06 ± 7.35 | 0.0–31.5 |
Table 2. Blood pressure, heart rate and cardiac hormones in cynomolgus monkeys.
| BP | Heart rate(Beat/min) | Cardiac hormone | ||||
|---|---|---|---|---|---|---|
| SBP (mmHg) | DBP (mmHg) | MBP (mmHg) | hANP (pg/ml) | hBNP (pg/ml) | ||
| Mean ± SD | ||||||
| Immature | 103.6 ± 14.05 | 54.09 ± 12.86 | 74.87 ± 13.04 | 163.19 ± 29.31 | 27.86 ± 23.10 | 2.26 ± 3.25 |
| Mature | 107.67 ± 15.94 | 54.73 ± 13.02 | 81.04 ± 14.09 | 162.30 ± 21.44 | 24.54 ± 1.66 | 5.72 ± 12.91 |
| Elderly | 110.55 ± 10.07 | 63.42 ± 9.29 | 81.08 ± 9.16 | 171.87 ± 30.54 | 26.87 ± 16.55 | 5.63 ± 7.57 |
| Total | 106.43 ± 12.83 | 56.12 ± 12.83 | 78.32 ± 13.16 | 164.54 ± 27.19 | 26.43 ± 20.60 | 4.15 ± 9.07 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; hANP, human atrial natriuretic peptide; hBNP, human brain natriuretic peptide.
Fig. 1.
Chest x-rays and electrocardiograms (ECGs) indicate no abnormal findings. In this study, we applied screening tests such as chest X-ray (A) and ECG (B) for all monkeys. This x-ray suggests that the monkey has no cardiac or pulmonary dysfunction (A). This ECG, which is enhanced to double the amplitude of each wave, shows no cardiac load or electrophysiological abnormalities (B). Similar x-ray and ECG results were seen for all monkeys.
This study proceeded according to the Rules for Animal Care and Management of the Tsukuba Primate Research Center, the Guiding Principles for Animal Experiments Using Nonhuman Primates formulated by the Primate Society of Japan [10, 23] and the Guide for the Care and Use of Laboratory Animals [13]. The Animal Welfare and Animal Care Committee of the National Institute of Biomedical Innovation, Health and Nutrition (NIBIOHN, Osaka, Japan) approved the study protocol.
Echocardiography procedures
All echocardiographic assessments proceeded on monkeys immobilized using intramuscular injection of ketamine hydrochloride at 10 mg/kg body weight (Daiichi Sankyo Propharma, Tokyo, Japan). Ketamine immobilization is a popular method for examination in nonhuman primates. We checked ECG and BP before the echocardiographic examination, and the results are shown in Table 2. Monkeys were placed in the right and left lateral recumbent positions using a cut-out table and echocardiographic images were acquired from below using Prosound SSD-4000 or Prosound α7 ultrasonography systems (Hitachi Health Care Manufacturing Co., Ltd., Kashiwa, Japan). Standard dimensional echocardiographic views were obtained from the right parasternal and left apical windows.
Measured items, techniques and technical terms followed the recommendations of the American Society of Echocardiography [19]. We initially measured the cardiac parameters of left atrial diameter (LAD), aortic diameter (AOD), interventricular septum (IVS), left ventricular inner dimension (LVID) and left ventricular posterior wall thickness (LVPW). The LAD and AOD were measured at the maximal expansion and diastolic phases, respectively. Other sections were measured twice at the diastolic and systolic phases. Cardiac function was assessed as stroke volume (SV; diastolic minus systolic left ventricular volume), cardiac output (CO; stroke volume × heart rate), fractional shortening of the left ventricle (FS; diastolic LVID - systolic LVID)/diastolic LVID) and left ventricular ejection fraction (EF; [diastolic minus systolic left ventricular volume]/diastolic left ventricular volume.
The outflow velocity of the aortic (AV) and pulmonary (PV) valves and the inflow velocity of the tricuspid valve (TV) were measured. We determined the inflow velocity of the mitral valve by measuring peak blood flow velocity during early diastole, E waves and late diastole caused by atrial contraction, A waves, and the ratio of E waves to A waves (E/A). When only one wave was found in the mitral valve, the maximum velocity was considered as E wave inflow velocity.
Statistical analysis
The monkeys were examined and then classified as immature (age <8 years old), mature (≥8 to<20 years old), and elderly (>20 years old) according to previously reported conventions [16, 24, 28]. These groups and sex differences were assessed using one-way analysis of variance (ANOVA) and Tukey corrections. Data are expressed as means with standard deviations. Relationships between parameters, body weight and age were analyzed using Pearson correlation coefficient. The value of “r” was shown as a correlation, and values of P<0.05 were considered indicative of statistically significant relationships in tables and graphs. All data were statistically analyzed using IBM SPSS Statistics 20 (IBM, Armonk, NY, USA).
All echocardiographic values were obtained from monkeys at Tsukuba Primate Research Center. All tables contain means and reference ranges for all monkeys, and three groups of reference ranges are shown. Correlation coefficients indicating significant relationships between body weight or age and other parameters are indicated. Reference ranges for measured parameters were determined by calculating 95% confidence intervals (CIs) to cover 99% of all future values.
Results
All monkeys were assessed BP and ECG, and we confirmed that no abnormal signs were evident during immobilization. No abnormal ECG results such as arrhythmias were present in any monkeys. BP remained stable and immobilization was adequately maintained, suggesting that ketamine immobilization had no adverse effects in this study. In addition, examination of cardiac hormones hANP and hBNP to detect cardiac abnormalities showed no out-range values (Table 2). In contrast, heart rate was unaffected by age (Table 2).
Cardiac structure
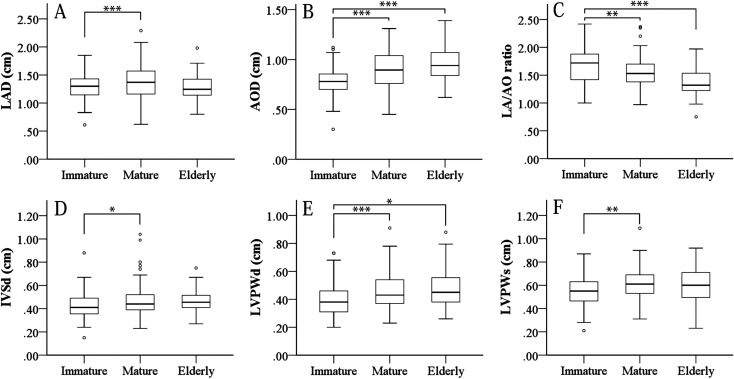
Table 3 and Figure 2 show that cardiac structure correlated with weight and age. Namely, all parameters for cardiac structures increased with growth. In particular, AOD, IVSd, LVPWd and LVPWs changed remarkably with growth, then stabilized after reaching maturity. In contrast, LAD did not appear to increase with age and the LA/AO ratio decreased with growth (Figs. 2A and C). These findings of cardiac expansion with growth are similar to previous findings [9]. In addition, cardiac structures displayed sexual differences in several parameters (Supplementary Table 1). In particular, LVID and LAD were suspected to be affected by the greater body size of males. Growth of body size with aging also differed between sexes. The immature to mature stages were particularly affected by sex, with LV size extended in males. However, these sizes became almost equal between sexes in the elderly stage (Supplementary Table 1).
Table 3. Mean values and correlations among cardiac structures.
| All age | Immature | Mature | Elderly | Correlation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Reference Range | Mean ± SD | Reference Range | Mean ± SD | Reference Range | Mean ± SD | Reference Range | Weight | Age | |
| LAD (cm) | 1.33 ± 0.26 | 1.30–1.36 | 1.30 ± 0.22 | 1.24–1.33 | 1.41 ± 0.26 | 1.33–1.44 | 1.28 ± 0.28 | 1.20–1.34 | .427*** | .021 |
| AOD (cm) | 0.87 ± 0.19 | 0.85–0.85 | 0.78 ± 0.14 | 0.75–0.81 | 0.92 ± 0.18 | 0.87–0.94 | 0.96 ± 0.20 | 0.90–1.01 | .421*** | .328*** |
| LA/Ao ratio | 1.58 ± 0.29 | 1.53–1.60 | 1.69 ± 0.29 | 1.60–1.73 | 1.56 ± 0.26 | 1.50–1.59 | 1.38 ± 0.27 | 1.30 -1.46 | -.040 | -.325*** |
| IVSd (cm) | 0.46 ± 0.12 | 0.44–0.47 | 0.43 ± 0.10 | 0.41–0.45 | 0.47 ± 0.13 | 0.44–0.49 | 0.49 ± 0.13 | 0.45–0.52 | .351*** | .188** |
| IVSs (cm) | 0.62 ± 0.15 | 0.60–0.64 | 0.60 ± 0.14 | 0.57–0.63 | 0.64 ± 0.17 | 0.60–0.66 | 0.63 ± 0.13 | 0.58 -0.67 | .323*** | .130* |
| LVIDd (cm) | 1.63 ± 0.34 | 1.59–1.68 | 1.54 ± 0.30 | 1.46–1.59 | 1.76 ± 0.32 | 1.69–1.81 | 1.56 ± 0.31 | 1.46–1.65 | .487*** | .056 |
| LVIDs (cm) | 1.03 ± 0.26 | 1.00–1.07 | 0.99 ± 0.20 | 0.93–1.02 | 1.11 ± 0.28 | 1.05–1.16 | 0.96 ± 0.24 | 0.87–1.03 | .359*** | -.030 |
| LVPWd (cm) | 0.44 ± 0.13 | 0.42–0.46 | 0.39 ± 0.11 | 0.38–0.43 | 0.45 ± 0.12 | 0.43–0.48 | 0.48 ± 0.15 | 0.44–0.53 | .324*** | .223*** |
| LVPWs (cm) | 0.60 ± 0.14 | 0.58–0.61 | 0.55 ± 0.11 | 0.53–0.58 | 0.62 ± 0.13 | 0.60–0.65 | 0.61 ± 0.16 | 0.55–0.66 | .321*** | .191*** |
*P<0.05, **P<0.01, ***P<0.001. IVSd, interventricular septum at diastole; IVSs, interventricular septum at systole; LA/AO, left atrium/aorta diameter; LVIDd, left ventricular inner dimension at diastole; LVIDs, left ventricular inner dimension at systole; LVPWd, left ventricular posterior wall thickness at diastole; LVPWs, left ventricular posterior wall thickness at systole.
Fig. 2.
Changes in cardiac structures including LA and AO diameter with age and weight. Graph shows cardiac structures related to LA and AO. Results of ANOVA (A–C). LAD remains essentially stable at all ages, with a slight tendency toward an increase in the mature group compared immature group (A). AOD and LA/AO ratio increased in all aging stages (B, C). Especially, aortic diameter at dilation increased remarkably with aging and growth (B) and LA/AO ratio concomitantly decreased (C). Diameter of LV walls (C, D). IVSd and LVPW increased until reaching maturity and then stabilized (D–F). Statistical outliers are plotted outside of the error bar. *P<0.05, **P<0.01, ***P<0.001; one-way ANOVA and Tukey’s test.
Cardiac function
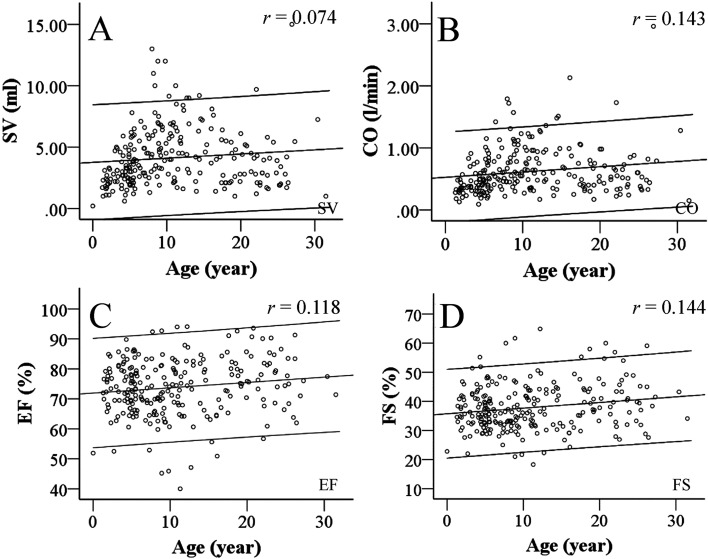
Table 4 shows mean cardiac function parameters and their correlations with body weight and age. Both SV and CO, like cardiac structures, correlated with body weight and increased with growth (Figs. 3A and B). On the other hand, EF was not associated with body weight (Table 4, Figs. 3C and D). However, only FS correlated slightly with aging despite being constant. In addition, several cardiac functional parameters differed between sexes (Supplementary Table 2). SV and CO showed particular increases with increasing body weight. In contrast, EF and FS were increased in females, unlike the influence of body weight.
Table 4. Mean values and correlations among cardiac function and inflow velocity.
| All age | Immature | Mature | Elderly | Correlation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Reference Range | Mean ± SD | Reference Range | Mean ± SD | Reference Range | Mean ± SD | Reference Range | Weight | Age | |
| SV (ml) | 4.13 ± 2.37 | 3.83–4.43 | 3.18 ± 1.55 | 2.88–3.51 | 4.99 ± 2.50 | 4.57–5.48 | 3.67 ± 2.58 | 2.86–4.44 | .527** | .074 |
| CO (l/min) | 0.62 ± 0.37 | 0.57–0.67 | 0.47 ± 0.25 | 0.43–0.53 | 0.70 ± 0.35 | 0.66–0.80 | 0.61 ± 0.77 | 0.47–0.78 | .481** | .143** |
| EF (%) | 73.87 ± 9.26 | 72.71–75.03 | 73.65 ± 7.26 | 72.25–75.36 | 73.58 ± 10.60 | 71.35–75.22 | 75.31 ± 8.40 | 73.02–78.35 | −.031 | .118 |
| FS (%) | 37.85 ± 7.78 | 36.88–38.83 | 36.75 ± 6.05 | 35.72–38.40 | 38.04 ± 8.67 | 36.37–39.50 | 38.95 ± 7.62 | 36.91–41.89 | .039 | .144* |
| AV outflow velocity (m/s) | 0.78 ± 0.17 | 0.76–0.80 | 0.82 ± 0.17 | 0.78–0.86 | 0.76 ± 0.16 | 0.72–0.78 | 0.76 ± 0.20 | 0.73–0.84 | −.023 | −.069 |
| PV outflow velocity (m/s) | 0.90 ± 0.21 | 0.87–0.92 | 0.92 ± 0.19 | 0.86–0.95 | 0.87 ± 0.20 | 0.82–0.90 | 1.00 ± 0.23 | 0.92–1.08 | .088 | .178** |
| E wave of MV (m/s) | 0.72 ± 0.20 | 0.69–0.74 | 0.8 ± 0.20 | 0.76–0.84 | 0.66 ± 0.17 | 0.63–0.70 | 0.67 ± 0.22 | 0.60–0.74 | −.250** | −.261** |
| A wave of MV (m/s) | 0.54 ± 0.17 | 0.51–0.57 | 0.52 ± 0.15 | 0.49–0.58 | 0.52 ± 0.16 | 0.50–0.58 | 0.54 ± 0.17 | 0.47–0.64 | .075 | .079 |
| E/A | 1.29 ± 0.46 | 1.21–1.37 | 1.51 ± 0.52 | 1.34–1.64 | 1.26 ± 0.40 | 1.15–1.33 | 0.95 ± 0.27 | 0.83–1.08 | −.287* | −.600* |
| TV inflow velocity (m/s) | 0.54 ± 0.21 | 0.51–0.57 | 0.47 ± 0.29 | 0.53–0.61 | 0.35 ± 0.30 | 0.48–0.57 | 0.31 ± 0.29 | 0.43–0.58 | −.003 | −.089 |
*P<0.01, **P<0.001. A wave of MV, atrial wave of mitral valve; AV outflow velocity, aorta valve outflow velocity; CO, cardiac output; E wave of MV, early wave of mitral valve; EF, ejection fraction; FS, fractional shortening of left ventricle; PV outflow velocity, pulmonary valve outflow velocity; TV inflow velocity, tricuspid valve inflow velocity.
Fig. 3.
Age-related changes in LV systolic function. This graph shows the relationship between LV function and aging. The X-axis range of coefficient lines indicates the 95%CI. Left ventricular systolic function was not obviously affected by aging, whereas SV (A), CO (B), EF (C) and FS (D) slightly increased with age. SV increases with aging and CO remains stable at all ages in humans, whereas our data show that SV and CO constantly increased with aging. Findings of EF and FS were similar to those in humans.
Inflow and outflow velocity
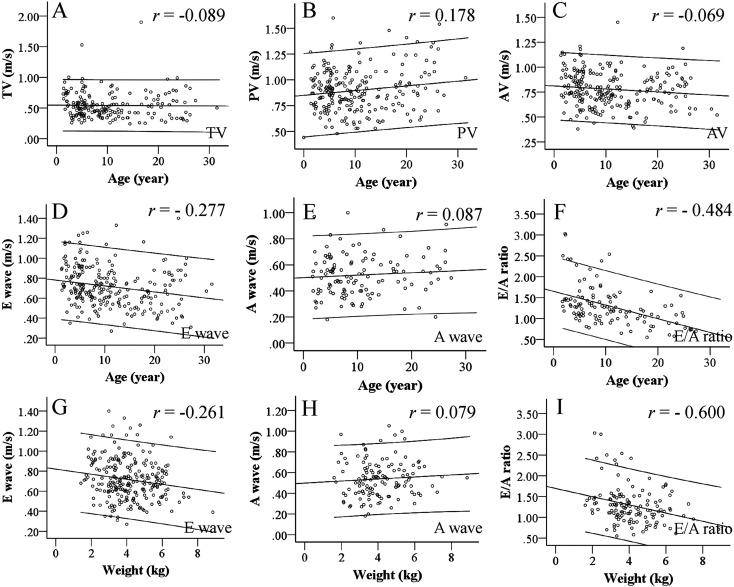
Table 4 and Figures 4A–C show that the inflow and outflow velocities of AV, PV and TV in the cardiac valves of cynomolgus monkeys did not fluctuate with growth and increasing body weight, whereas the E wave correlated negatively with age and body weight (Figs. 4D and G). The AV and TV and A wave did not change with aging (Figs. 4A–C, E and H). PV outflow velocity increased slightly with age (Table 4). The E/A ratio decreased with age and increased along with body weight (Figs. 4F and I), indicating that these parameters correlated with growth.
Fig. 4.
Inflow and outflow velocity according to age and body weight. This graph shows the relationships of in- or outflow velocity and aging. The x-axis range of coefficient lines indicates the 95%CI. Flow was stable in TV (A), slightly increased in PA (B), and somewhat decreased in AV with age (C). Like LV systolic functions, inflow and outflow velocity of cardiac valves were stable and not affected by aging due to various buffer mechanisms. E waves apparently decreased with aging (D), and E/A ratio showed the same tendency (F) due to the influence of changes in E waves. In contrast, A waves remained stable at all ages (E). E and A waves also decreased as body weight increased (G, H), but E/A ratio clearly decreased with age (I). These findings showed that atrial systolic function compensates for LV diastole function, and suggest that E waves consequently decrease.
E/A ratio varied in each age, but remained within the normal range for humans and other animals. In addition, cardiac stricture and SV were elevated in mature males (Supplementary Table 2). Those results were suggested to be related to the body size of monkeys.
Discussion
This report is the first to describe echocardiographic references for a large breeding colony of healthy cynomolgus monkeys with a broad range of ages and no cardiovascular dysfunction. Electrophysiological assessments have suggested that cardiac functions are similar between humans and cynomolgus monkeys [2]. Only a few reports have described echocardiographic reference values for cynomolgus monkeys [18, 29, 32, 36], and standardized echocardiographic reference values are incomplete, because those studies used small colonies and monkeys with a limited age range. However, echocardiography offers a graphic, real-time means of intravital imaging and has more potential for diagnosis than electrocardiography. Therefore, echocardiographic reference values for cynomolgus monkeys are urgently required for cardiovascular and other types of investigations. This study used 247 healthy cynomolgus monkeys that had been examined beforehand to ensure the absence of obvious pathology. All monkeys were immobilized by ketamine and lacked underlying diseases. BP showed no statistical difference in each age, but tended to increase with aging. Furthermore, the BP of macaques is reportedly lower than that of humans, and our data support that finding [11].
The indices of cardiac structures such as LAD and IVS significantly increased with body weight (Table 3). The AOD and LVPW enlarged with age between the immature and mature groups (Figs. 2B, E and F). The size of cardiac structures is considered to constantly change with growth, and our data also showed that cardiac volume increased with body weight [8, 9]. Those results were observed only in matured males (Supplementary Table 1). On the other hand, the LAD changed constantly and the LA/AO ratio obviously decreased with aging (Figs. 2A and C). These findings were similar to those in humans and other animals [30] and are conceivably a result of the increase in the size of cardiac structures. Also, the consistent, but not extreme increase in LVPW together with IVSd (Figs. 2D and E) resembles the situation in humans. This increase might similarly reflect the ratio of growth in cynomolgus monkeys as it does in humans [30].
Cardiac functions, especially SV and CO increased with body weight and age (Figs. 3A and B), which were thought to result from a concurrent increase in the size of the heart with physical growth. Those changes were particularly apparent in males (Supplementary Table 2). Previous studies have found that CO does not change, whereas SV increases with aging in humans [12, 25]. However, SV and CO slightly increased with aging and growth, then stabilized after reaching maturity. Heart rate affects CO because they are calculated as SV × heart rate. However, heart rates do not change with aging in cynomolgus monkeys [22]. We suggest that the nature of changes in CO in the present study was due to this influence. FS and EF, both representing important indices of cardiac function, did not change with age or weight (Figs. 3C and D), indicating that cardiac performance also did not change with age and weight. Neither FS nor EF is affected by the size of the body or heart in humans. Therefore, these results suggested that FS and EF can be used to diagnose cardiac disease at any age in cynomolgus monkeys [32]. That FS and EF in cynomolgus monkeys remained unchanged by aging is the same as findings in humans [7]. However, these results do not support the notion that cardiac systolic function remains unchanged because velocities reduced and cardiac interactions with the vascular system were altered. Cardiac functions (SV, CO, EF and FS) showed no significant differences between age groups (Table 4). This increase in cardiac functions was suggested to be related to BP elevation with aging, such as the effects of vessel stiffness. Cardiac functions showed sexual differences in some point of functions. In particular, SV and CO were high in males. On the other hand, EF and FS displayed significantly high levels in females (Supplementary Table 2). As previously reported in humans [35], females showed high EF, particularly in the senior stage. However, these results are reportedly affected by sex-related background factors of hormone and other physiological differences [19, 35]. Further discussion is required in this regard.
The inflow and outflow velocities of the TV, PV and AV did not change with body weight or age (Figs. 4A–C). Systolic functions such as SV, CO, FS and EF are maintained at rest, whereas diastolic functions of the MV change (Figs. 4D–I). E wave velocity significantly decreased with advancing age and increasing body weight (Figs. 4D and G), whereas the A wave was stable with all ages (Figs. 4E and H). The E/A ratio obviously decreased with aging in the present study (Figs. 4F and I). The filling ratio during early diastole decreases from a young age in human [4, 6, 20]. This is mainly due to an increase in isovolumic relaxation time. Additionally, these changes might be due to aging with valvular fibrosis and a decline in ventricular functions [14, 33]. Moreover, the decrease in MV inflow velocity with increasing body weight was thought to result from the distribution bias of lighter weight monkeys in the immature group. E/A ratio varied by age and sex (Supplementary Table 2). E/A ratio is an important parameter, indicating cardiac overload. However, we considered fluctuations in E/A ratio do not appear particularly relevant to the study of monkeys, because these changes remain within the normal range of reference values for humans and other animals.
The decline of cardiac functions (especially diastolic function) with aging are thought to result from diastolic disorders according to calcium ion dynamics in cardiac myocytes, cardiac fibrosis and changes in internuncial collagen with age [5, 15]. In addition, age-related changes in the endocrine environment such as the renin-angiotensin system are thought to cause diastolic functions to decline. However, the reasons for diastolic disorders remain unclear from the perspective of the molecular mechanisms of myocytes in cynomolgus monkeys. Hence, further molecular biology analyses are necessary.
We assumed that the inflow velocity from the MV to the left ventricle decreases due to a decrease in the E wave induced by declining cardiac diastolic function (Figs. 4D–I). On the other hand, the A wave tended to increase with aging, but these changes did not reach the level of statistical significance (Table 3). We estimated that the systolic function of the LA compensated for the declining diastolic function of the LV by increasing the A wave, and thus maintaining CO. Moreover, prolonged E wave deceleration was suggested. We were unable to measure E wave deceleration because cynomolgus monkeys have rapid heart rates. Compensatory buffer systems noted in the present study have also been identified in humans [22, 27]. Monkeys seem to have a similar cardiovascular system to that of humans and a buffering LV diastolic function that differs from that of other animals.
The present study clarified echocardiographic reference values, as well as age-dependent changes in cardiac function using echocardiography in cynomolgus monkeys over a wide range of ages and in both sexes. Echocardiography represents an important clinical diagnostic modality for humans and for medical studies of experimental animals. Additionally, interest in the effects of aging in cardiovascular systems is increasing. Our findings will be of value to clinical research in humans, and to experimental studies of nonhuman primates.
Supplementary Material
Acknowledgments
We thank Hiromi Ogawa and Yuko Katakai from the Corporation for Production and Research of Laboratory Primates for the care and handling of the monkeys, as well as Yoshitaka Ikeda and Harumi Shitara for their excellent technical assistance. This work was supported by JSPS KAKENHI Grant Numbers 19590834, 24615009 and 15K07789, as well as grants from the Japan Agency for Medical Research and Development (Grant no: 19ak0101047h0004).
References
- 1.Ageyama N., Kurosawa H., Fujimoto O., Uehara T., Hiroe M., Arano Y., Yoshida T., Yasutomi Y., Imanaka-Yoshida K.2019. Successful inflammation imaging of non-human primate hearts using an antibody specific for tenascin-C. Int. Heart J. 60: 151–158. doi: 10.1536/ihj.17-734 [DOI] [PubMed] [Google Scholar]
- 2.Ando K., Hombo T., Kanno A., Ikeda H., Imaizumi M., Shimizu N., Sakamoto K., Kitani S., Yamamoto Y., Hizume S., Nakai K., Kitayama T., Yamamoto K.2005. QT PRODACT: in vivo QT assay with a conscious monkey for assessment of the potential for drug-induced QT interval prolongation. J. Pharmacol. Sci. 99: 487–500. doi: 10.1254/jphs.QT-A4 [DOI] [PubMed] [Google Scholar]
- 3.Bashuda H., Kimikawa M., Seino K., Kato Y., Ono F., Shimizu A., Yagita H., Teraoka S., Okumura K.2005. Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates. J. Clin. Invest. 115: 1896–1902. doi: 10.1172/JCI23743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryg R.J., Williams G.A., Labovitz A.J.1987. Effect of aging on left ventricular diastolic filling in normal subjects. Am. J. Cardiol. 59: 971–974. doi: 10.1016/0002-9149(87)91136-2 [DOI] [PubMed] [Google Scholar]
- 5.Gazoti Debessa C.R., Mesiano Maifrino L.B., Rodrigues de Souza R.2001. Age related changes of the collagen network of the human heart. Mech. Ageing Dev. 122: 1049–1058. doi: 10.1016/S0047-6374(01)00238-X [DOI] [PubMed] [Google Scholar]
- 6.Gerstenblith G., Frederiksen J., Yin F.C., Fortuin N.J., Lakatta E.G., Weisfeldt M.L.1977. Echocardiographic assessment of a normal adult aging population. Circulation 56: 273–278. doi: 10.1161/01.CIR.56.2.273 [DOI] [PubMed] [Google Scholar]
- 7.Gutgesell H.P., Paquet M., Duff D.F., McNamara D.G.1977. Evaluation of left ventricular size and function by echocardiography. Results in normal children. Circulation 56: 457–462. doi: 10.1161/01.CIR.56.3.457 [DOI] [PubMed] [Google Scholar]
- 8.Hacker T.A., McKiernan S.H., Douglas P.S., Wanagat J., Aiken J.M.2006. Age-related changes in cardiac structure and function in Fischer 344 x Brown Norway hybrid rats. Am. J. Physiol. Heart Circ. Physiol. 290: H304–H311. doi: 10.1152/ajpheart.00290.2005 [DOI] [PubMed] [Google Scholar]
- 9.Henry W.L., Ware J., Gardin J.M., Hepner S.I., McKay J., Weiner M.1978. Echocardiographic measurements in normal subjects. Growth-related changes that occur between infancy and early adulthood. Circulation 57: 278–285. doi: 10.1161/01.CIR.57.2.278 [DOI] [PubMed] [Google Scholar]
- 10.Honjo S.1985. The Japanese Tsukuba Primate Center for Medical Science (TPC): an outline. J. Med. Primatol. 14: 75–89. [PubMed] [Google Scholar]
- 11.Hotchkiss C.E., Wang C., Slikker W., Jr2007. Effect of prolonged ketamine exposure on cardiovascular physiology in pregnant and infant rhesus monkeys (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 46: 21–28. [PubMed] [Google Scholar]
- 12.Izzo C., Carrizzo A., Alfano A., Virtuoso N., Capunzo M., Calabrese M., De Simone E., Sciarretta S., Frati G., Oliveti M., Damato A., Ambrosio M., De Caro F., Remondelli P., Vecchione C.2018. The Impact of Aging on Cardio and Cerebrovascular Diseases. Int. J. Mol. Sci. 19: E481. doi: 10.3390/ijms19020481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ILAR C. 2011. Guide for the care and use of laboratory animals (8th ed.). Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 14.Iwase M., Nagata K., Izawa H., Yokota M., Kamihara S., Inagaki H., Saito H.1993. Age-related changes in left and right ventricular filling velocity profiles and their relationship in normal subjects. Am. Heart J. 126: 419–426. doi: 10.1016/0002-8703(93)91061-I [DOI] [PubMed] [Google Scholar]
- 15.Jalil J.E., Doering C.W., Janicki J.S., Pick R., Shroff S.G., Weber K.T.1989. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ. Res. 64: 1041–1050. doi: 10.1161/01.RES.64.6.1041 [DOI] [PubMed] [Google Scholar]
- 16.Jayo M.J., Jerome C.P., Lees C.J., Rankin S.E., Weaver D.S.1994. Bone mass in female cynomolgus macaques: a cross-sectional and longitudinal study by age. Calcif. Tissue Int. 54: 231–236. doi: 10.1007/BF00301684 [DOI] [PubMed] [Google Scholar]
- 17.Koie H., Ageyama N., Ono F., Kanayama K., Sakai T., Sankai T.2005. Echocardiographic diagnosis of muscular ventricular septal defect in a cynomolgus monkey (Macaca fascicularis). Contemp. Top. Lab. Anim. Sci. 44: 26–28. [PubMed] [Google Scholar]
- 18.Korcarz C.E., Padrid P.A., Shroff S.G., Weinert L., Lang R.M.1997. Doppler echocardiographic reference values for healthy rhesus monkeys under ketamine hydrochloride sedation. J. Med. Primatol. 26: 287–298. doi: 10.1111/j.1600-0684.1997.tb00057.x [DOI] [PubMed] [Google Scholar]
- 19.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U.2015. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 16: 233–270. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 20.Miyatake K., Okamoto M., Kinoshita N., Owa M., Nakasone I., Sakakibara H., Nimura Y.1984. Augmentation of atrial contribution to left ventricular inflow with aging as assessed by intracardiac Doppler flowmetry. Am. J. Cardiol. 53: 586–589. doi: 10.1016/0002-9149(84)90035-3 [DOI] [PubMed] [Google Scholar]
- 21.Nakayama S., Koie H., Kanayama K., Katakai Y., Ito-Fujishiro Y., Sankai T., Yasutomi Y., Ageyama N.2018. Utility of arterial blood gas, CBC, biochemistry and cardiac hormones as evaluation parameters of cardiovascular disease in nonhuman primates. J. Vet. Med. Sci. 80: 1165–1173. doi: 10.1292/jvms.18-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama S., Koie H., Kato-Tateishi M., Pai C., Ito-Fujishiro Y., Kanayama K., Sankai T., Yasutomi Y., Ageyama N.2019. Establishment of a new formula for QT interval correction using a large colony of cynomolgus monkeys. Exp. Anim., Inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Primate Society of Japan. 1986. Guiding Principles for animal experiments using nonhuman primates. Primate Research 2: 111–113. doi: 10.2354/psj.2.111 [DOI] [Google Scholar]
- 24.Richtsmeier J.T., Cheverud J.M., Danahey S.E., Corner B.D., Lele S.1993. Sexual dimorphism of ontogeny in the crab-eating macaque (Macaca fascicularis). J. Hum. Evol. 25: 1–30. doi: 10.1006/jhev.1993.1035 [DOI] [Google Scholar]
- 25.Rodeheffer R.J., Gerstenblith G., Becker L.C., Fleg J.L., Weisfeldt M.L., Lakatta E.G.1984. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation 69: 203–213. doi: 10.1161/01.CIR.69.2.203 [DOI] [PubMed] [Google Scholar]
- 26.Roth G.S., Mattison J.A., Ottinger M.A., Chachich M.E., Lane M.A., Ingram D.K.2004. Aging in rhesus monkeys: relevance to human health interventions. Science 305: 1423–1426. doi: 10.1126/science.1102541 [DOI] [PubMed] [Google Scholar]
- 27.Ruan Q., Nagueh S.F.2005. Effect of age on left ventricular systolic function in humans: a study of systolic isovolumic acceleration rate. Exp. Physiol. 90: 527–534. doi: 10.1113/expphysiol.2005.030007 [DOI] [PubMed] [Google Scholar]
- 28.Schillaci M.A., Jones-Engel L., Lee B.P.Y.H., Fuentes A.2007. Morphology and somatometric growth of long-tailed macaques (Macaca fascicularis fascicularis) in Singapore. Biol. J. Linn. Soc. Lond. 92: 675–694. doi: 10.1111/j.1095-8312.2007.00860.x [DOI] [Google Scholar]
- 29.Sleeper M.M., Gaughan J.M., Gleason C.R., Burkett D.E.2008. Echocardiographic reference ranges for sedated healthy cynomolgus monkeys (Macaca fascicularis). J. Am. Assoc. Lab. Anim. Sci. 47: 22–25. [PMC free article] [PubMed] [Google Scholar]
- 30.Strait J.B., Lakatta E.G.2012. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail. Clin. 8: 143–164. doi: 10.1016/j.hfc.2011.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swinnen M., Vanhoutte D., Van Almen G.C., Hamdani N., Schellings M.W., D’hooge J., Van der Velden J., Weaver M.S., Sage E.H., Bornstein P., Verheyen F.K., VandenDriessche T., Chuah M.K., Westermann D., Paulus W.J., Van de Werf F., Schroen B., Carmeliet P., Pinto Y.M., Heymans S.2009. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation 120: 1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266 [DOI] [PubMed] [Google Scholar]
- 32.Tang H.L., Wang L.L., Cheng G., Wang L., Li S.2008. Evaluation of the cardiovascular function of older adult Rhesus monkeys by ultrasonography. J. Med. Primatol. 37: 101–108. doi: 10.1111/j.1600-0684.2007.00249.x [DOI] [PubMed] [Google Scholar]
- 33.Takenaka K., Dabestani A., Waffarn F., Gardin J.M., Henry W.L.1987. Effect of left ventricular size on early diastolic left ventricular filling in neonates and in adults. Am. J. Cardiol. 59: 138–141. doi: 10.1016/S0002-9149(87)80086-3 [DOI] [PubMed] [Google Scholar]
- 34.Tsusaki H., Yonamine H., Tamai A., Shimomoto M., Iwao H., Nagata R., Kito G.2005. Evaluation of cardiac function in primates using real-time three-dimensional echocardiography as applications to safety assessment. J. Pharmacol. Toxicol. Methods 52: 182–187. doi: 10.1016/j.vascn.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 35.Tsuchihashi-Makaya M., Hamaguchi S., Kinugawa S., Goto K., Goto D., Furumoto T., Yamada S., Yokoshiki H., Takeshita A., Tsutsui H.2011. Sex differences with respect to clinical characteristics, treatment, and long-term outcomes in patients with heart failure. Int. J. Cardiol. 150: 338–339. doi: 10.1016/j.ijcard.2011.03.042 [DOI] [PubMed] [Google Scholar]
- 36.Ueda Y., Gunther-Harrington C.T., Cruzen C.L., Robert J.A., Stern J.A.2017. Echocardiographic Parameters of Clinically Normal Geriatric Rhesus Macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 56: 361–368. [PMC free article] [PubMed] [Google Scholar]
- 37.Yan L., Ge H., Li H., Lieber S.C., Natividad F., Resuello R.R., Kim S.J., Akeju S., Sun A., Loo K., Peppas A.P., Rossi F., Lewandowski E.D., Thomas A.P., Vatner S.F., Vatner D.E.2004. Gender-specific proteomic alterations in glycolytic and mitochondrial pathways in aging monkey hearts. J. Mol. Cell. Cardiol. 37: 921–929. doi: 10.1016/j.yjmcc.2004.06.012 [DOI] [PubMed] [Google Scholar]
- 38.Yuki Y., Nochi T., Harada N., Katakai Y., Shibata H., Mejima M., Kohda T., Tokuhara D., Kurokawa S., Takahashi Y., Ono F., Kozaki S., Terao K., Tsukada H., Kiyono H.2010. In vivo molecular imaging analysis of a nasal vaccine that induces protective immunity against botulism in nonhuman primates. J. Immunol. 185: 5436–5443. doi: 10.4049/jimmunol.1001789 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.