Abstract
To investigate the effects of Co-Venenum Bufonis Oral Liquid (cVBOL) on radiation-induced esophagitis in rats. Irradiation (30 Gy) with X-RAD 225 x-ray was applied to induce esophagitis in 64 Wistar rats and treated by different methods. The body weight of rats either in RT group, cVBOL+RT, or EM+RT group was significantly decreased when compared with that in normal group (P<0.0001). After irradiation, histopathological studies, immunohistochemistry, and MRI scanning on esophagus were performed. Serum TNF-α,IL-6 and IL-10 were also determined by ELISA at 7, 14, 21 and 28 days after radiation treatment. The results demonstrated that radiation caused esophageal injury and thickening of esophageal tissue layers. The esophageal tissues after radiation treatment showed typical pathological changes of esophagitis. Radiation also caused esophagus edema. Treatment of cVBOL reduced the severity of histological esophageal lesion, decreased the expression of bFGF and TGF-β1, and lowered serum levels of inflammatory cytokines including TNF-α, IL-6 and IL-10 over 28 days after radiation treatment. In conclusion, cVBOL treatment is effective to prevent radiation induced esophagitis and reduces radiation induced esophagitis may be mediated through its ant-inflammatory effects.
Keywords: histopathology, magnetic resonance imaging (MRI), radiotherapy, radiation-induced esophagitis
Introduction
Radiation therapy or radiotherapy is one of the commonly used treatments for cancer patients. For example, radiotherapy is one of the mainstream treatments for advanced esophageal cancer, one of the most common malignant tumors of digestive tracts and the fourth leading cause of cancer death in China [8]. About 80% of the patients with esophageal cancer are treated with radiotherapy-based comprehensive treatment. Although a direct delivery of radiation to the tumor of the patient has been applied, radiotherapy induced acute and long-term damages to the surrounding normal tissues are still a common complication [1, 5, 21]. Radiation-induced esophagitis is one of the most common inflammatory reactions to radiotherapy in thoracic tumor [20, 24, 29].
Radiation induced esophageal injury is an aseptic inflammation of esophageal mucosa. The radiation mainly causes the esophageal mucosal injury. The main effector cells are monocytes and macrophages, which are over-activated in response to radiation stimulation and release a large number of inflammatory mediators, such as IL-6, IL-10 and TNF-α, leading to pathological damage [26]. The target cells of acute radiation-induced esophageal injury are mucosal basal cells. Radiation also induces oxidative stress through the formation of hydroxyl radicals by the decomposition of water molecules in esophageal tissue to cause the damage to normal esophageal tissues. Excessive oxygen free radicals can attack fatty acids, proteins and nucleic acids, resulting in decreased membrane fluidity, increased permeability, swelling of mitochondria and release of lysosomes, leading to tissue damage, causing and aggravating inflammatory reaction [13].
The pathological manifestations of radiation-induced esophagitis are congestion, edema, erosions, inflammatory exudative changes or even ulcers of the esophagus [3, 7]. The clinical manifestations were dysphagia, pain, burning sensation and aggravation after eating [15, 33]. Severe radiation induced esophagitis causes the patient to be afraid of radiotherapy, reducing the patient’s compliance and resulting in treatment failure [6].
To treat patients with radiation induced esophagitis, relieving severe pain and improving the quality of life of cancer patients becomes the main goals. In order to achieve this goal, medicines are used to relieve esophageal smooth muscle spasm, inhibit gastric acid secretion, induce detumescence by using high doses of anti-inflammatory medicines. however, the early use of antibiotics and hormones is not conform to rationale of clinical drug use, since radiation induced esophagitis is aseptic inflammation [2, 11, 25, 27]. Traditional Chinese medicine has been widely accepted by patients because of its low toxic side effects and its applicable in the early stages of esophagitis. Although traditional Chinese medicine has been used to treat patients with radiation induced esophagitis by reducing fever and detoxifying toxins, the basic research on the treatment of radiation induced esophagitis with traditional Chinese medicine has rarely been reported.
Venenum Bufonis was a Chinese medicine preparation widely used in clinical cancer therapy in China, which is also known as toad venom or Chan Su, derived from the dried white secretion of the skin and parotid venom glands of the toad bufo bufo gargarizans Cantor or bufo melanostictus schneider [14, 22, 31]. Venenum Bufonis used in traditional Chinese medicine, exhibits antipyretic, detoxicant, diuresis, stasis-eliminative and pus-discharging properties [19, 28]. It was demonstrated to have significant anti-cancer effects of a variety of cancers, including hepatic, gastric, pancreatic and esophageal carcinoma [12, 16, 18, 30]. Cinobufagin is the main active component in venenum bufonis, and as a monomer, it has clear molecular and structural formulas. Basic and clinical studies have shown that the pharmacological activity of cinobufagin is similar to that of venenum bufonis [32]. Venenum bufonis contains various compounds, including polypeptides, bufadienolides, indole alkaloids, and organic acids. Bufadienolides are widely recognized as the main active components of venenum bufonis; however, venenum bufonis also shows varying degrees of toxicity [4, 23]. To explore the efficiency of Chinese medicine in the treatment of radiation induced esophagitis,clinicians, through clinical observation, have found that Co-Venenum Bufonis Oral Liquid (cVBOL) is effective to prevent and treat radiation induced esophagitis. In this study, the effects of cVBOL on radiation induced esophagitis in rats were observed and the underlying mechanisms of therapeutic effects were studied.
Materials and Methods
Animals
This study included 64 female Wistar rats of 8-week-old (200.7 ± 2.155 g) obtained from Jinan PengYue experimental animal breeding Co., Ltd., Jinan, Shandong, China (Certificate of Quality No. SCXK (Lu) 20140007). The rats were quarantined for at least 3 days before exposure to irradiation. All groups received standard laboratory chow and water ad libitum. The experimental animal techniques and animal handling procedures were approved by the Institutional Animal Care and Use Committee of the Shandong Cancer Hospital and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory animals.
Drugs
The Co-Venenum Bufonis Oral Liquid (cVBOL) was processed and formulated in the Pharmacy Department of the Shandong Cancer Hospital. The concentration of crude medicinal components contained in the cVBOL was 0.491 g/ml by dissolved in water. The major components of cVBOL were Radix Scrophulariae, Paeoniae Radix Tree Peony Bark and Venenum Bufonis (Shanxi C&Y Pharmaceutical Group Co., Ltd., Datong, Shanxi, China). The major components of Esophagus Mixture (EM) [34] was 16 × 104 U gentamycin sulfate injection, 10 mg dexamethasone injection, 10 mg anisodamine injection mixed with 100 ml 1% procaine hydrochloride oral liquid. All solutions were administered orally. Rats were anesthetized with subcutaneous injection of 3% pentobarbital sodium administered (Sigma-Aldrich Co., LLC, St. Louis, MO, USA).
Animal models of radiation-induced esophagitis
The irradiation exposure was performed by using X-Ray 225 (Radsource, North Branford, CT, USA) with exposure dose rate of 219.93 cGy/min. According to the literature [10], the total irradiation dose was 30 Gy and the irradiated area was 2 cm×5 cm and 5 mm lead was used.
Rats were divided into four groups: (1) The treatment group (cVBOL+RT), in which rats were given 100g/ml.bw cVBOL through intra-esophageal perfusion with a stainless-steel feeding needle after irradiation exposure (2) The positive control group (EM+RT), in which rats were treated with Esophagus Mixture of 111.7g/ml.bw using the same route of administration as cVBOL after irradiation. (3) The radiation treatment only group (RT), in which rats was not subjected to any treatment following irradiation. (4) The normal group, in which rats did not receive irradiation and any treatment. Rats were sacrificed at 7, 14, 21, or 28 days after irradiation treatment.
General observation
The body weight of rats was measured every week after administration, and the behavioral and physiological indexes such as diet, drinking water, behavioral activities, rat fur, defecation and mental state were observed every day.
Blood collection
Wistar rats were anesthetized after an intraperitoneal injection of 3% pentobarbital sodium. All blood samples were obtained from different time points using capillary tubes introduced into the medial retro orbital venous plexus. The serum was separated by centrifugation at 3,000 rpm for 10 min. Samples were stored at −80°C until required as described previously.
Tissue collection and histopathological examination
Wistar rats were euthanized with carbon dioxide (CO2) and the esophagus was resected, tissue were immediately fixed in 10% formalin at room temperature for 24 h, embedded in paraffin and sectioned at 4 µm, mounted on glass microscope slides. Esophagus sections were stained with hematoxylin-eosin staining and examined using microscope and observation on epithelium and inner esophageal layer including lamina propria, muscularis mucosa and muscularis externa (BX35, OLYMPUS, Tokyo, Japan). The sections were stained with IHC and Observation indexes: (1) positive expression of whole epithelium; (2) positive cell rate. Observation methods: (1) the positive expression of epithelial layer was observed under low power microscope; (2) 10 visual fields were randomly selected under high power microscope for positive cell count performed using Image-ProPlus version 6.2 software and the visual fields were obtained by moving from left to right in order, each field was not repeated. The percentage of positive cells in each visual field was calculated and averaged.
Immunohistochemistry
Sections of esophagi were stained by immunohistochemistry. Then the sections were blocked with serum and incubated with antibodies against TGF-β1 (1:500) (Wuhan Servicebio Technology Co., Ltd., Wuhan, China), bFGF (1:200) (Wuhan Servicebio Technology Co., Ltd.) followed by secondary antibodies of HRP-conjugated Goat Anti-Rabbit (1:200) (Wuhan Servicebio Technology Co., Ltd.).
ELISA assay of cytokines
The serum TNF-α, interleukin-6 (IL-6) and interleukin-10 (IL-10) levels were determined by specific enzyme-linked immuno sorbent assay (ELISA) kit (CUSABIO, Wuhan, Hubei, China) following the procedure provided by ELISA kit. Samples were run in 96 well plates and determine absorbance using a microplate reader (SpectraMax i3, San Jose, CA, USA) at 450 nm and the concentrations of cytokines were obtained based on the standard curve.
Magnetic resonance imaging (MRI) scan and imaging analysis
All animals were scanned MRI (Discovery MR750W 3.0T, GE, Concord, MA, USA) at 7, 14, 21, 28 days after establishing the injury model. Briefly, animals were anesthetized with 3% pentobarbital sodium. Rats were placed prone onto the MRI table and secured. MRI scans using a T1-weighted with an echo time (TE) of 80 ms and repetition time (TR) of 3,000 ms was performed. All surviving animals received a final MRI scan to document image change in esophagus. Two radiology specialists independently evaluated all MRI images.
Statistical analysis
All data were analyzed by SPSS statistical software version 13.0 (SPSS, Chicago, IL, USA) and the data mean ± SD (X ± SD) was evaluated using one-way ANOVA at significance levels of P<0.05, <0.001, and <0.0001.
Result
Effect of cVBOL on general condition and body mass of rats
During the full period of experiments, the rats in each group had no abnormal behavior, bright and clean hair color, normal breath, stool shape, eating, drinking and activity. The groups of cVBOL+RT, EM+RT, RT was not significantly different with the normal group in 0 day (F=2.191, P=0.0981). The body weight of each rat was measured every day for 28 consecutive days. The data showed that the body weight of the four groups was not significantly different from each other before treatment (P<0.05). The body weight of rats in RT group was significantly lower than that in normal group on 7, 14, 21, 28 days (P<0.001). In addition, the body weights in normal group increased significantly compared with the cVBOL+RT in 7, 14, 21, 28 days (P<0.001). The body weights in normal group increased significantly compared with the EM+RT in 7, 14, 21, 28 days (P<0.05). These results suggest that the rats in RT group have shown manifest reduced and the RT model is successfully constructed (Table 1).
Table 1. Body weights of the rats at different times.
| Group | Time after radiation (Day) | ||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |
| cVBOL+RT Group | 200.7 ± 2.502 | 212.0 ± 1.702*** | 227.4 ± 3.119** | 244.7 ± 3.453** | 257.2 ± 4.564** |
| EM+RT Group | 200.8 ± 1.849 | 213.2 ± 4.152** | 228.1 ± 5.282* | 238.4 ± 6.749*** | 249.4 ± 5.537*** |
| RT Group | 200.9 ± 1.444 | 209.6 ± 4.311*** | 215.6 ± 6.620*** | 223.5 ± 6.788*** | 238.8 ± 8.950*** |
| Normal Group | 200.3 ± 2.410 | 217.4 ± 4.244 | 232.8 ± 5.713 | 252.7 ± 6.323 | 279.1 ± 6.872 |
| F | 2.191 | 17.89 | 10.57 | 19.81 | 30.15 |
| P | 0.0981 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
vs Normal group *P<0.05, **P<0.001, ***P<0.0001.
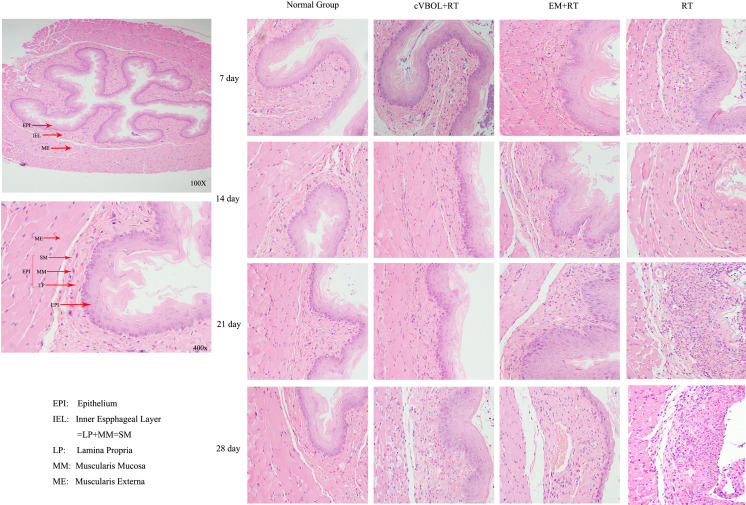
cVBOL reduces pathological changes after radiation treatment
There were no pathological lesions in the whole esophageal layers (muscularis mucosa, inner esophageal layer and epithelium) of the rat in normal group. The epithelium of the esophageal mucosa was intact and smooth, and there was no blood vessel congestion under the microscope. No infiltration of inflammatory cells in each layer of tissues was observed.
On the 7th day after irradiation treatment, the lesion of mucosal layer of the esophagus, the stratified flat epithelium was thickened, in which the lesion involved the submucosa in the model group (RT). Significant attenuated lesion in mucosal epithelium and reduced the squamous epithelium thickening was illustrated with the treatment group (cVBOL+RT).
On the 14th day of RT group, the much more serious damage in squamous epithelium of the mucosal epithelium and the spinous processes appeared. The cVBOL reduced the damage in epithelial layer of mucosa and increased thickness of the lamina propria.
On the 21st day in RT group, the esophagus demonstrated necrosis and exfoliation of mucosal epithelium, edema or vacuoles in basal cell layer, and moderate inflammatory cells infiltration in lamina propria, which were reduced by cVBOL treatment.
On the 28th day in RT group, severe necrosis and exfoliation of mucosal epithelium, multiple vacuoles in basal cell layer and ulceration foci in some cases were observed. Treatment of cVBOL improved the integrity of epithelial layer of mucosa, increased the thickness of the lamina propria, and reduced neutrophils infiltration (Fig. 1).
Fig. 1.
The pathological change in the esophageal tissue by radiotherapy (RT) X-ray 225. Esophageal tissue of Treatment group (cVBOL+RT), Control group (Esophagus Mixture+RT), Model group (RT) on 7th, 14th, 21st, 28th day (H&E×100, 400).
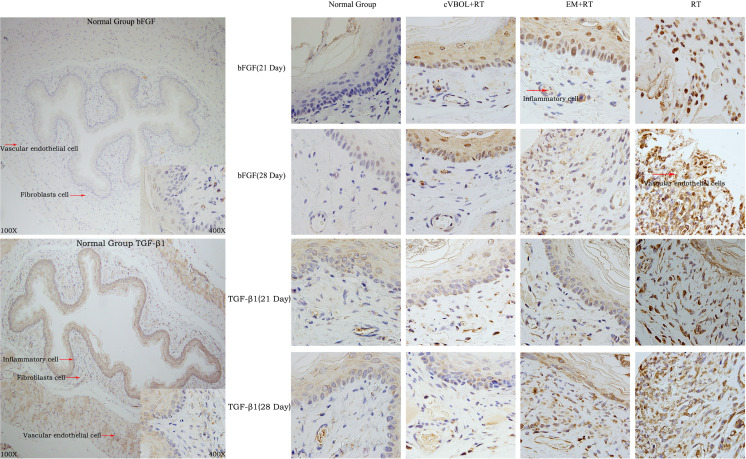
cVBOL reduces the expression of bFGF and TGF-β1 after radiation treatment
As shown in Fig. 2, IHC staining showed that bFGF and TGF-β1 were expressed in inflammatory cells, fibroblasts cells and vascular endothelial cells. The expression was mainly found in cytoplasm with brown granules. The number of cells that expressed bFGF or TGF-β1 did not significantly change over 28 days of period in normal group. Radiation treatment (RT group) significantly increased the number of cells that expressed bFGF or TGF-β1 from day 7 to day 28 when compared with normal group. Application of cVBOL (cVBOL+RT group) significantly lowered the number of cells that expressed bFGF or TGF-β1 at all 4 time points observed after radiation treatment (Table 2 and 3).
Fig. 2.
Immunohistochemistry results of the expression of bFGF and TGF-β1 in esophageal tissues of rats in each group. (A) Esophageal tissue of rat in the normal group (×400). (B) Esophageal tissue of rat in treatment group (cVBOL+RT, ×400). (C) Esophageal tissue of rat in the control group (EM+RT, ×400). (D) Esophageal tissue of rat in the model group (RT×400).
Table 2. Positive cells expression of bFGF results.
| Group | Time after radiation (Day) | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| cVBOL+RT Group | 26.6 ± 1.08†*** | 29.4 ± 2.06†††** | 43.6 ± 1.60†*** | 43.2 ± 3.60††*** |
| EM+RT Group | 29.2 ± 1.39†*** | 31.8 ± 1.39†††*** | 38.6 ± 1.03††*** | 44.2 ± 1.39††*** |
| RT Group | 40.6 ± 4.02** | 51.6 ± 1.89*** | 59.4 ± 3.09*** | 73.8 ± 4.18*** |
| Normal Group | 13.0 ± 0.89 | 13.4 ± 1.08 | 14.2 ± 0.58 | 14.8 ± 0.66 |
vs. RT group: †P<0.05, ††P<0.001,†††P<0.0001, vs Normal group: *P<0.05, **P<0.001, ***P<0.0001.
Table 3. Positive cells expression of TGF-β1 results.
| Group | Time after radiation (Day) | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| cVBOL+RT Group | 27.2 ± 0.86†*** | 30.6 ± 1.63†** | 38.4 ± 1.08†††* | 42.8 ± 1.43††*** |
| EM+RT Group | 26.8 ± 1.24†** | 31.8 ± 1.07††*** | 39.4 ± 1.50††*** | 43.4 ± 0.93†††*** |
| RT Group | 33.0 ± 1.30*** | 42.8 ± 1.39*** | 53.0 ± 1.30*** | 57.8 ± 1.63*** |
| Normal Group | 18.0 ± 0.95 | 19.4 ± 1.21 | 22.2 ± 0.97 | 22.8 ± 0.73 |
vs. RT group: †P<0.05, ††P<0.001,†††P<0.0001, vs Normal group: *P<0.05, **P<0.001, ***P<0.0001.
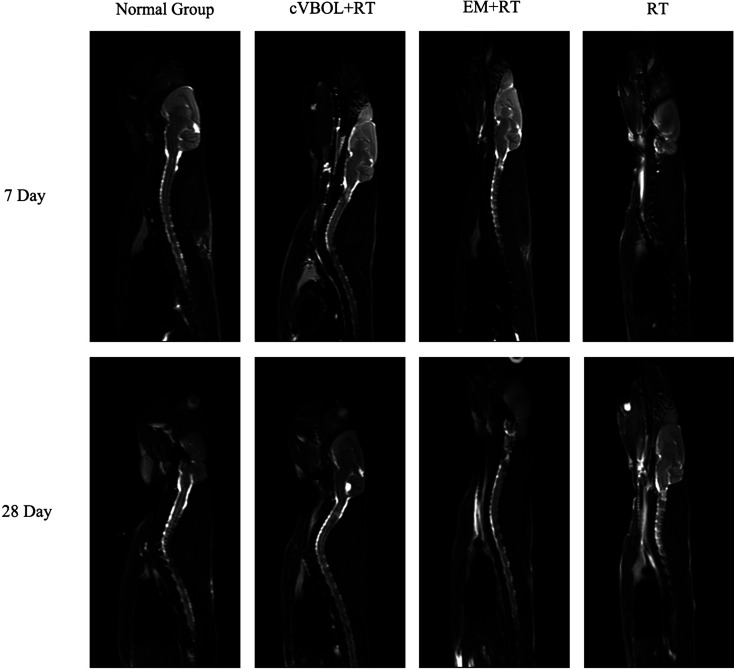
cVBOL reduces esophageal edema after radiation treatment
After establishing the model of radiation induced esophagitis in rats, magnetic resonance imaging (MRI) was used to monitor the progression of esophagitis and the effect of cVBOL treatment. As shown in Fig. 3, the lesion was shown as a wide high light intensity area in the model group. Irradiation induced esophageal mucosal edema and stenosis at the 28th day. While only slight esophagus edema was observed in the treatment group and the control group on the 28th day (Fig.3).
Fig. 3.
Comparative analysis of MRI on 7th and 28th day. MIR scan image in Normal group, Treatment group (cVBOL+RT), Control group (Esophagus Mixture+RT), Model group (RT)
cVBOL decreases the levels of IL-6,IL10 and TNF-α after radiation treatment
TNF-α, IL-6 and IL-10 function as important inflammatory cytokines in radiation induced esophagitis. The severity of radiation induced esophagitis could be evaluated directly by the change in these inflammatory cytokines. As shown in Table 4, 5, 6, radiation treatment induced progressive increase in serum levels of TNF-α, IL-6 and IL-10, which were decreased by cVBOL treatment, the effects were compatible to Esophagus Mixture treatment.
Table 4. Secretion of Wistar rat’s serum IL-6 factor at different time points.
| Group | Time after radiation (Day) | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| cVBOL+RT Group | 1.13 ± 0.07†††* | 1.53 ± 0.06††† | 3.69 ± 0.05†††*** | 4.76 ± 0.06†*** |
| EM+RT Group | 1.27 ± 0.04†††** | 1.87 ± 0.22††† | 3.65 ± 0.10†††*** | 4.68 ± 0.22 †*** |
| RT Group | 3.93 ± 0.28*** | 4.45 ± 0.19*** | 5.35 ± 0.13*** | 6.70 ± 0.39*** |
| Normal Group | 0.87 ± 0.04 | 1.33 ± 0.07 | 1.52 ± 0.07 | 1.97 ± 0.10 |
vs. RT group: †P<0.05, ††P<0.001,†††P<0.0001, vs Normal group: *P<0.05, **P<0.001, ***P<0.0001.
Table 5. Secretion of Wistar rat’s serum IL-10 factor at different time points.
| Group | Time after radiation (Day) | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| cVBOL+RT Group | 11.02 ± 0.25†** | 11.69 ± 0.37†* | 14.79 ± 0.55†††* | 16.47 ± 0.466†††*** |
| EM+RT Group | 11.42 ± 0.36†** | 12.39 ± 0.49†* | 15.85 ± 0.50†††** | 16.14 ± 0.58†††** |
| RT Group | 13.22 ± 0.42*** | 19.85 ± 3.75* | 22.03 ± 0.27*** | 29.82 ± 0.68*** |
| Normal Group | 7.66 ± 0.41 | 10.23 ± 0.20 | 11.41 ± 0.22 | 11.62 ± 0.23 |
vs. RT group: †P<0.05, ††P<0.001,†††P<0.0001, vs Normal group: *P<0.05, **P<0.001, ***P<0.0001.
Table 6. Secretion of Wistar rat’s serum TNF-α factor at different time points.
| Group | Time after radiation (Day) | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| cVBOL+RT Group | 9.510 ± 0.12†††*** | 11.43 ± 0.42†*** | 12.58 ± 0.11††*** | 13.39 ± 0.22†††*** |
| EM+RT Group | 8.678 ± 0.89†* | 10.61 ± 0.46†** | 12.34 ± 0.32††** | 13.36 ± 0.55†††** |
| RT Group | 13.26 ± 0.27*** | 15.86 ± 0.83*** | 27.88 ± 2.06*** | 42.85 ± 2.82*** |
| Normal Group | 4.733 ± 0.18 | 6.313 ± 0.29 | 8.163 ± 0.39 | 9.318 ± 0.28 |
vs. RT group: †P<0.05, ††P<0.001,†††P<0.0001, vs Normal group: *P<0.05, **P<0.001, ***P<0.0001.
Discussion
In this study, the role of a Chinese medicine, cVBOL, was investigated in radiation induced esophagitis in rats. The results indicated that cVBOL is effective in preventing or attenuating radiation induced esophageal damage.
Currently, the commonly used treatment for radiation induced esophagitis is antibiotics and topical anesthetics. The basis for use of these medicines is generally empirical and some of these drugs are not effective and even toxic. Treatment by using cortical hormones to relieve symptoms is also unlikely to cure. Lack of objective criteria or tools is a hurdle to evaluate and analyze the effects of a treatment [9]. In this respect, animal models of radiation induced esophagitis are a good choice for this purpose.
In animal experiments, Northway MG has been used opossums to establish the model of acute radiation induced esophagitis. Endoscopy, mucosal biopsy and esophageal barium film were used to test the pathological changes and evaluate the efficacy of drugs [17]. The changes of body weight, survival time, esophageal pathology and inflammatory cell score have also been used to evaluate the efficacy of the drug in the treatment of radiation induced esophagitis in C3H mice [10].
In this study, rats were used and radiation induced esophagitis was established, in which the progressive pathological and MIR changes in the esophagus were observed. We therefore evaluated the effectiveness of cVBOL in preventing and treating radiation esophagitis in this rat model. The results of the toxicity test used in this study showed that the cVBOL had little effect on the body mass of rats, the body weight in cVBOL+RT, EM+RT groups was lower than those in the normal group. The rats in the normal group did not receive radiation injury and drug treatment, while the rats in the RT group received radiation injury. In the later observation, it was found that the body weight of the rats in the RT group was significantly lower than that in the normal group. It shows that esophageal injury has a significant effect on the body weight of rats. The difference between the normal group and the cVBOL+RT group was caused by esophageal injury. There was no significant difference in body weight between the cVBOL+RT group and the EM+RT group, indicating that it was not the weight difference caused by drugs. This effect is consistent with that reported in literature [30].
The results showed that a Chinese medication combination, cVBOL, can significantly improve the general condition of rats, attenuate esophageal injury, and reduce the histological lesion after radiation treatment. Compared with the effectiveness of commonly used the combination (EM) that includes gentamycin, dexamethasone, anisodamine, and procaine, the effects of cVBOL is compatible to prevent radiation induced esophagitis. Radiation caused esophageal edema at 7 and 28 days after treatment illustrated by MRI. In addition, significant increase in serum inflammatory cytokines that include TNF-α, IL-6 and IL-10 after radiation treatment, indicating that radiation induces a systemic inflammatory response. Application of cVBOL can not only reduce esophageal edema, but also significantly down-regulate the levels of serum TNF-α, IL-6 and IL-10 in rats following radiation treatment, suggesting that anti-inflammatory reaction induced by radiation might be a mechanism through which cVBOL prevent radiation induced esophagitis.
In conclusion, the cVBOL has remarkable therapeutic effects on radiation-induced esophagitis in rats, suggesting that cVBOL is a potential alternative for the treatment of radiation induced esophagitis.
Acknowledgments
This study was supported in part by the General Programs of National Natural Science Foundation of China (Grants 81904186), Shandong Academy of Medical Sciences Foundation (Grant 2017-31, 2017-176).
References
- 1.Alevronta E., Ahlberg A., Mavroidis P., al-Abany M., Friesland S., Tilikidis A., Laurell G., Lind B.K.2010. Dose-response relations for stricture in the proximal oesophagus from head and neck radiotherapy. Radiother. Oncol. 97: 54–59. doi: 10.1016/j.radonc.2010.04.021 [DOI] [PubMed] [Google Scholar]
- 2.Akthar A.S., Golden D.W., Nanda R., Sharma M.R., Te H.S., Reddy K.G., Zhang X., Malik R.2016. Early and severe radiation esophagitis associated with concurrent sirolimus. J. Clin. Oncol. 34: e73–e75. doi: 10.1200/JCO.2013.50.1643 [DOI] [PubMed] [Google Scholar]
- 3.Bentzen S.M.2006. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat. Rev. Cancer 6: 702–713. doi: 10.1038/nrc1950 [DOI] [PubMed] [Google Scholar]
- 4.Bi Q.R., Hou J.J., Qi P., Ma C.H., Shen Y., Feng R.H., Yan B.P., Wang J.W., Shi X.J., Zheng Y.Y., Wu W.Y., Guo D.2016. Venenum Bufonis induces rat neuroinflammation by activiating NF-κB pathway and attenuation of BDNF. J. Ethnopharmacol. 186: 103–110. doi: 10.1016/j.jep.2016.03.049 [DOI] [PubMed] [Google Scholar]
- 5.Bradley J., Movsas B.2008. Radiation pneumonitis and esophagitis in thoracic irradiation. Cancer Treat. Res. 128: 43–64. doi: 10.1007/0-387-25354-8_4 [DOI] [PubMed] [Google Scholar]
- 6.Bradley J., Movsas B.2004. Radiation esophagitis: Predictive factors and preventive strategies. Semin. Radiat. Oncol. 14: 280–286. doi: 10.1016/j.semradonc.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 7.Brush J., Lipnick S.L., Phillips T., Sitko J., McDonald J.T., McBride W.H.2007. Molecular mechanisms of late normal tissue injury. Semin. Radiat. Oncol. 17: 121–130. doi: 10.1016/j.semradonc.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 8.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J.2016. Cancer statistics in China, 2015. CA Cancer J. Clin. 66: 115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 9.Cheng W.C., Shen M.F., Wu B.R., Chen C.Y., Chen W.C., Liao W.C., Chen C.H., Tu C.Y.2019. The prognostic predictors of patients with airway involvement due to advanced esophageal cancer after metallic airway stenting using flexible bronchoscopy. J. Thorac. Dis. 11: 3929–3940. doi: 10.21037/jtd.2019.08.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook J.A., Naz S., Anver M.R., Sowers A.L., Fabre K., Krishna M.C., Mitchell J.B.2018. Cancer Incidence in C3H Mice Protected from Lethal Total-Body Radiation after Amifostine. Radiat. Res. 189: 490–496. doi: 10.1667/RR14987.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coskun H., Andic F., Daglıoglu Y.K., Doran F., Sahin K., Tunalı C., Kucuk O.2017. Lycopene in the Prevention of Radiation-Induced Esophagitis. Nutr. Cancer 69: 319–329. doi: 10.1080/01635581.2017.1265133 [DOI] [PubMed] [Google Scholar]
- 12.Deng X., Sheng J., Liu H., Wang N., Dai C., Wang Z., Zhang J., Zhao J., Dai E.2019. Cinobufagin Promotes Cell Cycle Arrest and Apoptosis to Block Human Esophageal Squamous Cell Carcinoma Cells Growth via the p73 Signalling Pathway. Biol. Pharm. Bull. 42: 1500–1509. doi: 10.1248/bpb.b19-00174 [DOI] [PubMed] [Google Scholar]
- 13.Djordjević V.B.2004. Free radicals in cell biology. Int. Rev. Cytol. 237: 57–89. doi: 10.1016/S0074-7696(04)37002-6 [DOI] [PubMed] [Google Scholar]
- 14.Gao H., Popescu R., Kopp B., Wang Z.2011. Bufadienolides and their antitumor activity. Nat. Prod. Rep. 28: 953–969. doi: 10.1039/c0np00032a [DOI] [PubMed] [Google Scholar]
- 15.Hirota S., Tsujino K., Endo M., Kotani Y., Satouchi M., Kado T., Hishikawa Y., Obayashi K., Takada Y., Kono M., Abe M.2001. Dosimetric predictors of radiation esophagitis in patients treated for non-small-cell lung cancer with carboplatin/paclitaxel/radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 51: 291–295. doi: 10.1016/S0360-3016(01)01648-0 [DOI] [PubMed] [Google Scholar]
- 16.Kamano Y., Yamashita A., Nogawa T., Morita H., Takeya K., Itokawa H., Segawa T., Yukita A., Saito K., Katsuyama M., Pettit G.R.2002. QSAR evaluation of the Ch’an Su and related bufadienolides against the colchicine-resistant primary liver carcinoma cell line PLC/PRF/5(1). J. Med. Chem. 45: 5440–5447. doi: 10.1021/jm0202066 [DOI] [PubMed] [Google Scholar]
- 17.Lawson J.D., Otto K., Grist W., Johnstone P.A.S.2008. Frequency of esophageal stenosis after simultaneous modulated accelerated radiation therapy and chemotherapy for head and neck cancer. Am. J. Otolaryngol. 29: 13–19. doi: 10.1016/j.amjoto.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 18.Li M., Yu X., Guo H., Sun L., Wang A., Liu Q., Wang X., Li J.2014. Bufalin exerts antitumor effects by inducing cell cycle arrest and triggering apoptosis in pancreatic cancer cells. Tumour Biol. 35: 2461–2471. doi: 10.1007/s13277-013-1326-6 [DOI] [PubMed] [Google Scholar]
- 19.Li X.Y., Lu Y., Shan Q.X., Xia Q.2006. [Effect of cinobufacini on vascular contractile of rat thoracic aorta]. Zhejiang Da Xue Xue Bao Yi Xue Ban 35: 178–181. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 20.Maier M.T., Vilhelmsson A., Louie S.M., Vagena E., Nomura D.K., Koliwad S.K., Xu A.W.2018. Regulation of Hepatic Lipid Accumulation and Distribution by Agouti-Related Protein in Male Mice. Endocrinology 159: 2408–2420. doi: 10.1210/en.2018-00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu Y., Wang H., Wiktor-Brown D., Rugo R., Shen H., Huq M.S., Engelward B., Epperly M., Greenberger J.S.2010. Irradiated esophageal cells are protected from radiation-induced recombination by MnSOD gene therapy. Radiat. Res. 173: 453–461. doi: 10.1667/RR1763.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi F., Li A., Inagaki Y., Gao J., Li J., Kokudo N., Li X.K., Tang W.2010. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 4: 297–307. [PubMed] [Google Scholar]
- 23.Qi F., Li A., Inagaki Y., Kokudo N., Tamura S., Nakata M., Tang W.2011. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int. Immunopharmacol. 11: 342–349. doi: 10.1016/j.intimp.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 24.Shen L., Li X., Shan B., Zhang L., Gong Y., Dong Z., Wang Z.2013. Therapeutic effect of compound of White Peony Root Oral Liquids on radiation-induced esophageal toxicity via the expression of EGF and TGF-β1. Biomed. Rep. 1: 308–314. doi: 10.3892/br.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topkan E., Yavuz M.N., Onal C., Yavuz A.A.2009. Prevention of acute radiation-induced esophagitis with glutamine in non-small cell lung cancer patients treated with radiotherapy: evaluation of clinical and dosimetric parameters. Lung Cancer 63: 393–399. doi: 10.1016/j.lungcan.2008.06.015 [DOI] [PubMed] [Google Scholar]
- 26.Usami S., Motoyama S., Koyota S., Wang J., Hayashi-Shibuya K., Maruyama K., Takahashi N., Saito H., Minamiya Y., Takasawa S., Ogawa J., Sugiyama T.2010. Regenerating gene I regulates interleukin-6 production in squamous esophageal cancer cells. Biochem. Biophys. Res. Commun. 392: 4–8. doi: 10.1016/j.bbrc.2009.12.129 [DOI] [PubMed] [Google Scholar]
- 27.Vidal-Casariego A., Calleja-Fernández A., Ballesteros-Pomar M.D., Cano-Rodríguez I.2013. Efficacy of glutamine in the prevention of oral mucositis and acute radiation-induced esophagitis: a retrospective study. Nutr. Cancer 65: 424–429. doi: 10.1080/01635581.2013.765017 [DOI] [PubMed] [Google Scholar]
- 28.Wang S.S., Zhai X.F., Li B.2009. [Effects of cinobufacini injection on contents of serum thyroid-stimulating hormone and adrenaline in rats]. J. Chin. Integr. Med. 7: 228–231. (in Chinese) doi: 10.3736/jcim20090306 [DOI] [PubMed] [Google Scholar]
- 29.Werner-Wasik M., Yorke E., Deasy J., Nam J., Marks L.B.2010. Radiation dose-volume effects in the esophagus. Int. J. Radiat. Oncol. Biol. Phys. 76:(Suppl): S86–S93. doi: 10.1016/j.ijrobp.2009.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong X., Lu B., Tian Q., Zhang H., Wu M., Guo H., Zhang Q., Li X., Zhou T., Wang Y.2019. Inhibition of autophagy enhances cinobufagin‑induced apoptosis in gastric cancer. Oncol. Rep. 41: 492–500. [DOI] [PubMed] [Google Scholar]
- 31.Xu L., Wang S., Shen H., Feng Q., Zhang X., Ni H., Yao M.2019. Analgesic and toxic effects of venenum bufonis and its constituent compound cinobufagin: A comparative study. Neurotoxicol. Teratol. 73: 49–53. doi: 10.1016/j.ntt.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 32.Zhang D.M., Liu J.S., Tang M.K., Yiu A., Cao H.H., Jiang L., Chan J.Y., Tian H.Y., Fung K.P., Ye W.C.2012. Bufotalin from Venenum Bufonis inhibits growth of multidrug resistant HepG2 cells through G2/M cell cycle arrest and apoptosis. Eur. J. Pharmacol. 692: 19–28. doi: 10.1016/j.ejphar.2012.06.045 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z., Xu J., Zhou T., Yi Y., Li H., Sun H., Huang W., Wang D., Li B., Ying G.2014. Risk factors of radiation-induced acute esophagitis in non-small cell lung cancer patients treated with concomitant chemoradiotherapy. Radiat. Oncol. 9: 54. doi: 10.1186/1748-717X-9-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C.X., Hu X., Pan R.Q.2014. Curative effect analysis of esophagus mixture treatment for acute radiative oesophagitis. J. N. Sichuan Med. Coll. 29: 68–71. [Google Scholar]