Abstract
High fat diet (HFD) treated mouse is widely used as experimental animal model for hyperlipidemia and hyperglycemia study. Many factors contribute to establish animal model that meant to simulate high fat and glucose diet induced phenotypes. In the present study, four strains of experiment mouse treated by HFD were used to explore the impact of mouse strain on lipid profile, glucose level, and major inflammation cytokines. HFD fed Kunming and ICR mouse gained significantly higher body weight than control which was not shown by C57BL/6 and BALB/c mouse. All four strains fed by HFD has heavier liver and adipose tissue than control ones. Obvious fat droplets and enlarged adipose cells were observed in obese mouse of four strains. Additionally, obese mouse showed typical response to glucose and insulin load in OGTT and ITT. Serum TC, LDL-c, and TC/HDL-c ratio, but not TG, increased in all four strains. Major inflammatory cytokines and insulin level showed little changes in obese mouse as well (P<0.05) The present study could provide basic information for diet induced obesity developed by four commonly used experimental mouse strains.
Keywords: hyperglycemia, hyperlipidemia, inflammation, obesity, serum lipid profile
Introduction
Obesity is one of the most common metabolic disease in both developing and developed countries due to the prevalence of sedentary lifestyle and fast food containing high level of fat and glucose [22, 35]. Obesity in human correlates with many chronic diseases like hyperlipidemia, hyperglycemia, diabetes, low-grade inflammation, hypertension coronary artery disease, metabolic syndrome etc. [33]. Hence, it is necessary to learn how to curb development of obesity and reduce related symptoms.
Experiment animals are suitable substitutions for human to study obesity and its related biomarkers. Among the many used animals, mouse is widely applied to develop obesity due to several advantages. Experiment mouse is relatively cheap and commercially available for most labs [5]. Also, mouse growth faster and consume less feed than most experiment animals like hen, rabbit and pig etc. In most societies, mouse is regarded as a pest and cause less ethnics controversy than other animal like cow or monkey. Mouse in different strain shows much different phenotypic characteristics like growth rate, tendency of obesity, and the ability to regulate the blood glucose and lipid. So, when developing obese model using mouse, it is necessary learn how mouse in different strain response to high fat diet (HFD).
Kunming mouse was introduced from Hoffkine Research Institute of India by Professor Feifan Tang in 1944 [9]. It belongs to the Swiss mice strain and named after the place where it was first introduced, Kunming. It has strong disease resistance and adaptability, shows high reproductive and survival rate [37]. It is also very cheap in price as one commercial Kunming mouse is about 6 yuan in 2019, which is about 0.8 US dollar. Kunming mouse is now the most widely used strain for common biological study in China. More than 70% of mouse used for biology and medical experiment in China is Kunming strain. Hence, many researchers use Kunming mice to develop obese model by high fat and glucose diet (HFGD) feeding [10, 19].
Like Kunming mouse, ICR is an outbreeding mouse strain which was breed from Swiss mouse. ICR is suitable for common lab experiment in term of fast growth, strong body, docile temperament. Price of one ICR mouse is about 12 yuan in 2019, which is about 1.6 US dollar, higher than that of Kunming mouse. ICR mouse is also widely used for research of obesity, especially for assessing anti-obesity function of food and nutraceuticals [12, 39].
C57BL/6 mouse was bred in 1921 as inbred strain by C.C. Little using the strain of Lathrop [26]. It has black hair and is widely employed to study athetosis, birth defects, obesity, diabetes, cancer etc. C57BL/6 mouse has low incidence of cancer but could develop obesity, diabetes and atherosclerosis induced by food [8]. Price of one C57BL/6 mouse is about 28 yuan in 2019, which is about 4 US dollar, much higher than that of Kunming mouse.
Another widely used white mouse is BALB/c which is also an inbred strain used in research of immunology and physiology. BALB/c is docile in temperament and adapt well to living in group in a cage. Price of BALB/c is the same with C57BL/6 mouse in 2019. BALB/c is also widely used in the obesity researches [29].
All the four mouse strains are commonly used in the study of food induced obesity and its relevant symptoms such as hyperlipidemia, hyperglycemia, diabetes and low-grade inflammation. Many factors can impact on the development of obesity. Among them, the mouse strain used is one of the most important factors. However, little information is available about the performance of different mouse strains when used to develop obese model. There is fewer comparison and discussion of mouse strain when the results were analyzed. Additionally, even used the same mouse strain to develop obese model, discrepancies in major obese biomarkers are commonly reported with very few explanations.
Hence, in the present study four widely used mouse strains, Kunming, C57BL/6, BALB/c and ICR, were used to develop obesity. Major biomarkers including body weight, blood lipid profile, blood glucose level, serum inflammation level etc., were monitored and compared. The results of this study are helpful to build food induced obesity mouse and related researches.
Materials and Method
Mouse strains and grouping
All four strains of mouse (male) were purchased from Laboratory Animal Center of Anhui Medical University, Hefei, China with 14 mice for each strain. The age in weeks of Kunming, C57BL/6, BALB/c and ICR were 8 weeks, 6 weeks, 6 weeks and 6 weeks respectively. Average body weight for Kunming, C57BL/6, BALB/c and ICR were 30 ± 2 g, 18 ± 2 g, 18 ± 2 g and 20 ± 2 g, respectively. For each strain, 7 mice were grouped as control and 7 were used as model, raised in an animal cage with saw dust bedding. Conditions of mice house are as follows: 23 ± 2°C, 55 ± 5% relative humidity, 12 h light/dark cycle. All mice were fed with standard chow (26% kcal from fat, Shoobree, Jiangsu, China) for 1 week to acclimatize before experiment started. The ingredient composition of standard chow is tabulated in Supplementary Table 1. Then, one group of mice were continuing to fed with standard diet while the other group were switched to high fat diet. The experiment was performed for 10 weeks before all mice were sacrificed. Animal welfare and experimental procedures in this study were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The project was reviewed and approved by the Committee for Protection of Animal Care Committee at the Hefei University of Technology.
Composition of high fat diet
High fat diet was formulated according to previous publications [3]. The composition was displayed in Supplementary Table 2 (53% kcal from fat). Maintain feed was the standard diet which was used for the control group.
Body weight and organ index measurement
Body weight of mice were recorded every week during the experiment period. After mice were sacrificed, major organs were harvested and weighted.
Liver and adipose tissue histological observation
Tissue was harvested and fixed in 10% formalin, imbedded in paraffin, followed by dehydration in graded alcohol. Tissue slices of 5 µm thickness prepared and stained with hematoxylin-eosin (H&E), and observed on a light microscope with magnification of 200 times.
Blood glucose, insulin level and lipid profile
At the 8th week after the experiment initialed, oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were performed according to the previous reported method [21]. The area under curve (AUC) of OGTT and ITT lines were calculated using GraphPad Prism. After mice were sacrificed, blood serum was obtained by centrifuge. Concentrations of HDL-c, LDL-c, TG, T-CHO and insulin in serum were measured using commercial kits according to the instruction.
Inflammation profile
Concentrations of six inflammation cytokines commonly examined in obese mice model, IL-1β, IL-6, IL-10, TNF-α, MCP-1 and LPS, were measured in serum using commercial ELISA kits [1, 24].
Statistical analysis
All tests were conducted in triplicate. Data were presented as the means ± SD. Statistical analyses were performed with Student’s t-test with a statistical analysis software package (SPSS 22.0, Chicago, IL, USA). A value of P<0.05 was considered significantly different.
Results
Body weight and organ index
Mouse strain exerts great impact on the body weight gain during HFD treatment. For Kunming mice, from the second week after providing HFD, the body weight increased to a significant higher level than control (Fig. 1a). It should be noted that Kunming mouse is larger than other mice at the initial stage of the experiment. From the start to the end of the experiment, HFD treated Kunming mice are heavier than control. But for C57BL/6 mice, no difference revealed for the body weight between control and obesity model (Fig. 1b). The body weight increase profile was also similar for that of BALB/c mice (Fig. 1c). Hence in our present experiment conditions, HFD treated C57BL/6 and BALB/c mice failed to gain much higher body weight than control. On the other hand, HFD fed ICR mice accumulated much heavier body weight than control from the 6th week (Fig. 1d). Food intake was also monitored during the experiment. Food intake of obesity model was slightly lower than that of control but with no significant difference (Supplementary Table 3).
Fig. 1.
Body weight of mice fed with normal chow (CON) and high fat diet (MOD) of the four mice strain: a is body weight change of Kunming mice; b is body weight change of C57BL/6 mice; c is body weight change of BALB/c mice and d is body weight change of ICR mice.
Excessive lipid induced by diet will be stored in liver and adipose tissues in the obese mice which has been widely reported in more consistent way. Hence heavier liver and adipose tissue are also regarded as important indicators of obesity. In our result, liver and adipose tissue in obese mice is heavier than control ones in most of mice strain (Figs. 2a and b). For Kunming mice, liver index showed no significant diffidence even though obese mice liver was heavier than control in average number (Fig. 2a). Liver indices in all other 3 mice strains were significantly higher than that of control ones. Especially for ICR mice, liver index of obese mice was significantly higher than that of control at the P value smaller than 0.01. Weight of adipose tissue index for Kunming, C57BL/6 and ICR obese mice was higher than that of control (Fig. 2b). But BALB/c showed no difference of adipose tissue index between obese and control ones. It should be also noted that adipose tissue index varied significantly among both control and treated mice.
Fig. 2.
Liver and fat index of mice fed with normal chow (CON) and HFD (MOD) of four mouse strains: Kunming, C57BL/6, BALB/c and ICR. Different superscript letters among bars denote significant difference (P<0.05) according to Tukey’s test.
Histological feature of liver and adipose tissue
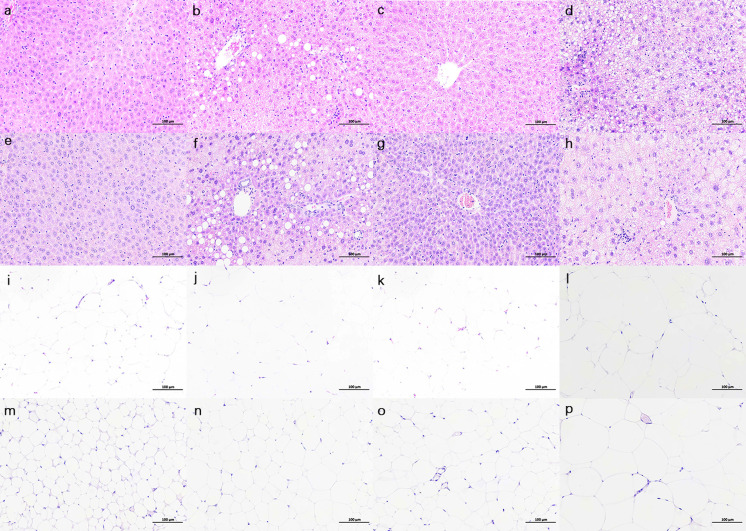
Liver sections of mice in control group were similar in terms of neatly arranged liver cells with no accumulation of lipid droplets and inflammation cell infiltration (Figs. 3a, c, e and g). However, in obese mice liver sections many lipid droplets presented alongside the liver cells (Figs. 3b, d, f and h). In liver section of Kunming mice, very large lipid droplets outside the liver cells as well as smaller lipid droplets inside the liver cells could be observed (Fig. 3b). Lipid accumulation in liver is similar for C57BL/6 and BALB/c mice. For ICR mice, it is noted that much smaller lipid droplets exist inside the liver cells which should be ascribed to different way of lipid storage in the liver cells. Inflammatory cell infiltration is also one important trait of obese mice liver. Liver in obese mice of four strains showed significant inflammatory cell infiltration. At the same time, adipose tissues in all HFD treated mice showed significantly different properties compared with normal ones in terms of larger adipose cells (Figs. 3j, i, n and p). In addition, as important biochemical markers of liver injury, ALT and AST activity in liver was also examined as shown in Fig. 4. For ALT, mouse strain exerts strong impact as obese mice exhibited higher ALT activity than control ones except for BALB/c mice. AST showed little difference between control and model mice.
Fig. 3.
Histomorphology photomicrographs of the liver (hematoxylin and eosin-stained) and fat tissue: a is liver of Kunming mice fed with normal chow; b is liver of Kunming mice fed with HFD; c is liver of C57BL/6 mice fed with normal chow; d is liver of C57BL/6 mice fed with HFD; e is liver of BALB/c mice fed with normal chow; f is liver of BALB/c mice fed with HFD; g is liver of ICR mice fed with normal chow, h is liver of ICR mice fed with HFD; i to p is fat tissue of mice in the same sequence. Scale bar is 100 µm
Fig. 4.
Activity of glutamic-pyruvic transaminase (ALT) and glutamic oxalacetic transaminase (AST) (a and b respectively) in liver of control (CON) and obese (MOD) mice of four mouse strains: Kunming, C57BL/6, BALB/c and ICR. Different superscript letters among bars denote significant difference (P<0.05) according to Tukey’s test.
Oral glucose tolerant test (OGTT) and insulin tolerant test (ITT)
For Kunming, C57BL/6 and ICR, obese mice showed higher peak glucose level and prolonged period to lower the glucose level (Figs. 5a, c and g). BALB/c obese mice presented similar peak glucose number but higher level after 30 min in the test (Fig. 5e). OGTT AUC of obese Kunming, C57BL/6, BALB/c and ICR were significantly higher than respective control values which indicate that HFD interferes the glucose metabolism of all the four mice strains (Figs. 5b, d, f and h).
Fig. 5.
Change of blood glucose concentration (a, c, e and g respectively) in oral glucose tolerant test (OGTT) of control (CON) and obese (MOD) mice of four mouse strains: Kunming, C57BL/6, BALB/c and ICR with their OGTT-AUC values (b, d, f and h respectively). Different superscript letters among bars denote significant difference (P<0.05) according to Tukey’s test.
ITT results revealed that after insulin injection, blood glucose level decreased for both control and model mice of four strain (Fig. 6). For C57BL/6, BALB/c and ICR mice, blood glucose level remained at low level after 30 min of insulin administration (Figs. 6c, e and g). The value for Kunming mice increased compared with the low value at 30 min. AUC value seems more accurate to reveal the insulin resistance state of mice. All four strains of mice fed with HFD showed higher ITT AUC value than control ones.
Fig. 6.
Change of blood glucose concentration (a, c, e and g respectively) in insulin tolerant test (ITT) of control (CON) and obese (MOD) mice of four mouse strains: Kunming, C57BL/6, BALB/c and ICR with their ITT-AUC values (b, d, f and h respectively). Different superscript letters among bars denote significant difference (P<0.05) according to Tukey’s test.
Serum lipid profile
Lipid profile of obese mice is also greatly impacted by HFD. TC value of obese mice in all four strains was significantly higher than that of control groups (Fig. 7a). For TG level, it is notable that there is no significant difference between CON and MOD for all four mice strains (Fig. 7b). LDL-c levels of obese mice in all four strains were significantly higher than the respective control group (Fig. 7c). The value for all control groups showed no difference. HDL-c is regarded as a beneficial factor of lowering level of blood lipid. However, our result revealed that HDL-c of obese mice is similar or higher than that of control ones (Supplementary Fig. 1). For ICR mice, HDL-c concentration of control and obese were at the same level. But for the rest three strain, HDL-c concentration of model was higher than control.
Fig. 7.
Serum concentration of TC, TG, LDL-c and TC/HDL-c ratio (a, b, c and d respectively) of control (CON) and obese (MOD) mice of four mouse strains: Kunming, C57BL/6, BALB/c and ICR. Different superscript letters among bars denote significant difference (P<0.05) according to Tukey’s test.
The TC/HDL-c ratio was calculated as depicted in Fig. 7d. In all four mice strains, the TC/HDL-c ratios in obese mice are significantly higher than that of control ones, indicating TC/HDL-c ratio is a more accurate parameter for obesity.
Major serum inflammatory cytokines
In the present study, IL-1β, TNF-α, IL-6 and MCP-1 were examined as representative pro-inflammatory cytokines, and IL-10 was analyzed as anti-inflammatory cytokine [14]. But results showed that for inflammatory cytokines examined, there seems no significantly different revealed for all four mouse strains (Fig. 8 and Supplementary Fig. 2). LPS and insulin level also showed no significant different between obese and control mice (Supplementary Fig. 2).
Fig. 8.
Serum concentration of IL-1β, TNF-α, IL-6 and MCP-1 (a, b, c and d respectively) of control (CON) and obese (MOD) mice of four mouse strains: Kunming, C57BL/6, BALB/c and ICR. Different superscript letters among bars denote significant difference (P<0.05) according to Tukey’s test.
Discussion
Many researches used body weight as the indicator of obesity. In previous report, significant body weight gain compared with control was considered as criteria of successfully constructed obese mice model [2, 20]. While HFD induced body weight increase, significantly higher body weight than control is not always consistence with obesity and its relevant symptoms [28]. It was found that HFD induced obesity in C57BL/6J and BALB/c mice, which are obesity prone mice sub-strain of C57BL/6 and BALB/c respectively, and increased their body weight. Body weight of model was higher than control but the difference was not significant [29]. Similar result has been reported by Mi Ra Lee et al. as HFD treated C57BL/6 mice were heavier than control but with no significant difference [8].
Body weight results indicate that Kunming mice and ICR mice could accumulate body weight more effectively than C57BL/6 and BALB/c mice when fed with HFD. But when considering the temperament of mice, it seems that Kunming mice were more energetic and fractious. More fighting and injuries among Kunming mice were recorded than all other 3 strains of mice. On the other hand, ICR mice were considered as most docile and easy to management during the experiment. No fighting and injuries were noted among ICR mice. But this important feature has not been widely considered and reported for developing obese mice model.
All four strain of mouse fed with HFD presented heavier liver and adipose tissue than control due to excess lipid storage as revealed via histological observation. Histological changes of liver and adipose could predict the obesity with more precision than body weight [17, 30].
OGTT was undertaken to examined the glucose lowering capacity of mice when administrated with high dosage of glucose. Obese mice usually showed slower and weaker ability to lower the enhanced blood glucose when challenged with high glucose load [17]. It was obvious that HFD treatment deteriorate the ability of mice to control glucose when challenged with high load. Insulin resistant is one hallmark of obesity which involve of low-grade inflammation and blockage of several insulin signal pathways [4]. ITT demonstrated similar result with that of OGTT in which blood glucose level of HFD fed mice was higher than that of control. The same trend was also showed in the AUC value of each test.
It is generally accepted that HFD treatment disturbs serum lipid profile with higher TC, TG, LDL-c and lower HDL-c concentration [16]. But the lipid profile of obese mouse is not unanimous reflected in the literature. TG result revealed in the present study contradicts with many previous reports as obese mice usually present higher level of TG [20]. However, some reports also support the present TG results in mouse strain like C57BL/6 which need further investigation [7]. HDL-c is regarded as a beneficial factor of lowering level of blood lipid. It is widely believed that obese mice have lower level of HDL-c as reported in several studies [23]. But some previous published results support the present HDL-c result. For instances it was found that HFD increased the level of HDL-c in obese C57BL/6 mice [7, 13, 18], which was also revealed in obese Kunming mice [19], BALB/c [32] and ICR mice [11]. In fact higher HDL-c concentration in obese Sprague Dawley rat is also reported in previous study [36].
Very little information is available to explain the HDL-c value in obese mice. It was hypothesized that when fed with HFD, the total concentration of lipoprotein increased to transport lipid in serum. Not only the LDL-c, but also HDL-c concentration increased in the obese mice. The observed contradiction may be caused by several factors, such as experimental setting, method of measuring lipoprotein etc. HFD treatment increased the level of serum glucose level, TG level and TC level but also HDL-c level in both male and female ICR mice [15]. Instead, TC/HDL-c ratio was applied as a better index for obesity and hyperlipidemia as reported in several previous publications [15, 38]. The present study support that TC/HDL-c seem a more effective index for evaluating serum lipid status.
It is commonly considered that HFD induced obesity up-regulate several pro-inflammatory cytokines and down-regulate anti-inflammatory cytokines in serum [27]. Higher IL-1β, TNF-α, IL-6 and MCP-1 concentration in obese mince is expected as reported previously [31, 34]. It is indicated that other methods like qPCR analysis of mRNAs level of aforementioned cytokines in tissues like liver and adipose tissue reflect the inflammatory status more precisely [6, 25]. But qPCR and western blot technique are not readily available for all labs and ELISA is still more widely used for common analysis need. In fact, it seems that even HFD could greatly increase the body weight and accumulate significant amount of lipid in liver and adipose tissue, it hardly affects the major inflammatory cytokines level in serum as reflected in our previous study and other un-published results [21].
Therefore, in the present study four widely used mouse strains, including Kunming, C57BL/6, BALB/c and ICR. It found that mouse strain may impact on the body weight increment, and weight ratio of adipose tissue. All four HFD treated mouse strains showed significant lipid accumulation in liver and adipose tissue. OGTT and ITT indicated that all four obese mouse strains response to glucose and insulin load in similar manner. Serum TC and LDL-c but not TG increased in all obese mouse strains. TC/HDL-c ratio is a more effective index than HDL-c that presented no significant difference compared with control mice. Major inflammatory cytokines, LPS and insulin also showed no significantly different between control and obese mice of all four strains. It is also widely accepted that sex may impact on the mouse obesity development that male mouse tends to gain more body weight and fat weight than female which will be considered in our further study [29]. In conclusion, mouse strain exerts limited effect on major physiology parameters of obesity. But considering the ease of animal management, ICR seems most qualified among the tested strains.
Conflict of Interests
The authors declare no competing financial interests.
Supplementary Material
Acknowledgments
This work is supported by National Natural Science Foundation of China (31700015), Fundamental Research Funds for the Central Universities (JZ2018HGTB0244) and Anhui Natural Science Foundation (1808085QC66).
References
- 1.Agrawal S., Gollapudi S., Su H., Gupta S.2011. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 31: 472–478. doi: 10.1007/s10875-010-9507-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chae H.S., Kim Y.M., Bae J.K., Sorchhann S., Yim S., Han L., Paik J.H., Choi Y.H., Chin Y.W.2016. Mangosteen extract attenuates the metabolic disorders of high-fat-fed mice by activating AMPK. J. Med. Food 19: 148–154. doi: 10.1089/jmf.2015.3496 [DOI] [PubMed] [Google Scholar]
- 3.Chen L., Liu L., Li C., Hu C., Su F., Liu R., Zeng M., Zhao D., Liu J., Guo Y., Long J.2017. A mix of apple pomace polysaccharide improves mitochondrial function and reduces oxidative stress in the liver of high-fat diet-induced obese mice. Mol. Nutr. Food Res. 61: 201600433. doi: 10.1002/mnfr.201600433 [DOI] [PubMed] [Google Scholar]
- 4.Czech M.P.2017. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 23: 804–814. doi: 10.1038/nm.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans J.P., Sutton P.A., Winiarski B.K., Fenwick S.W., Malik H.Z., Vimalachandran D., Tweedle E.M., Costello E., Palmer D.H., Park B.K., Kitteringham N.R.2016. From mice to men: Murine models of colorectal cancer for use in translational research. Crit. Rev. Oncol. Hematol. 98: 94–105. doi: 10.1016/j.critrevonc.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 6.Fang X., Ge K., Song C., Ge Y., Zhang J.2018. Effects of n-3PUFAs on autophagy and inflammation of hypothalamus and body weight in mice. Biochem. Biophys. Res. Commun. 501: 927–932. doi: 10.1016/j.bbrc.2018.05.084 [DOI] [PubMed] [Google Scholar]
- 7.Ivanovic N., Minic R., Dimitrijevic L., Radojevic Skodric S., Zivkovic I., Djordjevic B.2015. Lactobacillus rhamnosus LA68 and Lactobacillus plantarum WCFS1 differently influence metabolic and immunological parameters in high fat diet-induced hypercholesterolemia and hepatic steatosis. Food Funct. 6: 558–565. doi: 10.1039/C4FO00843J [DOI] [PubMed] [Google Scholar]
- 8.Jayaprakasam B., Olson L.K., Schutzki R.E., Tai M.H., Nair M.G.2006. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 54: 243–248. doi: 10.1021/jf0520342 [DOI] [PubMed] [Google Scholar]
- 9.Jiang L.H., Shi Y., Wang L.S., Yang Z.R.2009. The influence of orally administered docosahexaenoic acid on cognitive ability in aged mice. J. Nutr. Biochem. 20: 735–741. doi: 10.1016/j.jnutbio.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 10.Kang D., Su M., Duan Y., Huang Y.2019. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 10: 5032–5045. doi: 10.1039/C9FO00604D [DOI] [PubMed] [Google Scholar]
- 11.Kawakami T., Hanao N., Nishiyama K., Kadota Y., Inoue M., Sato M., Suzuki S.2012. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol. Appl. Pharmacol. 258: 32–42. doi: 10.1016/j.taap.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Kim K.S., Jang M.J., Fang S., Yoon S.G., Kim I.Y., Seong J.K., Yang H.I., Hahm D.H.2019. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids 51: 245–254. doi: 10.1007/s00726-018-2659-7 [DOI] [PubMed] [Google Scholar]
- 13.Lee H.S., Nam Y., Chung Y.H., Kim H.R., Park E.S., Chung S.J., Kim J.H., Sohn U.D., Kim H.C., Oh K.W., Jeong J.H.2014. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 118: 7–14. doi: 10.1016/j.lfs.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.M., Yoon Y., Yoon H., Park H.M., Song S., Yeum K.J.2017. Dietary anthocyanins against obesity and inflammation. Nutrients 9: 1089. doi: 10.3390/nu9101089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei F., Zhang X.N., Wang W., Xing D.M., Xie W.D., Su H., Du L.J.2007. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 31: 1023–1029. doi: 10.1038/sj.ijo.0803502 [DOI] [PubMed] [Google Scholar]
- 16.Li J., Pang B., Shao D., Jiang C., Hu X., Shi J.2020. Artemisia sphaerocephala Krasch polysaccharide mediates lipid metabolism and metabolic endotoxaemia in associated with the modulation of gut microbiota in diet-induced obese mice. Int. J. Biol. Macromol. 147: 1008–1017. [DOI] [PubMed] [Google Scholar]
- 17.Li X., Yu X., Sun D., Li J., Wang Y., Cao P., Liu Y.2017. Effects of polar compounds generated from the deep -frying process of palm oil on lipid metabolism and glucose tolerance in Kunming mice. J. Agric. Food Chem. 65: 208–215. doi: 10.1021/acs.jafc.6b04565 [DOI] [PubMed] [Google Scholar]
- 18.Li X., Chen Y., Li S., Chen M., Xiao J., Xie B., Sun Z.2019. Oligomer procyanidins from lotus seedpod regulate lipid homeostasis partially by modifying fat emulsification and digestion. J. Agric. Food Chem. 67: 4524–4534. doi: 10.1021/acs.jafc.9b01469 [DOI] [PubMed] [Google Scholar]
- 19.Liu R., Zhang J., Liu W., Kimura Y., Zheng Y.2010. Anti-Obesity effects of protopanaxdiol types of Ginsenosides isolated from the leaves of American ginseng (Panax quinquefolius L.) in mice fed with a high-fat diet. Fitoterapia 81: 1079–1087. doi: 10.1016/j.fitote.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Liu R., Zheng Y., Cai Z., Xu B.2017. Saponins and flavonoids from Adzuki bean (Vigna angularis L.) Ameliorate high-fat diet-induced obesity in ICR mice. Front. Pharmacol. 8: 687. doi: 10.3389/fphar.2017.00687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Zong S., Li J.2020. Attenuation effects of bulk and nanosized zno on glucose, lipid level, and inflammation profile in obese mice. Appl. Biochem. Biotechnol. 190: 475–486.. [DOI] [PubMed] [Google Scholar]
- 22.Lu J., Bi Y., Ning G.2016. Curbing the obesity epidemic in China. Lancet Diabetes Endocrinol. 4: 470–471. doi: 10.1016/S2213-8587(16)30007-9 [DOI] [PubMed] [Google Scholar]
- 23.Ma M., Liu G.H., Yu Z.H., Chen G., Zhang X.2009. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 113: 872–877. doi: 10.1016/j.foodchem.2008.03.064 [DOI] [Google Scholar]
- 24.Martin-Cordero L., Garcia J.J., Giraldo E., De la Fuente M., Manso R., Ortega E.2009. Influence of exercise on the circulating levels and macrophage production of IL-1β and IFNgamma affected by metabolic syndrome: an obese Zucker rat experimental animal model. Eur. J. Appl. Physiol. 107: 535–543. doi: 10.1007/s00421-009-1140-4 [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Fernández L., González-Muniesa P., Laiglesia L.M., Sáinz N., Prieto-Hontoria P.L., Escoté X., Odriozola L., Corrales F.J., Arbones-Mainar J.M., Martínez J.A., Moreno-Aliaga M.J.2017. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 31: 2135–2145. doi: 10.1096/fj.201600859R [DOI] [PubMed] [Google Scholar]
- 26.Mekada K., Abe K., Murakami A., Nakamura S., Nakata H., Moriwaki K., Obata Y., Yoshiki A.2009. Genetic differences among C57BL/6 substrains. Exp. Anim. 58: 141–149. doi: 10.1538/expanim.58.141 [DOI] [PubMed] [Google Scholar]
- 27.Mito N., Hosoda T., Kato C., Sato K.2000. Change of cytokine balance in diet-induced obese mice. Metabolism 49: 1295–1300. doi: 10.1053/meta.2000.9523 [DOI] [PubMed] [Google Scholar]
- 28.Nawaz S., Shareef M., Shahid H., Mushtaq M., Sajid S., Sarfraz M.2017. Lipid lowering effect of synthetic phenolic compound in a high- fat diet (HFD) induced hyperlipidemic mice. Matrix Sci. Pharm. 1: 12–16. doi: 10.26480/msp.01.2017.12.16 [DOI] [Google Scholar]
- 29.Nishikawa S., Yasoshima A., Doi K., Nakayama H., Uetsuka K.2007. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp. Anim. 56: 263–272. doi: 10.1538/expanim.56.263 [DOI] [PubMed] [Google Scholar]
- 30.Norris G.H., Porter C.M., Jiang C., Millar C.L., Blesso C.N.2017. Dietary sphingomyelin attenuates hepatic steatosis and adipose tissue inflammation in high-fat-diet-induced obese mice. J. Nutr. Biochem. 40: 36–43. doi: 10.1016/j.jnutbio.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 31.Shang H., Sun J., Chen Y.Q.2016. Clostridium butyricum CGMCC0313.1 modulates lipid profile, insulin resistance and colon homeostasis in obese mice. PLoS One 11: e0154373. doi: 10.1371/journal.pone.0154373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva F.M.C., Oliveira E.E., Gouveia A.C.C., Brugiolo A.S.S., Alves C.C., Correa J.O.A., Gameiro J., Mattes J., Teixeira H.C., Ferreira A.P.2017. Obesity promotes prolonged ovalbumin-induced airway inflammation modulating T helper type 1 (Th1), Th2 and Th17 immune responses in BALB/c mice. Clin. Exp. Immunol. 189: 47–59. doi: 10.1111/cei.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studentsova V., Mora K.M., Glasner M.F., Buckley M.R., Loiselle A.E.2018. Obesity/Type II diabetes promotes function-limiting changes in murine tendons that are not reversed by restoring normal metabolic function. Sci. Rep. 8: 9218. doi: 10.1038/s41598-018-27634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y., Gao L., Guo Y., Xu Y.C.2017. Short-term phlorizin treatment attenuates adipose tissue inflammation without alerting obesity in high-fat diet fed mice. J. Food Biochem. 41: 12407. doi: 10.1111/jfbc.12407 [DOI] [Google Scholar]
- 35.Vandevijvere S., Chow C.C., Hall K.D., Umali E., Swinburn B.A.2015. Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bull. World Health Organ. 93: 446–456. doi: 10.2471/BLT.14.150565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu D., Xu M., Lin L., Rao S., Wang J., Davey A.K.2012. The effect of isosteviol on hyperglycemia and dyslipidemia induced by lipotoxicity in rats fed with high-fat emulsion. Life Sci. 90: 30–38. doi: 10.1016/j.lfs.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 37.Zhang G., Yao G.1997. A survey on the genetic background document of Chinese Kunming mouse (KM mouse). Chin. J. Lab Anim. Sci. 7: 246–251. [Google Scholar]
- 38.Zhou Q., Wu J., Tang J., Wang J.J., Lu C.H., Wang P.X.2015. Beneficial effect of higher dietary fiber intake on plasma HDL-C and TC/HDL-C ratio among chinese rural-to-urban migrant workers. Int. J. Environ. Res. Public Health 12: 4726–4738. doi: 10.3390/ijerph120504726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuhua Z., Zhiquan W., Zhen Y., Yixin N., Weiwei Z., Xiaoyong L., Yueming L., Hongmei Z., Li Q., Qing S.2015. A novel mice model of metabolic syndrome: the high-fat-high-fructose diet-fed ICR mice. Exp. Anim. 64: 435–442. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.