Abstract
Retinoic acid (RA), the biologically active metabolite of vitamin A, regulates a vast spectrum of biological processes, such as cell differentiation, proliferation, apoptosis, and morphogenesis. microRNAs (miRNAs) play a crucial role in regulating gene expression by binding to messenger RNA (mRNA) which leads to mRNA degradation and/or translational repression. Like RA, miRNAs regulate multiple biological processes, including proliferation, differentiation, apoptosis, neurogenesis, tumorigenesis, and immunity. In fact, RA regulates the expression of many miRNAs to exert its biological functions. miRNA and RA regulatory networks have been studied in recent years. In this manuscript, we summarize literature that highlights the impact of miRNAs in RA-regulated molecular networks included in the PubMed.
1. Introduction
Retinoic acid (RA) is a metabolite of vitamin A that has a vast spectrum of biological processes, including differentiation, proliferation, apoptosis, and morphogenesis (Hu, Xu, Sun, Teng,& Xiao, 2017;Wang et al., 2012). While all-trans-RA is a major form, other isomers such as 13-cis-RA and 9-cis-RA are also present (Ruhl, Krezel, & de Lera, 2018; Tang & Russell, 1990). In this paper, RA is used to refer to all-trans-RA. Pharmacologically, retinoids including RA are used for acne and cancer treatment (Dobrotkova, Chlapek, Mazanek, Sterba, & Veselska, 2018; Leyden, Stein-Gold, & Weiss, 2017). Its effects in treating inflammation, allergy, and autoimmune diseases have also been revealed (Oliveira, Teixeira, & Sato, 2018).
The action of RA is mediated through the nuclear receptors: retinoic acid receptor (RARα, β, and γ) and retinoid x receptors (RXRα, β, and γ). Because RXR is an essential partner of many nuclear receptors, RA can regulate the function of other nuclear receptors such as peroxisome proliferator activate receptor and farnesoid x receptor. This allows RA to regulate lipid metabolism (Bushue & Wan, 2010; Chen, Wang, & Wan, 2010; Dai et al., 2003; Goto, 2019; Gyamfi, He, French, Damjanov, & Wan, 2008; Hu et al., 2017; Krezel, Ruhl, & de Lera, 2019; Liu, Wang, & Lin, 2019; Martin, Ma, & Bernard, 2019; Ruhl et al., 2018; Wan et al., 2000, 2000, 2003; Watanabe & Kakuta, 2018). Many RAR and RXR-regulated genes have been identified using genome-wide approaches such as chromatin immunoprecipitation followed by sequencing (Al Tanoury et al., 2014; Fang et al., 2013; He et al., 2013; Hu et al., 2013; Hua, Kittler, & White, 2009; Kiss et al., 2017; Liu, Ly, Hu, & Wan, 2014; Penvose, Keenan, Bray, Ramlall, & Siggers, 2019; Tang et al., 2011). In addition to transcriptional regulation, oral administration of RA alters gut microbiota and has an impact on liver regeneration (Liu, Hu, & Wan, 2016). Thus, it is important to understand mechanisms by which RA exerts its effects.
miRNA is small non-coding RNA that silences messenger RNA (mRNA). Its functions via base-pairing with complementary sequences within mRNA. As a result, the targeted mRNA is reduced by either cleavage or destabilization, or has its translational efficiency reduced (Fig. 1). miRNAs regulate the expression of as much as one-third of human gene-encoded proteins (Ganapathy & Ezekiel, 2019). Like RA, miRNAs regulate multiple biological and disease processes (Ganapathy & Ezekiel, 2019; Ivey & Srivastava, 2015). miRNAs have been approved by the FDA as diagnostic tools and for treatment (Dave et al., 2019). This review paper focuses on the miRNAs that mediate or affect the effects of RA. In some cases, RA regulates the expression of miRNA through its receptors such as RARα and RARβ, which bind to the regulatory region of certain miRNAs to alter their expression levels. In other cases, miRNA may target the receptors of RA and affect the effect of RA (Fig. 2). The literature search was limited to the papers included in the PubMed using mammalian cells or animal models.
Fig. 1.
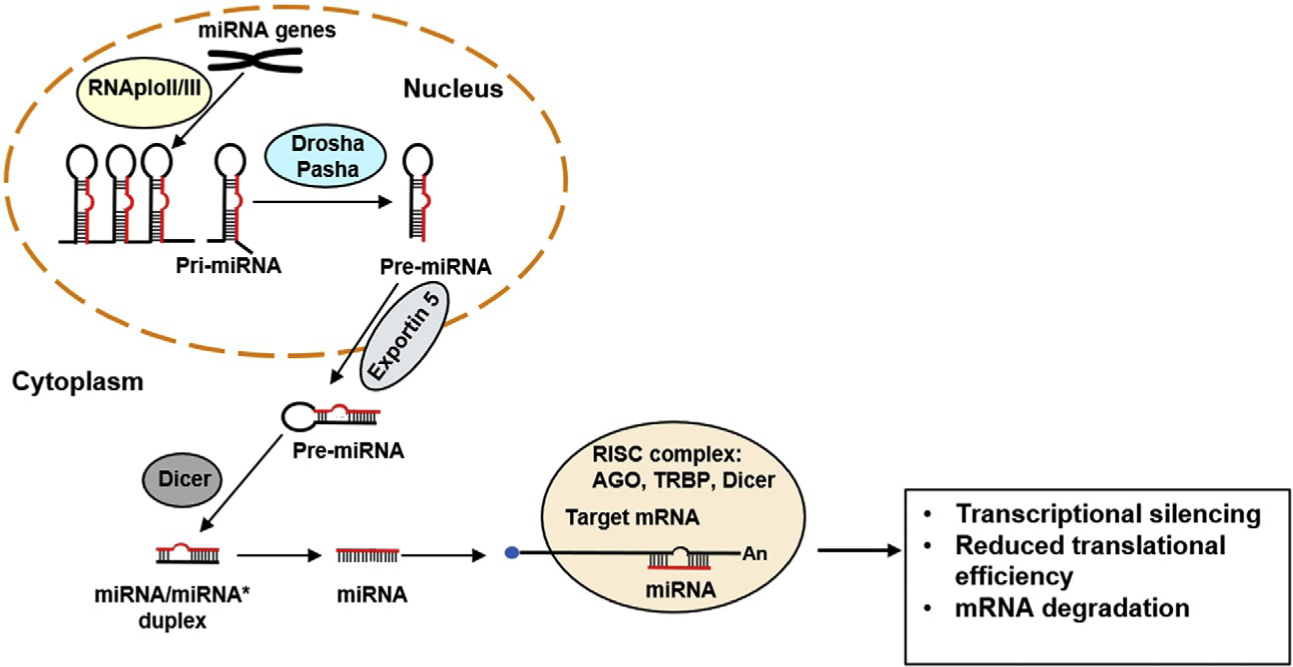
miRNA Biogenesis. miRNA is transcribed into pri-mRNA, which is converted to pre-miRNA by the protein complex Drosha-Pasha. Pre-miRNA is then transported out of the nucleus via the protein exportin 5. The endoribonuclease dicer cleaves pre-miRNA to form a miRNA duplex. Then, unwinding of the two strands and asymmetric assembly of one strand into RISC (RNA-induced silencing complex) occurs. The RISC complex acts via transcriptional silencing, reduced translational efficiency, or mRNA degradation.
Fig. 2.
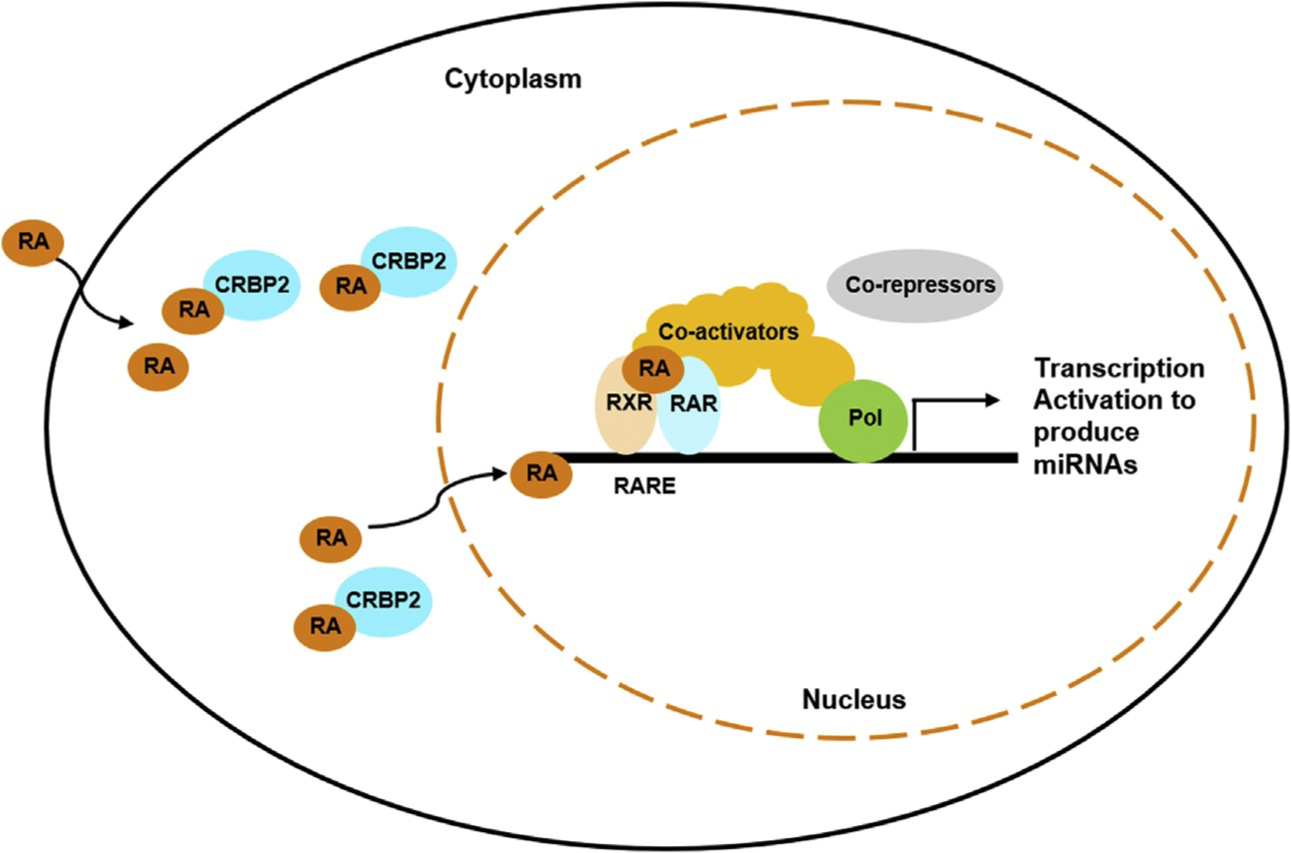
Retinoic acid and its receptor-mediated gene expression. RA is transported into the cell and bind to CRABP2 (cellular RA binding protein). In the nucleus, RA binds to nuclear receptors RXR and RAR heterodimer, which recognizes the RA receptor responsive element (RARE) located on the target gene, to regulate gene expression. The transcriptional activation requires the recruitment of coactivators and the departure of co-suppressors. Once miRNA is produced, it can silence the RA receptors, the transcriptional co-regulators, or other specific targets to modulate the effects of RA.
2. miRNAs that are implicated in RA-induced differentiation
2.1. Embryonic stem cell differentiation
Dicer is an endoribonuclease that cleaves double stranded RNA critical for miRNA biogenesis. In Dicer-deficient embryonic stem cells, increased dimethylation of histone H3 at lysine 9 (H3K9me2) at over 900 CpG islands has been noted, indicating the significance of miRNAs in epigenome regulation during differentiation. Additionally, the balance between the transcriptionally favorable tri-methylation histone H3 at lysine 4 (H3K4me3) and the unfavorable tri-methylation histone H3 at lysine 27 (H3K27me3) is shifted in the Dicer-deficient epigenome. Homeobox (HOX) transcription factors play pivotal roles in many aspects of cellular physiology, embryonic development, and tissue homeostasis. In response to RA treatment, elevated H3K27me3 is found in the promoters of the caudal type homeobox 2 (Cdx2) and homeobox A1 (Hoxa1) genes of Dicer-deficient embryonic stem cells. However, forced expression of let-7g in Dicer-deficient embryonic cells counteracts the effect of H3K27me3 on the expression of the Cdx2 and Hoxa1 genes. Let-7g counteracts the effects by reducing the enhancer of zeste homolog 2(Ezh2), a histone-lysine N-methyltransferase enzyme, which thereby leads to the differentiated phenotype (Tennakoon, Wang, Coarfa, Cooney, & Gunaratne, 2013).
miRNAs inhibit mouse embryonic stem cell self-renewal and stabilize their differentiated states. In mouse embryonic stem cells, RA upregulates miRNA-134, miR-296, and miR-470, which induces differentiation by targeting the pluripotency genes Nanog, octamer-binding transcription factor 4 (Oct4), and sex determining region Y-box 2 (Sox2) (Tay et al., 2008; Tay, Zhang, Thomson, Lim, & Rigoutsos, 2008). miRNA-134 inhibits Nanog and the liver receptor homolog 1 (LRH1), both of which positively regulate Oct4 (Tay, Tam, et al., 2008). In addition, miR-134, miR-296, and miR-470 are all involved in the lineage specific repression of mouse embryonic stem cell renewal (Zhang et al., 2015). Elevated forkhead box protein M1 (FOXM1) contributes to the maintenance of human stem cell pluripotency by directly regulating the Oct4 promoter. miR-134 over-expression reduces FOXM1 and promotes the differentiation effect of RA (Chen et al., 2015). Additionally, by reducing either miR-200b or miR-200c, RA inhibits the expression of pluripotency genes and increases the ectodermal marker Nestin, a type VI intermediate filament protein (Zhang, Gao, et al., 2015).
2.2. Neuronal differentiation
The significance of miR-10 in neuronal differentiation has been revealed. The hsa-miR-10 family is substantially increased (~95-fold) during the RA-induced neuronal differentiation of human embryonic stem cells (Parsons, 2012). In addition, during RA-induced neural lineage specific progression, hsa-miR-302 family is reduced and the expression of the Hox gene, hsa-miR-10, and let-7 are induced (Parsons, Parsons, & Moore, 2012). Similarly, the expression of Hoxd4 and miR-10b is coordinated in the RA-induced neural differentiation of P19 cells. This suggests the importance of miR-10b (Phua et al., 2011). Through the let-7-dependent mechanism, phosphorylation of a highly conserved RNA-binding protein called LIN28A fosters neural differentiation of P19 cells (Liu et al., 2017). Moreover, the differentiation of embryonic stem cells is also accompanied with increased miR-10a-5p, miR-219–5p, and miR-219-2-3p. The increase of miR-219 and its mimics also promote mouse embryonic stem cell differentiation into neural cells (Wu et al., 2017). In contrast, miR-125b-2 over-expression suppresses this differentiation (Deng, Zhang, Xu, & Ma, 2015).
Sirtuin 1 (SIRT1) plays a negative role in inducing the differentiation of induced pluripotent stem cells (iPSCs) into neural stem cells. When SIRT1 is inhibited, the differentiation continues and is marked by increased miR-34a, which silences SIRT1 (Hu et al., 2014). In mouse neuroblastoma N2a cells, P19 embryonal carcinoma cells, and the mouse brain, increased miR-124 reduces RARγ, thereby inhibiting neurite outgrowth (Wang, Yao, Lu, Li, & Ma, 2010) (Su, Gu, Zhang, Li, & Wang, 2020). There are many other miRNAs that may be implicated in neural cell differentiation (Hu et al., 2017). RA treatment of adipose-derived mesenchymal stem cells leads to changes in 76 miRNAs that have a greater than twofold difference (Hu et al., 2017).
2.3. Spermatogonia differentiation
Tyrosine-protein kinase Kit, a receptor tyrosine kinase protein, plays an essential role in cell proliferation, hematopoiesis, etc. The tyrosine-kinase receptor Kit is also essential for the maintenance of primordial germ cells in both sexes, and RA-induced differentiation of spermatogonia is accompanied by Kit induction. Recent data reveals that RA-induced miR-26b promotes the transition from Kit-negative to Kit-positive spermatogonia (Tu et al., 2018). miR-26b can silence a transcriptional factor named promyelocytic leukemia zinc finger protein (Plzf) in undifferentiated spermatogonia. It also downregulates the methylcytosine dioxygenase (Tet3) gene, thus reducing 5-hydroxymethylcytosine in spermatogonia. Together, through various mechanisms including epigenetic regulation, RA regulates spermatogonia differentiation (Tu et al., 2018).
While glial cell-derived neurotrophic factor (GDNF) is essential for the self-renewal of spermatogonia stem cells, RA is critical for the differentiation of spermatogonia. miR-202–3p, which is abundantly found in the testis, can be induced by GDNF but is reduced by RA. miR-202–3p prevents stem cells from premature differentiation by suppressing RNA binding fox-1 homolog 2 (Rbfox2). In addition, the inhibition of miR-202–3p, but not of miR-202–5p, induces the differentiation of spermatogonia stem cells (Chen et al., 2017).
RA-induced differentiation of spermatogonia has reduced miR-146, which targets the mediator complex subunit 1 (Med1), a co-activator of RARs and RXRs. miR-146 can also inhibit the expression of stimulated by retinoic acid 8 (Stra8) and the spermatogenesis- and oogenesis-specific basic helix-loop-helix 2 (Sohlh2) (Huszar & Payne, 2013). Thus, RA-reduced miR-146 is critically important for RA-induced differentiation of spermatogonia. In addition, RA also reduces miR-17–92 (Mirc1) and miR-106b-25 (Mirc3), both of which function cooperatively to promote mouse spermatogonia differentiation (Tong, Mitchell, McGowan, Evanoff, & Griswold, 2012).
2.4. Pre-adipocytes and myoblast differentiation
RA inhibits the differentiation of 3T3-L1 pre-adipocytes by reducing lipid metabolism (Stoecker, Sass, Theis, Hauner, & Pfaffl, 2017). Such an action is through miR-27a and miR-96 induction that leads to the rearrangement of the actin cytoskeleton as well as inhibition of the citric acid cycle (Stoecker et al., 2017).
Excess RA also inhibits myoblast differentiation and proliferation. In an embryonic tongue-derived myoblast C2C12 cell line, RA induce miR-27b-3p, which silences the α-dystrobrevin (DTNA) gene to inhibit proliferation and differentiation (Li et al., 2017). In addition, RA-induced miR-31–5p targets the Ca2+/calmodulin-dependent protein kinase II δ gene (CamkIIδ) and is implicated in the RA-induced myogenic abnormalities of tongue (Liu et al., 2017). miR-133a, 210, and 34a are also involved in the RA-induced myoblasts differentiation and proliferation (Vecellio et al., 2012).
miR-10a targets the histone deacetylase 4 (Hdac4) gene and has a critical role in the RA-induced differentiation of mouse embryonic stem cells into smooth muscle cells (Huang et al., 2010). In addition, nuclear factor kappa B (NFκB), through binding to the miR-10a promoter, has a significant role in the differentiation process. Inhibiting the nuclear translocation of NFκB reduces miR-10a and prevents the RA-induced differentiation smooth muscle cells (Huang et al., 2010).
2.5. Hematopoietic progenitor cell differentiation
RA induces miR-223, which acts as a translational inhibitor to regulate lineage specification and differentiation of mouse hematopoietic stem cells (Chen, Li, Lodish, & Bartel, 2004). Transcription factors nuclear factor I-A (NFI-A) and CCAAT/enhancer-binding protein-alpha (C/EBPα) compete for binding to the miR-223 promoter. In response to RA treatment, the induction of miR-223 requires the release of NF1-A and the binding of C/EBPα. Taken together, the RA regulated differentiation of granulocytes is mediated by miR-223 expression controlled by two transcriptional factors (Fazi et al., 2005).
3. miRNAs that are implicated in RA-regulated inflammatory signaling and immunity
Signal-regulatory protein α (SIRPα) is essential for modulating leukocyte inflammatory responses. The SIRPα gene is a commonly targeted by miR-17, miR-20a, and miR-106a. RA reduces these miRNAs and increases SIRPα expression in SIRPα-negative promyelocytic cells. However, bacterial lipopolysaccharides (LPS) increases miR-17, miR-20a, and miR-106a in macrophages, which leads to SIRPα reduction and macrophage activation. Thus, miR-17, miR-20a, and miR-106a regulate macrophage inflammatory responses by repressing SIRPα. (Zhu et al., 2013).
RA-induced miR-10a is abundantly found in naturally occurring regulatory T cells (Treg). miR-10a inhibits the development of inducible T regulatory cells (iTregs) into follicular helper T cells by suppressing both the transcriptional repressor B-cell lymphoma 6 (Bcl6) and the co-repressor nuclear receptor co-repressor 2 (Ncor2). In addition, miR-10a attenuates the differentiation process into the T(H)17 subset of helper T cells (Takahashi et al., 2012). In contrast to RA, transforming growth factor-β (TGF-β)-induced iTregs in vitro are unstable and do not have miR-10a. Thus, RA-induced miR-10a is selectively expressed and plays a role in stabilizing iTregs (Jeker et al., 2012).
T-cell receptor (TCR) signaling and TGF-β regulate the production of peripheral regulatory T (pTreg) cell. The expression of miR-31 is triggered by TCR signaling but inhibited by TGF-β. miR-31 directly targets the retinoic acid-inducible protein 3 (Gprc5a), a member of the type 3G protein-coupled receptor family. The deficiency of this protein leads to the impairment of pTreg-cell induction as well as an increased severity of experimental autoimmune encephalomyelitis (Zhang et al., 2015). Therefore, by targeting Gprc5a, miR-31 reduces the production of pTreg (Zhang, Ke, et al., 2015).
RA induces the secretion of exosomes in gut-tropic T cells that causes an upregulation of integrin α4β7 that binds to mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1). Addressins are the ligands to the homing receptors of lymphocytes, which determine which tissue the lymphocyte will enter. miRNA profiling suggests that exosomes that originate from the gut tropic T cells contain a large quantity of miRNAs, such as miR-132 and miR212, which target NKX2.3, a transcription factor important for the expression of MAdCAM-1 (Park et al., 2019).
Metabolism controls immune cell fate and function. miR-33 not only represses genes involved in cholesterol efflux and fatty acid oxidation, but also polarization of immune cells. Macrophage-specific miR-33 deletion promotes M2 macrophage polarization. Interestingly, inhibiting miR-33 enhances the expression of RA-producing enzyme aldehyde dehydrogenase family 1, subfamily A2 (ALDH1A2), as well as the activity of retinal dehydrogenase in macrophages. Such findings are in consistent with the ability of RA to foster iTregs. Furthermore, treating hypercholesterolemic mice with miR-33 inhibitors leads to accumulation of inflammation-suppressing M2 macrophages and forkhead box P3+ (foxp3+) Tregs in plaques. This treatment thereby inhibits the progression of atherosclerosis. Together, inhibiting miR-33 benefits metabolism and inflammation (Ouimet et al., 2015).
4. miRNAs that are implicated in RA-induced differentiation of cancer cells
4.1. Embryonic carcinoma
RA has a profound effect in inducing the differentiation of embryonic carcinoma F9 cells into endodermal cells (Wan, Orrison, Lieberman, Lazarovici, & Ozato, 1987; Wan, Wang, & Wu, 1994; Wu, Wang, & Wan, 1992). In the F9 cell line, RA induces miR-485, which silences the α/β-hydrolase domain-containing protein 2 (Abhd2). The reduction of Abhd2 leads to ERK1/2 phosphorylation and differentiation of embryonic carcinoma cells (Yu, Zhang, Liu, Liu, & Guo, 2019).
4.2. Brain tumors
RA treatment enhances gap junctions which leads to an increased miR-124–3p-mediated antiproliferation in glioblastoma cells (Suzhi et al., 2015). RA also induces glioma cell death by elevating miR-302b, and silences E2F3, a transcriptional glioma growth regulator (Chen et al., 2014). In addition, reduced miR-452 found in gliomas promotes their stem-like features and tumorigenesis because miR-452 inhibits many stemness regulators like polycomb complex protein BMI-1, lymphoid enhancer binding factor 1 (LEF1), and transcription factor 4 (TCF4), (Liu et al., 2013).
4.3. Neuroblastoma
Several miRNAs have changes in their expression levels when the human neuroblastoma cell line SH-SY5Y is treated with RA and a dopamine cocktail (Das & Bhattacharyya, 2014). Among them, miR-432 plays a key role in differentiation of human neuroblastoma cells by suppressing NESTIN and the repressor element-1-silencing transcription factor (REST) corepressor 1 (RCOR1) (Das & Bhattacharyya, 2014). RA induces neuronal differentiation via miR-29b and miR-664a-5p (Jauhari, Singh, & Yadav, 2018; Watanabe, Yamaji, & Ohtsuki, 2018). miR-29b reduces the P53 inhibitor P85α, which in turns increases miR-145 to silence OCT4, SOX2, and kruppel-like factor 4 (KLF4) during SH-SY5Y differentiation (Jauhari et al., 2018). Further studies are required to understand the function of miR-664a-5p (Watanabe et al., 2018).
RA has anti-metastatic and anti-migratory activities via miR-10a and miR-10b, both of which target the SR-family splicing factor SFRS1 (SF2/ASF) (Meseguer, Mudduluru, Escamilla, Allgayer, & Barettino, 2011). They also induce neuronal differentiation by silencing NCOR2 which inhibits neurite growth (Foley et al., 2011). Moreover, other RA-induced miRNAs, including miR-9 and miR-103, control neuroblastoma differentiation by reducing a differentiation inhibitor molecule called Inhibitor of DNA-binding 2 (Id2) (Annibali et al., 2012). RA can also decrease miR-17 in SH-SY5Y cells. miR-17 is involved in the regulation of the mitogen-activated protein kinase (MAPK) signaling pathway, synaptic plasticity, and other markers of neuronal differentiation (Beveridge, Tooney, Carroll, Tran, & Cairns, 2009). RA- and brain-derived neurotrophic factor (BDNF)-induced miR-125b and miR-124a also positively regulate the differentiation of SH-SY5Y cells and neurite outgrowth of human neural progenitor ReNcell VM cells by targeting multiple genes (Le et al., 2009).
The mutant amyloid precursor protein (APP)L17C [the leucine (L) residue 17 is replaced by a cysteine (C) residue in amyloid β peptide (Aβ)] increases amyloid precursor protein dimerization which leads to reduced neurite outgrowth. The overexpression of a mutant amyloid precursor protein (APP)L17C in human neuroblastoma SH-SY5Y cells inhibits neurite outgrowth (Luu et al., 2019). miR-34a expression is greatly decreased in APPL17C compared to APPWT cells, and overexpression of miR-34a restores neurite outgrowth in the mutant cells (Luu et al., 2019). Thus, miR-34a expression has a pivotal role in APP-mediated neurite outgrowth (Luu et al., 2019).
DNA demethylation mediated by miRNAs promotes RA-induced differentiation of neuroblastoma cells. The ectopic overexpression of miR-152 that targets the DNA methyltransferase 1 (DNMT1), contributes to the differentiated phenotypes, including reduced invasiveness and anchorage-independent growth in a human neuroblastoma cell line SK-N-BE (Das et al., 2010).
Other miRNAs are implicated in the differentiation of neuroblastoma cells. During the differentiation of NT2 cells, the stemness phenotype-associated miR-302 is reduced, but let-7, miR-125b, and miR-132 are increased (Pallocca et al., 2013). miR-128 is also increased that in turn reduces Reelin and Doublecortin, where both are involved in the migratory potential of neural cells (Evangelisti et al., 2009). Moreover, during the RA-induced differentiation of neuroblastoma BE (2)-C cells, tumor suppressor miR-449a is increased. miR-449a targets the microfibril associated protein 4 (MFAP4), plakophilin 4 (PKP4), and TRNA splicing endonuclease subunit 15 (TSEN15), which are associated with poor survival of neuroblastoma patients (Zhao et al., 2015). Moreover, miR-449a induces cell cycle arrest by inhibiting the cyclin dependent kinase 6 (CDK6) and lymphoid enhancer binding factor 1 (LEF1) (Zhao et al., 2015).
RA-induced differentiation of the human neuroblastoma SK-N-BE cells have overexpressed miR-34a that directly targets the E2F3 and lowers its protein. E2F3 is a potent transcriptional inducer for cell-cycle progression. In addition, overexpression of miR-34a in neuroblastoma cell lines prevents cell proliferation by inducing the caspase-dependent apoptotic pathway. Furthermore, miR-34a is reduced in primary neuroblastoma tumors and cell lines when compared to normal adrenal tissue (Welch, Chen, & Stallings, 2007). miR-184 is also implicated in the apoptosis pathway as RA-induced miR-184 promotes the apoptosis of human neuroblastoma cell lines including KELLY or SK-N-AS (Chen & Stallings, 2007).
4.4. Hematopoietic malignancies
Acute promyelocytic leukemia (APL) is caused by translocation of the promyelocytic leukemia (PML) gene located on chromosome 15 and the RARα gene on chromosome 17, which generates a fusion oncogene PML/RARα and differentiation arrest.
In hematopoietic malignancies, high c-Myc expression is correlated with a poor prognosis (Delgado & Leon, 2010). It has been shown that reduced PML/RARα due to loss of c-Myc is miRNA-dependent. In addition, tumor suppressor let-7 has been identified to target both the c-Myc and the PML/RARα (Ding et al., 2016). Moreover, PML/RARα binds to the RA responsive element of the host genes such as the let-7c (Careccia et al., 2009; Pelosi et al., 2014). However, RA treatment induces a conformational change in the host promoter thus regulating let-7c (Pelosi et al., 2014). Taken together, RA by inducing let-7c, which silences PML/RARα, can lead to the differentiation of APL (Ding et al., 2016).
The synergism between c-Myc and miR-17–19b, a truncated version of the miR-17–92 cluster, is well-established for tumor initiation (He et al., 2005; Mu et al., 2009). RA not only reduces c-Myc mRNA but also miR-17–92 to induce APL cell differentiation (Yu et al., 2019). In the APL NB4 cell line, RA induces miR-10b, miR-194, miR-195, miR-196a, and others. Many of them are transcriptionally silenced by PML/RARα which leads to oncogenesis (Saumet et al., 2009). To add on, a long non-coding RNA named HOTAIRM1 increases the RA-induced degradation of PML/RARα via the autophagy pathway. In fact, HOTAIRM1 can function as a miRNA sponge to regulate the expression of autophagy-related genes by competing for miRNA bindings sites (Chen et al., 2017).
Other mechanisms are involved in RA-mediated differentiation of APL. Knockdown adenylate cyclase 9 (ADCY9), which converts ATP into cAMP, inhibits RA-induced differentiation of APL. Similarly, miR-181a, which directly targets ADCY9 and reduces cAMP, has a negative role in RA-induced APL differentiation (Zhuang et al., 2014). miR-17–5p also inhibits RA-induced APL cell differentiation (Yu, Hu, et al., 2019). In contrast, RA induces granulocytic differentiation through miR-382–5p that silences the suppressor gene phosphatase and tensin homolog (PTEN) (Liu et al., 2019).
By regulating miRNA, RA treats APL (van Gils, Verhagen, & Smit, 2017). RA treatment causes a loss of imprinting on chromosome 14q32 that regulates the overexpression of many miRNAs (Manodoro et al., 2014). RA treatment of APL cells increases many tumor suppressor miRNAs, such as the let-7 family (Garzon et al., 2007; van Gils et al., 2017). RA treatment of APL cells also increases miR-107, which silences the NFI-A (Fazi et al., 2005) (Garzon et al., 2007). RA also reduces many miRNAs such as miR-17 and miR-25 in APL cells (Garzon et al., 2007; Rossi et al., 2010). Moreover, by reducing miR-301a-3p to increase ubiquitin-specific protease 48 (USP48), RA is effective in APL differentiation (Li et al., 2018).
RA increases miR-145 in myeloid leukemia cells which thereby reduces RAS-responsive element-binding protein 1 (RREB1), which prevents granulocytic differentiation of myeloid leukemia cells (Yao et al., 2019). In addition, miR-29a, which targets the CDK6, and miR-142–3p, which silences the TGF-β-activated kinase 1/MAP3K7 binding protein 2 (TAB2), contributes to RA-induced granulocytic differentiation of HL-60, THP-1, or NB4 cells. Forced expression of either miR-29a or miR-142–3p in hematopoietic stem cells induces myeloid differentiation (Wang et al., 2012). Ectopic miR-638 overexpression, which targets the cyclin-dependent kinase 2 (CDK2), also increases RA-induced differentiation of leukemic cell lines and primary acute myeloid leukemia blasts (Lin et al., 2015). Moreover, RA increases the expression of tumor suppressor miR-663 and inhibits HL-60 cell proliferation. Exotic overexpression of miR-663 also induces HL-60 cell differentiation (Jian et al., 2011).
Autophagy regulated by miRNA has a role in the regulation of myeloid differentiation. Autophagy genes (ATG) are frequently repressed in primary acute myeloid leukemia patients. These reduced levels are likely due to increased miR-106a, which targets a transcriptional factor for several ATG genes (Jin et al., 2018). By mediating other mechanisms such as regulation of transcriptional machinery, miRNA is important for differentiation. In malignant myeloid cells, transcription factor CDX2 induces miR-125b expression. Increased miR-125b prevents the differentiation of myeloid cells and promotes leukemogenesis by inhibiting the core binding factorβ (CBFB) translation. RA treatment decreases CDX2 activity, reduces miR-125b, and increases the CBFB during myeloid cell differentiation and in patients. Thus, miR-125b is important for RA-induced differentiation (Lin et al., 2011).
HOXB4, which has a function in stem cell expansion, along with miR10a, is highly expressed in atypical myeloproliferative neoplasms. However, overexpression of miR-10a has no effect on proliferation, differentiation or self-renewal of normal hematopoietic progenitors. (Dumas et al., 2018). RA reduces HOXA9 in acute leukemia cells leading to the inhibition of proliferation and promotion of differentiation of leukemia cells. The downregulation also interferes with tumor growth by modulating certain miRNAs. For example, during the leukemia cell differentiation induced by HOXA9 downregulation, miR-663 and miR-494 are increased and miR-10a and miR-181 are decreased (Chen, Yu, Lv, & Zhang, 2017).
4.5. Breast cancer
Retinoids are promising for breast cancer prevention and treatment. In estrogen receptor (ER)-positive breast carcinoma cells (MCF-7), RA induces miR-21 to counteract the anti-proliferative action of RA but may have a benefit in reducing cell motility. The pro-inflammatory cytokine IL1B, the adhesion molecule ICAM-1, and the tissue-type plasminogen activator (PLAT) are the identified miR-21 target genes (Terao et al., 2011). In addition, miR-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) that is implicated in cell migration, as well as the programmed cell death 4 (PDCD4), and the mammary serine protease inhibitor (Maspin) (Terao et al., 2011). Thus, miR-21 inhibitors may be used for breast cancer treatment.
Other miRNAs are implicated in RA-mediated anti-breast cancer effects. Nearly 70% of human breast carcinomas have reduced HOXA5. In addition, loss of HOXA5 in human breast cancer correlates with the progression to higher-grade lesions, suggesting its tumor suppressor effect (Jeannotte, Gotti, & Landry-Truchon, 2016). However, via miR-130a reduction, RA induces HOXA5 in breast cancer tissue (Yang, Miao, Mei, & Wu, 2013). RA also acts as a inducer for miR-10a, which counteracts the reduced miR-10a and RARβ in breast cancer cells (Khan et al., 2015). To add on, RA increases miR-210 and miR-23a/24–2 but decreases miR-17/92 and miR-424/450b in breast cancer cells. However, estrogen can counteract the effect of RA on expression of those four miRNAs (Saumet et al., 2012).
In a specific subgroup of HER2-positive breast cancer cells, SKBR3, RA and Lapatinib treatment regulates the expression of 174 miRNAs (Fisher et al., 2015). Together, RA-regulated miRNAs have a significant impact on growth or mobility of SKBR3 cells (Fisher et al., 2015).
4.6. Digestive system cancers
RARβ is an upstream regulator and downstream effector of miR-22. In other words, RARβ can transcriptionally induces miR-22, which in turns epigenetically regulate the expression and function of RARβ and another orphan receptor named NUR77. As a result, nuclear NUR77 exports to cytosol converting its oncogenic effect into apoptotic effect thereby killing colon cancer cells (Hu et al., 2019). RA by itself or in combination with synthetic and natural HDAC inhibitors including short-chain fatty acids can induce miR-22. Thus, in addition to RARβ-mediated transcription, epigenetic mechanism also regulates miR-22 expression. Additionally, it is interesting that miR-22 targets multiple protein deacetylases including HDAC1, HDAC4, as well as SIRT1 and has HDAC inhibitory effect used to fight for cancer. Thus, RA-induced miR-22 is a tumor suppressor for colon cancer (Hu et al., 2019). Furthermore, reduced miR-22 is found in many other types of cancers including liver, colon, etc., and is considered as a tumor suppressor (Yang, Hu, Liu, & Wan, 2015). In consistency with the role of miR-22 being a tumor suppressor, miR-22 has been characterized as a metabolic silencer (Hu et al., 2020).
In metastatic pancreatic adenocarcinoma cells, miR-10a is overexpressed and regulates the metastatic behavior by suppressing HOXB1 and HOXB3. As mentioned above, miR-10a is important for RA-mediated differentiation of neuronal cells. However, in pancreatic adenocarcinoma, RAR antagonists reduces miR-10a and has an anti-cancer effect (Weiss et al., 2009).
Reduced miR-452 promotes stem-like features and tumorigenesis in glioma as mentioned above (Liu et al., 2013). However, increased miR-452 significantly promotes hepatocellular carcinoma (HCC) cell proliferation in vitro and in vivo (Zheng et al., 2016). miR-452 is overexpressed in the chemo-resistant hepatospheres and human HCCs (Zheng et al., 2016). Thus, miR-452 over-expression predicts poor survival for liver cancer patients (Zheng et al., 2016). Moreover, RA combined with doxorubicin reduces miR-452 and inhibits HCC metastasis (Zheng et al., 2016). Furthermore, miR-452 silences the Sox7, which suppresses the Wnt/β-catenin signaling pathway (Chan, Mak, Leung, Chan, & Ngan, 2012; Zheng et al., 2016).
4.7. Other cancers
As a tumor suppressor, reduced RARβ can be an indicator for prognosis. In papillary thyroid carcinoma, reduced RARβ is associated with increased miR-146b-5p (Czajka et al., 2016). Overexpression of miR-146a-5p and miR-146b-5p results in reduced RARβ mRNA (Czajka et al., 2016). In contrast, toll-like receptor 3 (TLR3) activation induces RARβ re-expression and tumor inhibition, which is due to increased miR-29b, −29c, −148b, and −152 as well as re-expression of epigenetically silenced genes (Galli et al., 2013).
Another miRNA that targets RARβ is miR-29b. Tuberous sclerosis complex (TSC) is an incurable multisystem disease featured by mTORC1-hyperactive tumors. It has been found that by targeting RARβ, miR-29b has an oncogenic effect in TSC2-deficient cells. In addition, inhibition of miR-29b suppresses oncogenic property of TSC2-deficient tumors in vivo (Liu et al., 2019).
In addition to RARβ, miRNAs also affect the expression of other RA receptors. miR-27a overexpression, which silences RARα and RXRα, is found in aggressive rhabdomyosarcomas cells (Tombolan et al., 2015). Additionally, RA can enhance the binding of RARα to the miR-27a promoter, leading to the inactivation of miR-27a transcription, which increases glycogen synthase kinase-3β (GSK-3β) in laryngeal cancer cells. However, the mechanism by which RARα reduces miR-27a remains to be understood (Chen et al., 2017).
The effects of other retinoids in regulating miRNA have been studied. For example, the treatment effect of 9-cis RA, a ligand for RXR, is revealed in an adrenocortical xenograft mouse model. 9-cis RA together with mitotane reduce circulating hsa-miR-483–5p in the adrenocortical xenograft mouse model and can potentially be a predictor of treatment efficacy (Nagy et al., 2015). Additionally, tomato-derived bioactive antioxidants such as carotenoids and lycopene also inhibit tumor growth. In PC3 prostate cancer cells, lycopene increases let-7f-1 to silence the AKT2 and induce apoptosis (Li, Chen, Zhao, Hao, & An, 2016).
5. miRNAs that are implicated in other RA-regulated health issues
5.1. Liver diseases
Overexpression of miR-34a in human hepatoblastoma HepG2 cells reduces RXRα mRNA and protein. An inversely correlated expression pattern exists between miR-34a and RXRα protein in 14 liver specimens, with fibrotic livers having increased miR-34a but reduced RXRα. p53 can up-regulate miR-34a and promote liver fibrosis. Thus, by reducing RXRα, miR-34a has a role in liver fibrosis (Oda et al., 2014).
5.2. Atherosclerosis
miR-10a is a potential diagnostic molecule because reduced miR-10a in aortic endothelium and serum is linked with atherosclerosis (Lee et al., 2018). Moreover, RARα/RXRα-specific agonists induce miR-10a in vascular endothelial cells and inhibit atherosclerotic lesion formation. Thus, the RA-induced miR-10a can be a potential target for treating atherosclerosis (Lee et al., 2018).
5.3. Congenital diseases
Through hypermethylation, miR-124a is reduced and p38 is deactivated in RA-induced spina bifida fetuses compared with healthy rats. In addition, p38 deactivation is accompanied by increased apoptosis suggesting the role of miR-124a in RA-induced congenital defect (Qin et al., 2017). RA also reduces miR-9/9*, miR-124a, and miR-125b, all of which are implicated in the development of spina bifida in rat models (Zhao et al., 2008). The up-regulation of miR-9, miR-124a, and miR-138 as well as down-regulation of miR-134 are found in the amniotic fluid of RA-induced spinal bifida fetuses compared with that of control fetuses (Qin et al., 2016).
5.4. Aging
miRNAs that are implicated in stress-induced premature senescence have been identified in primary cultures of human diploid fibroblasts and human trabecular meshwork cells. Senescence is associated with reduced miR-15 and miR-106b as well as increased miR-182 and −183. In addition, miR-106b and miR-182 target the p21CDKN1A and the RARG, respectively, and are thereby implicated in senescence (Li, Luna, Qiu, Epstein, & Gonzalez, 2009).
5.5. Myopia
Mutation of the paired box gene 6 (PAX6) gene, which controls oculogenesis, is implicated in the development of myopia. RA induces miR-328, which targets the PAX6, in retinal pigment epithelial cells leading to increased retinal pigment epithelial cell proliferation and reduced scleral cell proliferation. Thus, the reduction of miR-328 contributes to the prevention or treatment of myopia (Chen et al., 2012).
5.6. Alzheimer’s disease
Overexpression of miR-138 is found in the N2a/APP cells expressing human amyloid precursor proteins (APP) 695 protein (APP695, APPwt) and HEK293/tau cells (human embryonic kidney 293 cells expressing human tau protein). In HEK293/tau cells, increased miR-138, which directly targets the RARα, activates GSK-3β, and enhances tau phosphorylation. In contrast, elevated RARα inhibits GSK-3β and reduces tau phosphorylation (Wang et al., 2015).
6. Conclusion
miRNA has a significant role in mediating the functions of RA. Among the above-mentioned miRNAs, there are a few such as let-7, miR-10, and miR-17 are implicated in multiple biological processes and disease models.
The let-7 family has a wide variety of implications. Increased Let-7g leads to the differentiated phenotype of Dicer-deficient mouse ESCs. Furthermore, the let-7 family has a variety of effects on neuronal lineage progression cells. It induces neuronal differentiation and is elevated in differentiated neuronal cultures. They can also act as tumor suppressors and lead to the differentiation of APL cells. Moreover, let-7f-1 induced by lycopene causes apoptosis.
The miR-10 family also has a wide variety of implications after RA induction. For example, reduced miR-10a is linked to atherosclerosis and can be a diagnostic tool. Furthermore, miR-10a promotes the differentiation of mouse embryonic stem cells into smooth muscle cells. miR-10a is also found in T cells and stabilizes iTregs that prevents their development into helper T cells. In addition, miR-10a level is low in breast cancer cells but is overexpressed in metastatic pancreatic adenocarcinoma cells.
miR-17 is generally decreased by RA. It is involved in the regulation of the MAPK signaling pathway, synaptic plasticity, and other markers of neuronal differentiation in neuroblastoma SH-SY5Y cells. It is also decreased in APL cell differentiation and in breast cancer cells. In addition, reduced levels of miR-17 also promotes the macrophage inflammatory responses such as infiltration, phagocytosis, and pro-inflammatory cytokine secretion. miR-17–92 also functions cooperatively with miR-106b-25 to regulate mice spermatogonia differentiation.
Since miRNA can target many genes and pathways, it would be challenging to use them for disease treatment and prevention. Additionally, certain miRNAs such as miR-452 has opposite effects based on the tumor types. Additional studies are required to dissect their functions and human relevance.
Funding
This study is supported by grants funded by National Institutes of Health U01CA179582 and R01CA222490.
References
- Al Tanoury Z, Piskunov A, Andriamoratsiresy D, Gaouar S, Lutzing R, Ye T, et al. (2014). Genes involved in cell adhesion and signaling: A new repertoire of retinoic acid receptor target genes in mouse embryonic fibroblasts. Journal of Cell Science, 127(Pt. 3), 521–533. [DOI] [PubMed] [Google Scholar]
- Annibali D, Gioia U, Savino M, Laneve P, Caffarelli E, & Nasi S (2012). A new module in neural differentiation control: Two microRNAs upregulated by retinoic acid, miR-9 and −103, target the differentiation inhibitor ID2. PLoS One, 7(7), e40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Tran N, & Cairns MJ (2009). Downregulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cellular Signalling, 21(12), 1837–1845. [DOI] [PubMed] [Google Scholar]
- Bushue N, & Wan YJ (2010). Retinoid pathway and cancer therapeutics. Advanced Drug Delivery Reviews, 62(13), 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careccia S, Mainardi S, Pelosi A, Gurtner A, Diverio D, Riccioni R, et al. (2009). A restricted signature of miRNAs distinguishes APL blasts from normal promyelocytes. Oncogene, 28(45), 4034–4040. [DOI] [PubMed] [Google Scholar]
- Chan DW, Mak CS, Leung TH, Chan KK, & Ngan HY (2012). Downregulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget, 3(12), 1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cai T, Zheng C, Lin X, Wang G, Liao S, et al. (2017). MicroRNA-202 maintains spermatogonial stem cells by inhibiting cell cycle regulators and RNA binding proteins. Nucleic Acids Research, 45(7), 4142–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Hsi E, Hu CY, Chou WW, Liang CL, & Juo SH (2012). MicroRNA-328 may influence myopia development by mediating the PAX6 gene. Investigative Ophthalmology & Visual Science, 53(6), 2732–2739. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, & Bartel DP (2004). MicroRNAs modulate hematopoietic lineage differentiation. Science, 303(5654), 83–86. [DOI] [PubMed] [Google Scholar]
- Chen Y, Meng L, Yu Q, Dong D, Tan G, Huang X, et al. (2015). The miR-134 attenuates the expression of transcription factor FOXM1 during pluripotent NT2/D1 embryonal carcinoma cell differentiation. Experimental Cell Research, 330(2), 442–450. [DOI] [PubMed] [Google Scholar]
- Chen PH, Shih CM, Chang WC, Cheng CH, Lin CW, Ho KH, et al. (2014). MicroRNA-302b-inhibited E2F3 transcription factor is related to all trans retinoic acid-induced glioma cell apoptosis. Journal of Neurochemistry, 131(6), 731–742. [DOI] [PubMed] [Google Scholar]
- Chen Y, & Stallings RL (2007). Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Research, 67(3), 976–983. [DOI] [PubMed] [Google Scholar]
- Chen S, Sun YY, Zhang ZX, Li YH, Xu ZM, & Fu WN (2017). Transcriptional suppression of microRNA-27a contributes to laryngeal cancer differentiation via GSK-3beta-involved Wnt/beta-catenin pathway. Oncotarget, 8(9), 14708–14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Wang WT, Huang W, Fang K, Sun YM, Liu SR, et al. (2017). The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death and Differentiation, 24(2), 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang K, & Wan YJ (2010). Retinoids activate RXR/CAR-mediated pathway and induce CYP3A. Biochemical Pharmacology, 79(2), 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yu J, Lv X, & Zhang L (2017). HOXA9 is critical in the proliferation, differentiation, and malignancy of leukaemia cells both in vitro and in vivo. Cell Biochemistry and Function, 35(7), 433–440. [DOI] [PubMed] [Google Scholar]
- Czajka AA, Wojcicka A, Kubiak A, Kotlarek M, Bakula-Zalewska E, Koperski L, et al. (2016). Family of microRNA-146 regulates RARbeta in papillary thyroid carcinoma. PLoS One, 11(3), e0151968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Wu Y, Leng AS, Ao Y, Robel RC, Lu SC, et al. (2003). RXRalpha-regulated liver SAMe and GSH levels influence susceptibility to alcohol-induced hepatotoxicity. Experimental and Molecular Pathology, 75(3), 194–200. [DOI] [PubMed] [Google Scholar]
- Das E, & Bhattacharyya NP (2014). MicroRNA-432 contributes to dopamine cocktail and retinoic acid induced differentiation of human neuroblastoma cells by targeting NESTIN and RCOR1 genes. FEBS Letters, 588(9), 1706–1714. [DOI] [PubMed] [Google Scholar]
- Das S, Foley N, Bryan K, Watters KM, Bray I, Murphy DM, et al. (2010). MicroRNA mediates DNA demethylation events triggered by retinoic acid during neuroblastoma cell differentiation. Cancer Research, 70(20), 7874–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave VP, Ngo TA, Pernestig AK, Tilevik D, Kant K, Nguyen T, et al. (2019). MicroRNA amplification and detection technologies: Opportunities and challenges for point of care diagnostics. Laboratory Investigation, 99(4), 452–469. [DOI] [PubMed] [Google Scholar]
- Delgado MD, & Leon J (2010). Myc roles in hematopoiesis and leukemia. Genes & Cancer, 1(6), 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Zhang Y, Xu C, & Ma D (2015). MicroRNA-125b-2 overexpression represses ectodermal differentiation of mouse embryonic stem cells. International Journal of Molecular Medicine, 36(2), 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Wang ZC, Zheng Y, Hu Z, Li Y, Luo DF, et al. (2016). C-Myc functions as a competing endogenous RNA in acute promyelocytic leukemia. Oncotarget, 7(35), 56422–56430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrotkova V, Chlapek P, Mazanek P, Sterba J, & Veselska R (2018). Traffic lights for retinoids in oncology: Molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy. BMC Cancer, 18(1), 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas PY, Mansier O, Prouzet-Mauleon V, Koya J, Villacreces A, Brunet de la Grange P, et al. (2018). MiR-10a and HOXB4 are overexpressed in atypical myeloproliferative neoplasms. BMC Cancer, 18(1), 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelisti C, Florian MC, Massimi I, Dominici C, Giannini G, Galardi S, et al. (2009). MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. The FASEB Journal, 23(12), 4276–4287. [DOI] [PubMed] [Google Scholar]
- Fang Y, Liu HX, Zhang N, Guo GL, Wan YJ, & Fang J (2013). NURBS: A database of experimental and predicted nuclear receptor binding sites of mouse. Bioinformatics, 29(2), 295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, et al. (2005). A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell, 123(5), 819–831. [DOI] [PubMed] [Google Scholar]
- Fisher JN, Terao M, Fratelli M, Kurosaki M, Paroni G, Zanetti A, et al. (2015). MicroRNA networks regulated by all-trans retinoic acid and Lapatinib control the growth, survival and motility of breast cancer cells. Oncotarget, 6(15), 13176–13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley NH, Bray I, Watters KM, Das S, Bryan K, Bernas T, et al. (2011). MicroRNAs 10a and 10b are potent inducers of neuroblastoma cell differentiation through targeting of nuclear receptor corepressor 2. Cell Death and Differentiation, 18(7), 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Paone A, Fabbri M, Zanesi N, Calore F, Cascione L, et al. (2013). Toll-like receptor 3 (TLR3) activation induces microRNA-dependent reexpression of functional RARbeta and tumor regression. Proceedings of the National Academy of Sciences of the United States of America, 110(24), 9812–9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy A, & Ezekiel U (2019). Phytochemical modulation of MiRNAs in colorectal cancer. Medicines (Basel), 6(2), 48 10.3390/medicines6020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, et al. (2007). MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene, 26(28), 4148–4157. [DOI] [PubMed] [Google Scholar]
- Goto T (2019). A review of the studies on food-derived factors which regulate energy metabolism via the modulation of lipid-sensing nuclear receptors. Bioscience, Biotechnology, and Biochemistry, 83(4), 579–588. [DOI] [PubMed] [Google Scholar]
- Gyamfi MA, He L, French SW, Damjanov I, & Wan YJ (2008). Hepatocyte retinoid X receptor alpha-dependent regulation of lipid homeostasis and inflammatory cytokine expression contributes to alcohol-induced liver injury. The Journal of Pharmacology and Experimental Therapeutics, 324(2), 443–453. [DOI] [PubMed] [Google Scholar]
- He Y, Gong L, Fang Y, Zhan Q, Liu HX, Lu Y, et al. (2013). The role of retinoic acid in hepatic lipid homeostasis defined by genomic binding and transcriptome profiling. BMC Genomics, 14, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. (2005). A microRNA polycistron as a potential human oncogene. Nature, 435(7043), 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, French SW, Chau T, Liu HX, Sheng L, Wei F, et al. (2019). RARbeta acts as both an upstream regulator and downstream effector of miR-22, which epigenetically regulates NUR77 to induce apoptosis of colon cancer cells. The FASEB Journal, 33(2), 2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Guo Y, Chen C, Li Q, Niu X, Guo S, et al. (2014). Repression of SIRT1 promotes the differentiation of mouse induced pluripotent stem cells into neural stem cells. Cellular and Molecular Neurobiology, 34(6), 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liu HX, He Y, Fang Y, Fang J, & Wan YJ (2013). Transcriptome profiling and genome-wide DNA binding define the differential role of fenretinide and all-trans RA in regulating the death and survival of human hepatocellular carcinoma Huh7 cells. Biochemical Pharmacology, 85(7), 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liu HX, Jena PK, Sheng L, Ali MR, & Wan YJ (2020). miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction. JHEP Reports, 2(2), 100093 10.1016/j.jhepr.2020.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Xu P, Sun B, Teng G, & Xiao Z (2017). Deep sequencing reveals complex mechanisms of microRNA regulation during retinoic acid-induced neuronal differentiation of mesenchymal stem cells. Genomics, 109(3–4), 302–311. [DOI] [PubMed] [Google Scholar]
- Hua S, Kittler R, & White KP (2009). Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell, 137(7), 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Xie C, Sun X, Ritchie RP, Zhang J, & Chen YE (2010). miR-10a contributes to retinoid acid-induced smooth muscle cell differentiation. The Journal of Biological Chemistry, 285(13), 9383–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar JM, & Payne CJ (2013). MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biology of Reproduction, 88(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey KN, & Srivastava D (2015). microRNAs as developmental regulators. Cold Spring Harbor Perspectives in Biology, 7(7), a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhari A, Singh T, & Yadav S (2018). Expression of miR-145 and its target proteins are regulated by miR-29b in differentiated neurons. Molecular Neurobiology, 55(12), 8978–8990. [DOI] [PubMed] [Google Scholar]
- Jeannotte L, Gotti F, & Landry-Truchon K (2016). Hoxa5: A key player in development and disease. Journal of Developmental Biology, 4(2), 13 10.3390/jdb4020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeker LT, Zhou X, Gershberg K, de Kouchkovsky D, Morar MM, Stadthagen G, et al. (2012). MicroRNA 10a marks regulatory T cells. PLoS One, 7(5), e36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian P, Li ZW, Fang TY, Jian W, Zhuan Z, Mei LX, et al. (2011). Retinoic acid induces HL-60 cell differentiation via the upregulation of miR-663. Journal of Hematology & Oncology, 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Britschgi A, Schlafli AM, Humbert M, Shan-Krauer D, Batliner J, et al. (2018). Low autophagy (ATG) gene expression is associated with an immature AML blast cell phenotype and can be restored during AML differentiation therapy. Oxidative Medicine and Cellular Longevity, 2018, 1482795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Wall D, Curran C, Newell J, Kerin MJ, & Dwyer RM (2015). MicroRNA-10a is reduced in breast cancer and regulated in part through retinoic acid. BMC Cancer, 15, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Czimmerer Z, Nagy G, Bieniasz-Krzywiec P, Ehling M, Pap A, et al. (2017). Retinoid X receptor suppresses a metastasis-promoting transcriptional program in myeloid cells via a ligand-insensitive mechanism. Proceedings of the National Academy of Sciences of the United States of America, 114(40), 10725–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel W, Ruhl R, & de Lera AR (2019). Alternative retinoid X receptor (RXR) ligands. Molecular and Cellular Endocrinology, 491, 110436. [DOI] [PubMed] [Google Scholar]
- Le MT, Xie H, Zhou B, Chia PH, Rizk P, Um M, et al. (2009). MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Molecular and Cellular Biology, 29(19), 5290–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Yang TL, Huang YH, Lee CI, Chen LJ, Shih YT, et al. (2018). Induction of microRNA-10a using retinoic acid receptor-alpha and retinoid x receptor-alpha agonists inhibits atherosclerotic lesion formation. Atherosclerosis, 271, 36–44. [DOI] [PubMed] [Google Scholar]
- Leyden J, Stein-Gold L, & Weiss J (2017). Why topical retinoids are mainstay of therapy for acne. Dermatology and Therapy, 7(3), 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen L, Zhao W, Hao J, & An R (2016). MicroRNA-let-7f-1 is induced by lycopene and inhibits cell proliferation and triggers apoptosis in prostate cancer. Molecular Medicine Reports, 13(3), 2708–2714. [DOI] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, & Gonzalez P (2009). Alterations in microRNA expression in stress-induced cellular senescence. Mechanisms of Ageing and Development, 130(11–12), 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Tang Y, Liu B, Cong W, Liu C, & Xiao J (2017). Retinoid acid-induced microRNA-27b-3p impairs C2C12 myoblast proliferation and differentiation by suppressing alpha-dystrobrevin. Experimental Cell Research, 350(2), 301–311. [DOI] [PubMed] [Google Scholar]
- Li L, Wang Y, Zhang X, Song G, Guo Q, Zhang Z, et al. (2018). Deubiquitinase USP48 promotes ATRA-induced granulocytic differentiation of acute promyelocytic leukemia cells. International Journal of Oncology, 53(2), 895–903. [DOI] [PubMed] [Google Scholar]
- Lin Y, Li D, Liang Q, Liu S, Zuo X, Li L, et al. (2015). miR-638 regulates differentiation and proliferation in leukemic cells by targeting cyclin-dependent kinase 2. The Journal of Biological Chemistry, 290(3), 1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KY, Zhang XJ, Feng DD, Zhang H, Zeng CW, Han BW, et al. (2011). miR-125b, a target of CDX2, regulates cell differentiation through repression of the core binding factor in hematopoietic malignancies. The Journal of Biological Chemistry, 286(44), 38253–38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen M, Li L, Gong L, Zhou H, & Gao D (2017). Extracellular signal-regulated kinases (ERKs) phosphorylate Lin28a protein to modulate P19 cell proliferation and differentiation. The Journal of Biological Chemistry, 292(10), 3970–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen K, Wu J, Shi L, Hu B, Cheng S, et al. (2013). Downregulation of miR-452 promotes stem-like traits and tumorigenicity of gliomas. Clinical Cancer Research, 19(13), 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Hu Y, & Wan YJ (2016). Microbiota and bile acid profiles in retinoic acid-primed mice that exhibit accelerated liver regeneration. Oncotarget, 7(2), 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HJ, Lam HC, Baglini CV, Nijmeh J, Cottrill AA, Chan SY, et al. (2019). Rapamycin-upregulated miR-29b promotes mTORC1-hyperactive cell growth in TSC2-deficient cells by downregulating tumor suppressor retinoic acid receptor beta (RARbeta). Oncogene, 38, 7367–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Liu C, Cong W, Li N, Zhou N, Tang Y, et al. (2017). Retinoid acid-induced microRNA-31–5p suppresses myogenic proliferation and differentiation by targeting CamkIIdelta. Skeletal Muscle, 7(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Ly I, Hu Y, & Wan YJ (2014). Retinoic acid regulates cell cycle genes and accelerates normal mouse liver regeneration. Biochemical Pharmacology, 91(2), 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Y, & Lin L (2019). Small molecules for fat combustion: Targeting obesity. Acta Pharmaceutica Sinica B, 9(2), 220–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhong L, Yuan Z, Yao J, Zhong P, Liu J, et al. (2019). miR-382–5p modulates the ATRA-induced differentiation of acute promyelocytic leukemia by targeting tumor suppressor PTEN. Cellular Signalling, 54, 1–9. [DOI] [PubMed] [Google Scholar]
- Luu L, Ciccotosto GD, Vella LJ, Cheng L, Roisman LC, Multhaup G, et al. (2019). Amyloid precursor protein dimerisation reduces neurite outgrowth. Molecular Neurobiology, 56(1), 13–28. [DOI] [PubMed] [Google Scholar]
- Manodoro F, Marzec J, Chaplin T, Miraki-Moud F, Moravcsik E, Jovanovic JV, et al. (2014). Loss of imprinting at the 14q32 domain is associated with microRNA over-expression in acute promyelocytic leukemia. Blood, 123(13), 2066–2074. [DOI] [PubMed] [Google Scholar]
- Martin N, Ma X, & Bernard D (2019). Regulation of cellular senescence by retinoid X receptors and their partners. Mechanisms of Ageing and Development, 183, 111131. [DOI] [PubMed] [Google Scholar]
- Meseguer S, Mudduluru G, Escamilla JM, Allgayer H, & Barettino D (2011). MicroRNAs-10a and −10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ASF). The Journal of Biological Chemistry, 286(6), 4150–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Han YC, Betel D, Yao E, Squatrito M, Ogrodowski P, et al. (2009). Genetic dissection of the miR-17~92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes & Development, 23(24), 2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Baghy K, Hunyadi-Gulyas E, Micsik T, Nyiro G, Racz G, et al. (2015). Evaluation of 9-cis retinoic acid and mitotane as antitumoral agents in an adrenocortical xenograft model. American Journal of Cancer Research, 5(12), 3645–3658. [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Nakajima M, Tsuneyama K, Takamiya M, Aoki Y, Fukami T, et al. (2014). Retinoid X receptor alpha in human liver is regulated by miR-34a. Biochemical Pharmacology, 90(2), 179–187. [DOI] [PubMed] [Google Scholar]
- Oliveira LM, Teixeira FME, & Sato MN (2018). Impact of retinoic acid on immune cells and inflammatory diseases. Mediators of Inflammation, 2018, 3067126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, et al. (2015). MicroRNA-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. The Journal of Clinical Investigation, 125(12), 4334–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallocca G, Fabbri M, Sacco MG, Gribaldo L, Pamies D, Laurenza I, et al. (2013). miRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biology and Toxicology, 29(4), 239–257. [DOI] [PubMed] [Google Scholar]
- Park EJ, Prajuabjinda O, Soe ZY, Darkwah S, Appiah MG, Kawamoto E, et al. (2019). Exosomal regulation of lymphocyte homing to the gut. Blood Advances, 3(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons XH (2012). MicroRNA profiling reveals distinct mechanisms governing cardiac and neural lineage-specification of pluripotent human embryonic stem cells. Journal of Stem Cell Research & Therapy, 2(3), 124 10.4172/2157-7633.1000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons XH, Parsons JF, & Moore DA (2012). Genome-scale mapping of microRNA signatures in human embryonic stem cell neurogenesis. Molecular Medicine & Therapeutics, 1(2), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi A, Careccia S, Sagrestani G, Nanni S, Manni I, Schinzari V, et al. (2014). Dual promoter usage as regulatory mechanism of let-7c expression in leukemic and solid tumors. Molecular Cancer Research, 12(6), 878–889. [DOI] [PubMed] [Google Scholar]
- Penvose A, Keenan JL, Bray D, Ramlall V, & Siggers T (2019). Comprehensive study of nuclear receptor DNA binding provides a revised framework for understanding receptor specificity. Nature Communications, 10(1), 2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua SL, Sivakamasundari V, Shao Y, Cai X, Zhang LF, Lufkin T, et al. (2011). Nuclear accumulation of an uncapped RNA produced by Drosha cleavage of a transcript encoding miR-10b and HOXD4. PLoS One, 6(10), e25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Li L, Zhang D, Liu QL, Chen XR, Yang HY, et al. (2016). Altered microRNA expression profiles in a rat model of spina bifida. Neural Regeneration Research, 11(3), 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Li L, Zhang D, Liu QL, Chen XR, Yang HY, et al. (2017). Preliminary investigation of methylation status of microRNA-124a in spinal cords of rat fetuses with congenital spina bifida. The Journal of Maternal-Fetal & Neonatal Medicine, 30(1), 23–28. [DOI] [PubMed] [Google Scholar]
- Rossi A, D’Urso OF, Gatto G, Poltronieri P, Ferracin M, Remondelli P, et al. (2010). Non-coding RNAs change their expression profile after Retinoid induced differentiation of the promyelocytic cell line NB4. BMC Research Notes, 3, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl R, Krezel W, & de Lera AR (2018). 9-Cis-13,14-dihydroretinoic acid, a new endogenous mammalian ligand of retinoid X receptor and the active ligand of a potential new vitamin A category: Vitamin A5. Nutrition Reviews, 76(12), 929–941. [DOI] [PubMed] [Google Scholar]
- Saumet A, Vetter G, Bouttier M, Antoine E, Roubert C, Orsetti B, et al. (2012). Estrogen and retinoic acid antagonistically regulate several microRNA genes to control aerobic glycolysis in breast cancer cells. Molecular BioSystems, 8(12), 3242–3253. [DOI] [PubMed] [Google Scholar]
- Saumet A, Vetter G, Bouttier M, Portales-Casamar E, Wasserman WW, Maurin T, et al. (2009). Transcriptional repression of microRNA genes by PML-RARA increases expression of key cancer proteins in acute promyelocytic leukemia. Blood, 113(2), 412–421. [DOI] [PubMed] [Google Scholar]
- Stoecker K, Sass S, Theis FJ, Hauner H, & Pfaffl MW (2017). Inhibition of fat cell differentiation in 3T3-L1 pre-adipocytes by all-trans retinoic acid: Integrative analysis of transcriptomic and phenotypic data. Biomolecular Detection and Quantification, 11, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Gu X, Zhang Z, Li W, & Wang X (2020). Retinoic acid receptor gamma is targeted by microRNA-124 and inhibits neurite outgrowth. Neuropharmacology, 163, 107657 10.1016/j.neuropharm.2019.05.034. [DOI] [PubMed] [Google Scholar]
- Suzhi Z, Liang T, Yuexia P, Lucy L, Xiaoting H, Yuan Z, et al. (2015). Gap junctions enhance the antiproliferative effect of microRNA-124–3p in glioblastoma cells. Journal of Cellular Physiology, 230(10), 2476–2488. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciume G, Muljo SA, et al. (2012). TGF-beta and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nature Immunology, 13(6), 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Chen Y, Meyer C, Geistlinger T, Lupien M, Wang Q, et al. (2011). A comprehensive view of nuclear receptor cancer cistromes. Cancer Research, 71(22), 6940–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang GW, & Russell RM (1990). 13-cis-retinoic acid is an endogenous compound in human serum. Journal of Lipid Research, 31(2), 175–182. [PubMed] [Google Scholar]
- Tay YM, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, et al. (2008). MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells, 26(1), 17–29. [DOI] [PubMed] [Google Scholar]
- Tay Y, Zhang J, Thomson AM, Lim B, & Rigoutsos I (2008). MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature, 455(7216), 1124–1128. [DOI] [PubMed] [Google Scholar]
- Tennakoon JB, Wang H, Coarfa C, Cooney AJ, & Gunaratne PH (2013). Chromatin changes in dicer-deficient mouse embryonic stem cells in response to retinoic acid induced differentiation. PLoS One, 8(9), e74556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao M, Fratelli M, Kurosaki M, Zanetti A, Guarnaccia V, Paroni G, et al. (2011). Induction of miR-21 by retinoic acid in estrogen receptor-positive breast carcinoma cells: Biological correlates and molecular targets. The Journal of Biological Chemistry, 286(5), 4027–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombolan L, Zampini M, Casara S, Boldrin E, Zin A, Bisogno G, et al. (2015). MicroRNA-27a contributes to rhabdomyosarcoma cell proliferation by suppressing RARA and RXRA. PLoS One, 10(4), e0125171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong MH, Mitchell DA, McGowan SD, Evanoff R, & Griswold MD (2012). Two miRNA clusters, Mir-17–92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biology of Reproduction, 86(3), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J, Zhang P, Shui Luk AC, Liao J, Chan WY, Qi H, et al. (2018). MicroRNA-26b promotes transition from Kit(−) to Kit(+) mouse spermatogonia. Experimental Cell Research, 373(1–2), 71–79. [DOI] [PubMed] [Google Scholar]
- van Gils N, Verhagen H, & Smit L (2017). Reprogramming acute myeloid leukemia into sensitivity for retinoic-acid-driven differentiation. Experimental Hematology, 52, 12–23. [DOI] [PubMed] [Google Scholar]
- Vecellio M, Meraviglia V, Nanni S, Barbuti A, Scavone A, DiFrancesco D, et al. (2012). In vitro epigenetic reprogramming of human cardiac mesenchymal stromal cells into functionally competent cardiovascular precursors. PLoS One, 7(12), e51694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YJ, An D, Cai Y, Repa JJ, Hung-Po Chen T, Flores M, et al. (2000). Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Molecular and Cellular Biology, 20(12), 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YJ, Cai Y, Lungo W, Fu P, Locker J, French S, et al. (2000). Peroxisome proliferator-activated receptor alpha-mediated pathways are altered in hepatocyte-specific retinoid X receptor alpha-deficient mice. The Journal of Biological Chemistry, 275(36), 28285–28290. [DOI] [PubMed] [Google Scholar]
- Wan YJ, Han G, Cai Y, Dai T, Konishi T, & Leng AS (2003). Hepatocyte retinoid X receptor-alpha-deficient mice have reduced food intake, increased body weight, and improved glucose tolerance. Endocrinology, 144(2), 605–611. [DOI] [PubMed] [Google Scholar]
- Wan YJ, Orrison BM, Lieberman R, Lazarovici P, & Ozato K (1987). Induction of major histocompatibility class I antigens by interferons in undifferentiated F9 cells. Journal of Cellular Physiology, 130(2), 276–283. [DOI] [PubMed] [Google Scholar]
- Wan YJ, Wang L, & Wu TC (1994). The expression of retinoid X receptor genes is regulated by all-trans- and 9-cis-retinoic acid in F9 teratocarcinoma cells. Experimental Cell Research, 210(1), 56–61. [DOI] [PubMed] [Google Scholar]
- Wang XS, Gong JN, Yu J, Wang F, Zhang XH, Yin XL, et al. (2012). MicroRNA-29a and microRNA-142–3p are regulators of myeloid differentiation and acute myeloid leukemia. Blood, 119(21), 4992–5004. [DOI] [PubMed] [Google Scholar]
- Wang X, Tan L, Lu Y, Peng J, Zhu Y, Zhang Y, et al. (2015). MicroRNA-138 promotes tau phosphorylation by targeting retinoic acid receptor alpha. FEBS Letters, 589(6), 726–729. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang Z, Lin S, Zheng W, Wang R, Jin S, et al. (2012). Revealing a natural marine product as a novel agonist for retinoic acid receptors with a unique binding mode and inhibitory effects on cancer cells. The Biochemical Journal, 446(1), 79–87. [DOI] [PubMed] [Google Scholar]
- Wang C, Yao N, Lu CL, Li D, & Ma X (2010). Mouse microRNA-124 regulates the expression of Hes1 in P19 cells. Frontiers in Bioscience (Elite Edition), 2, 127–132. [DOI] [PubMed] [Google Scholar]
- Watanabe M, & Kakuta H (2018). Retinoid X receptor antagonists. International Journal of Molecular Sciences, 19(8), 2354 10.3390/ijms19082354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Yamaji R, & Ohtsuki T (2018). MicroRNA-664a-5p promotes neuronal differentiation of SH-SY5Y cells. Genes to Cells, 23(3), 225–233. [DOI] [PubMed] [Google Scholar]
- Weiss FU, Marques IJ, Woltering JM, Vlecken DH, Aghdassi A, Partecke LI, et al. (2009). Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology, 137(6) 2136–2145.e7. [DOI] [PubMed] [Google Scholar]
- Welch C, Chen Y, & Stallings RL (2007). MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene, 26(34), 5017–5022. [DOI] [PubMed] [Google Scholar]
- Wu TC, Wang L, & Wan YJ (1992). Retinoic acid regulates gene expression of retinoic acid receptors alpha, beta and gamma in F9 mouse teratocarcinoma cells. Differentiation, 51(3), 219–224. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhao J, Fu B, Yin S, Song C, Zhang J, et al. (2017). Retinoic acid-induced upregulation of miR-219 promotes the differentiation of embryonic stem cells into neural cells. Cell Death & Disease, 8(7), e2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Hu Y, Liu HX, & Wan YJ (2015). MiR-22-silenced cyclin A expression in colon and liver cancer cells is regulated by bile acid receptor. The Journal of Biological Chemistry, 290(10), 6507–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Miao L, Mei Y, & Wu M (2013). Retinoic acid-induced HOXA5 expression is co-regulated by HuR and miR-130a. Cellular Signalling, 25(6), 1476–1485. [DOI] [PubMed] [Google Scholar]
- Yao J, Zhong L, Zhong P, Liu D, Yuan Z, Liu J, et al. (2019). RAS-responsive element-binding protein 1 blocks the granulocytic differentiation of myeloid leukemia cells. Oncology Research, 27(7), 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Hu Y, Wu Y, Fang C, Lai J, Chen S, et al. (2019). The c-Myc-regulated miR-17–92 cluster mediates ATRA-induced APL cell differentiation. Asia-Pacific Journal of Clinical Oncology, 15(6), 364–370. [DOI] [PubMed] [Google Scholar]
- Yu M, Zhang L, Liu Y, Liu D, & Guo Z (2019). Retinoic acid induces differentiation of mouse F9 embryonic carcinoma cell by modulating the miR-485 targeting of Abhd2. International Journal of Molecular Sciences, 20(9), 2071 10.3390/ijms20092071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gao Y, Yu M, Wu H, Ai Z, Wu Y, et al. (2015). Retinoic acid induces embryonic stem cell differentiation by altering both encoding RNA and microRNA expression. PLoS One, 10(7), e0132566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ke F, Liu Z, Bai J, Liu J, Yan S, et al. (2015). MicroRNA-31 negatively regulates peripherally derived regulatory T-cell generation by repressing retinoic acid-inducible protein 3. Nature Communications, 6, 7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ma X, Sung D, Li M, Kosti A, Lin G, et al. (2015). microRNA-449a functions as a tumor suppressor in neuroblastoma through inducing cell differentiation and cell cycle arrest. RNA Biology, 12(5), 538–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Sun DG, Wang J, Liu SR, Zhang CY, Zhu MX, et al. (2008). Retinoic acid downregulates microRNAs to induce abnormal development of spinal cord in spina bifida rat model. Child’s Nervous System, 24(4), 485–492. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Liu J, Yang Z, Wu L, Xie H, Jiang C, et al. (2016). MicroRNA-452 promotes stem-like cells of hepatocellular carcinoma by inhibiting Sox7 involving Wnt/beta-catenin signaling pathway. Oncotarget, 7(19), 28000–28012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Pan C, Li L, Bian Z, Lv Z, Shi L, et al. (2013). MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein alpha. The Journal of Allergy and Clinical Immunology, 132(2), 426–436.e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang LK, Xu GP, Pan XR, Lou YJ, Zou QP, Xia D, et al. (2014). MicroRNA-181a-mediated downregulation of AC9 protein decreases intracellular cAMP level and inhibits ATRA-induced APL cell differentiation. Cell Death & Disease, 5, e1161. [DOI] [PMC free article] [PubMed] [Google Scholar]


