Abstract
Camellia oil has become an important plant oil in China in recent years, but its effects on non-alcoholic fatty liver disease (NAFLD) have not been documented. In this study, the effects of camellia oil, soybean oil, and olive oil on NAFLD were evaluated by analyzing the fatty acid profiles of the plant oils, the serum lipids and lipoproteins of rats fed different oils, and by cytological and ultrastructural observation of the rats’ hepatocytes. Analysis of fatty acid profiles showed that the polyunsaturated fatty acid (PUFA) n-6/n-3 ratio was 33.33 in camellia oil, 12.50 in olive oil, and 7.69 in soybean oil. Analyses of serum lipids and lipoproteins of rats showed that the levels of total cholesterol and low-density lipoprotein cholesterol in a camellia oil-fed group (COFG) were lower than those in an olive oil-fed group (OOFG) and higher than those in a soybean oil-fed group (SOFG). However, only the difference in total cholesterol between the COFG and SOFG was statistically significant. Cytological observation showed that the degree of lipid droplet (LD) accumulation in the hepatocytes in the COFG was lower than that in the OOFG, but higher than that in the SOFG. Ultrastructural analysis revealed that the size and number of the LDs in the hepatocytes of rats fed each of the three types of oil were related to the degree of damage to organelles, including the positions of nuclei and the integrity of mitochondria and endoplasmic reticulum. The results revealed that the effect of camellia oil on NAFLD in rats was greater than that of soybean oil, but less than that of olive oil. Although the overall trend was that among the three oil diets, those with a lower n-6/n-3 ratio were associated with a lower risk of NAFLD, and the effect of camellia oil on NAFLD was not entirely related to the n-6/n-3 ratio and may have involved other factors. This provides new insights into the effect of oil diets on NAFLD.
Keywords: Camellia oil, Fatty acid, Lipid droplet, Hepatocyte ultrastructure, Organelle
1. Introduction
Camellia oleifera Abel. belongs to the Theaceae family, which is native to south and southeastern China (Zeng and Endo, 2019). The species produces the highest yield of seed oil (known as camellia oil) within the Theaceae family and therefore has become the focus of some research attention over recent decades (Yang et al., 2016; Zeng and Endo, 2019). Studies have shown that in camellia oil more than 76% of the fatty acids are oleic acid (Yang et al., 2016), compared with more than 50% of those in olive oil (Oğraş et al., 2016). Moreover, camellia oil contains a variety of substances with biological activity, such as flavonoids (Liu et al., 2014), polyphenols (Wang et al., 2017b), squalene (Wang et al., 2017a), and sesamin. Previous studies also have shown that camellia oil has antioxidant, anti-inflammatory, antimicrobial, gastroprotective, and hepatoprotective bioactivity (Cheng et al., 2015). In 2017, 600 000 tons of oil was processed from 2.43 million tons of C. oleifera seeds in China, based on data from the National Bureau of Statistics of China (Boudour-Benrachou et al., 2017). At present, the cultivated area of C. oleifera is increasing every year.
Soybean oil is second to camellia oil in terms of the amount of dietary plant oil produced in the world (Boudour-Benrachou et al., 2017). Soybean oil consists of five fatty acids, including 55% linoleic acid (18:2), 18% oleic acid (18:1), 13% linolenic acid (18:3), 10% palmitic acid (16:0), and 4% stearic acid (18:0) (Clemente and Cahoon, 2009). Further studies have shown that soybean fat is high in polyunsaturated fatty acids (PUFAs), including linoleic and linolenic acids. It contains a substantial amount of monounsaturated fatty acids (MUFAs; e.g., oleic acid) and moderate amounts of saturated fatty acids (SFAs; e.g., palmitic and stearic acids) (Prabakaran et al., 2018). Linoleic acid is the predominant fatty acid, accounting for over 53% of the total fatty-acid content of soybeans (Messina, 1997).
Non-alcoholic fatty liver disease (NAFLD) is characterized by hepatocellular fat deposition and is closely related to nutritional factors (Juárez-Hernández et al., 2016). Among dietary fatty acids, MUFAs have a dual role, including reducing oxidized low-density lipoprotein (LDL), low-density lipoprotein cholesterol (LDL-C), and total cholesterol (TC) concentrations, as well as increasing high-density lipoprotein (HDL), in various human and murine models. By comparison, the PUFAs exhibit anti-obesity, anti-steatosis, and anti-inflammatory effects. The n-3 fatty acids, such as α-linolenic acid (18:3n-3), inhibit the synthesis of triglycerides (TGs) and very-low-density lipoproteins (VLDLs) in the liver, and have a major effect in lowering TG levels in the blood (Han, 2012). The daily intake and the ratio of n-6 to n-3 fats affect lipid metabolism in the liver (Ferramosca and Zara, 2014). This is because n-3 PUFAs in the diet can limit the hepatic storage of triacylglycerol, and a lower dietary n-6/n-3 ratio is an ideal way to improve human health (Monteiro et al., 2014). Although camellia oil is used as a daily edible oil and its anti-inflammatory or anti-oxidative effect has been widely studied (Xiao et al., 2017), there have been few investigations of the effects of its fatty acids on fatty liver disease.
In this study, therefore, we analyzed the fatty acid profile of camellia oil, and compared the effects of camellia, olive, and soybean oils on serum lipids. The results revealed the cytological and ultrastructural characteristics of NAFLD caused by each of the three oils in rats. This will provide a valuable reference for understanding the effects of edible oil composition on NAFLD and human health.
2. Materials and methods
2.1. Analysis of fatty acid profile of plant oils
Camellia oil was obtained from the Zhejiang Changfa Cereals Oil and Food Co., Ltd. (Quzhou, China), soybean oil from the Zhejiang Yihai Kerry Food Industry Co., Ltd. (Hangzhou, China), and olive oil from the Olive Garden Longnan Technology Development Co., Ltd. (Longnan, China).
For analyzing the fatty acid profiles of the three kinds of oil, reference standards of 37 fatty acid methyl esters (gas chromatography (GC) standard) were purchased from Sigma Co., Ltd. (Shanghai, China). The standards included: methyl butyrate; methyl laurate; methyl octanoate; methyl decanoate; methyl hexanoate; methyl undecanoate; methyl tridecanoate; methyl myristate; methyl 9-tetradecenoate; methyl pentadecanoate; methyl cis-10-decarbonate; methyl palmitoleate; methyl palmitate; methyl heptadecanoate; methyl stearate; methyl cis-10-heptachenoate; methyl inveroleate; methyl oleate; methyl antilinoleate; methyl γ-linolenate; methyl linoleate; methyl arachidate; methyl cis-11-eicosenoate; methyl linolenate; methyl heneicosanoate; cis-8,11,14-eicosatrienoic acid methyl ester; cis-11,14-eicosadienoic acid methyl ester; methyl behenate; cis-11,14,17-eicosatrienoic acid methyl ester; methyl arachidonate; methyl tricosanoate; methyl cis-13-docosenoate; methyl all-cis-5,8,11,14,17-eicosapentaenoate; methyl cis-13,16-docosadienoate; methyl cis-15-tetracosenoate; methyl tetracosanoate; all-cis-4,7,10,13,16,19-mocosahexaenoic acid methyl ester. The fatty acid profiles of the three oil samples were analyzed by a gas chromatograph (GC-2014, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector (FID) in compliance with the Food Safety National Standard of China (NHFPC and CFDA, 2016). Each GC sample was tested in triplicate.
2.2. Animal model and diets
Forty-two adult male Sprague-Dawley (SD) rats (each weighing about 250 g) were obtained from the Zhejiang Chinese Medical University Laboratory Animal Research Center (Hangzhou, China). The animals were housed in individual cages in rooms with controlled temperature (20–22 °C) and humidity of (55±5)%, and with a 12-h light/dark cycle. After a one-week period of adaptation to the diet and the feeding level, the rats were randomly divided into six groups: one group was fed a regular chow diet group (RCDG) (Table 1) and the other five groups were fed a high-fat diet (HFD) (Table 2). The groups were fed these diets for five weeks, with water and food supplied ad libitum. Thereafter, the RCDG continued to be fed regular chow diet (RCD), while the five HFD groups were fed with an RCD, HFD, camellia oil diet (COD), soybean oil diet (SOD), or olive oil diet (OOD) (Table 2). Food and water were supplied as described above. After twelve weeks, the rats were anesthetized and sacrificed for biochemical, cytological, and ultrastructural analyses.
Table 1.
Composition and energy density of the regular chow diet
| Component | Mass fraction (%) | Energy (%) |
| Crude fiber | 1.80 | 0 |
| Crude protein | 19.20 | 20.60 |
| Crude fat | 4.90 | 12.00 |
| Crude ash | 4.80 | 0 |
| Moisture | 9.92 | 0 |
| Calcium | 1.08 | 0 |
| Total phosphorus | 0.71 | 0 |
| Vitamin mix, V10001 | 0.01 | 0.20 |
| Nitrogen free extract | 58.00 | 67.20 |
|
| ||
| Energy (kcal/g diet) | 3.62 | |
The regular chow diet (RCD) was purchased from the Synergy Pharmaceutical Biological Engineering Co., Ltd. (SHOBREE; Jiangsu, China). 1 kcal=4.18 kJ
Table 2.
Composition of the high-fat diet and three plant oil diets
| Ingredient | HFD |
OOD |
COD |
SOD |
||||
| Mass (g) | Energy (kcal) | Mass (g) | Energy (kcal) | Mass (g) | Energy (kcal) | Mass (g) | Energy (kcal) | |
| Casein, 80 mesh | 200.00 | 800 | 200.00 | 800 | 200.00 | 800 | 200.00 | 800 |
| L-Cystine | 3.00 | 12 | 3.00 | 12 | 3.00 | 12 | 3.00 | 12 |
| Corn starch | 212.00 | 848 | 212.00 | 848 | 212.00 | 848 | 212.00 | 848 |
| Maltodextrin 10 | 71.00 | 284 | 71.00 | 284 | 71.00 | 284 | 71.00 | 284 |
| Sucrose | 113.00 | 452 | 113.00 | 452 | 113.00 | 452 | 113.00 | 452 |
| Cellulose, BW200 | 50.00 | 0 | 50.00 | 0 | 50.00 | 0 | 50.00 | 0 |
| Cocoa butter | 155.00 | 1395 | 0 | 0 | 0 | 0 | 0 | 0 |
| Olive oil | 0 | 0 | 180.00 | 1620 | 0 | 0 | 0 | 0 |
| Camellia oil | 0 | 0 | 0 | 0 | 180.00 | 1620 | 0 | 0 |
| Soybean oil | 25.00 | 225 | 0 | 0 | 0 | 0 | 180.00 | 1620 |
| Mineral mix, S10021 | 10.00 | 0 | 10.00 | 0 | 10.00 | 0 | 10.00 | 0 |
| Di-calcium phosphate | 13.00 | 0 | 13.00 | 0 | 13.00 | 0 | 13.00 | 0 |
| Calcium carbonate | 5.50 | 0 | 5.50 | 0 | 5.50 | 0 | 5.50 | 0 |
| Potassium citrate | 16.50 | 0 | 16.50 | 0 | 16.50 | 0 | 16.50 | 0 |
| Vitamin mix, V10001 | 10.00 | 40 | 10.00 | 40 | 10.00 | 40 | 10.00 | 40 |
| Choline bitartrate | 2.00 | 0 | 2.00 | 0 | 2.00 | 0 | 2.00 | 0 |
| Cholesterol | 11.25 | 0 | 11.25 | 0 | 11.25 | 0 | 11.25 | 0 |
| 2-Thiouracil | 1.90 | 0 | 1.90 | 0 | 1.90 | 0 | 1.90 | 0 |
| Sodium cholate | 4.50 | 0 | 4.50 | 0 | 4.50 | 0 | 4.50 | 0 |
| FD&C red dye No. 40# | 0.05 | 0 | 0.05 | 0 | 0.05 | 0 | 0.05 | 0 |
| FD&C blue dye No. 1# | 0.05 | 0 | 0.05 | 0 | 0.05 | 0 | 0.05 | 0 |
| FD$ yellow dye No. 5# | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Total | 903.75 | 4056 | 903.75 | 4056 | 903.75 | 4056 | 903.75 | 4056 |
The high-fat diet (HFD; product No. D12109T), olive oil diet (OOD; D19052002), camellia oil diet (COD; D19052001), and soybean oil diet (SOD; D19052001) were purchased from Changzhou SYSE (Shu-yi-shu-er) Biotechnology Co., Ltd. (Jiangshu, China).
Different products marked by different trace dyes (for fast recognition in use)
2.3. Blood collection and biochemical analysis
The rats were anesthetized with 10% chloral hydrate (0.03 mL/kg), and then weighed. Fasting blood samples (2 mL) were obtained from the abdominal aorta of each animal. Each blood sample was collected into a tube and centrifuged at 3000 r/min at 4 °C for 10 min to obtain the serum. The serum TC, TG, high-density lipoprotein cholesterol (HDL-C), and LDL-C in each sample were measured using an automatic biochemistry analyzer (Hitachi 3100, Tokyo, Japan).
2.4. Cytological observation of livers
After the rats were sacrificed, their livers were removed at the ventral aspect of the body and dried with gauze before being weighed. A sample of tissue from each liver was fixed immediately in 10% neutral formalin. After at least 3 d of fixation, the tissue samples were processed using an automatic tissue processor and embedded in paraffin wax. Thin sections (4 μm) were stained with hematoxylin and eosin. Cytological characteristics were observed and photographed using a light microscope (Nikon Eclipse E100, Japan) with a NIKON DS-U3 imaging system.
2.5. Ultrastructure observation of livers
After recording the weight of the liver, a piece of tissue (1 mm×3 mm×1 mm) was cut out and fixed in glutaraldehyde, as described by Zhang et al. (2012). Tissues were washed, post-fixed, and embedded, after passing through a dehydration series. Ultrathin sections were cut using a Reichart-Jung Ultracut E ultramicrotome with a diamond knife. Ultrathin sections were stained with uranyl acetate and lead citrate, and examined using a transmission electron microscope at 80 kV and a Gatan 832 CCD camera (Hitachi H-7650, Tokyo, Japan).
2.6. Statistical analysis
All statistical analysis was performed using the SPSS statistical program for Windows, Version 26 (SPSS Inc., Chicago, Ill., USA) and the level of significance for the Student’s t-test or analysis of variance (ANOVA) test was set at P<0.05.
3. Results
3.1. Fatty acid profiles of plant oils
The fatty acid composition and content of the three kinds of oil are presented in Table 3. The fatty acid composition comprised a mixture of SFAs and unsaturated fatty acids (USFAs), classified as MUFAs and PUFAs according to the number of unsaturated bonds. Although the five major fatty acids were identified in the three samples, oleic acid (18:1n-9), linoleic acid (18:2n-6), and palmitic acid (16:0) were the major fatty acids in camellia oil, and similarly in olive oil (Table 3). The fatty acid composition of olive oil was also similar to that reported by Boudour-Benrachou et al. (2017). In soybean oil, the major fatty acids were linoleic acid (18:2n-6), oleic acid (18:1n-9), palmitic acid (16:0), and α-linolenic acid (18:3n-3).
Table 3.
Fatty acid composition and content of three kinds of oil
| Fatty acid | Content (%) |
||
| Soybean oil | Camellia oil | Olive oil | |
| 16:0 | 10.93±0.06 | 8.76±0.05 | 12.87±0.04 |
| 16:1 | 1.14±0.04 | ||
| 18:0 | 3.57±0.16 | 0.93±0.48 | 1.04±0.17 |
| 18:1n-9 | 22.36±0.01 | 79.45±0.33 | 74.52±0.12 |
| 18:2n-6 | 56.10±0.33 | 9.90±0.04 | 8.91±0.01 |
| 18:3n-3 | 7.03±0.10 | 0.34±0.05 | 0.68±0.04 |
| 20:0 | 0.27±0.02 | ||
| 20:1n-9 | 0.62±0.08 | 0.22±0.01 | |
| SFA | 14.59±0.23 | 9.86±0.43 | 14.53±0.15 |
| USFA | 85.41±0.23 | 90.14±0.43 | 85.47±0.15 |
| MUFA | 22.36±0.01 | 79.94±0.34 | 75.43±0.26 |
| PUFA | 63.05±0.23 | 10.21±0.09 | 10.04±0.33 |
|
| |||
| n-6/n-3 | 7.69±0.01 | 33.33±0.01 | 12.50±0.01 |
Data are expressed as mean±standard deviation (SD), n=3. 16:0, palmitic acid; 16:1, palmitoleic acid; 18:0, stearic acid; 18:1n-9, oleic acid; 18:2n-6, linoleic acid; 18:3n-3, α-linolenic acid; 20:0, arachidic acid; 20:1n-9, gondoic acid; SFA, saturated fatty acid; USFA, unsaturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid
Among the three kinds of oil, camellia oil contained the highest content of USFAs ((90.14±0.43)%) and the lowest content of SFAs ((9.86±0.43)%) compared with soybean oil with (85.41±0.23)% USFAs and (14.59±0.23)% SFAs, and olive oil with (85.47±0.15)% USFAs and (14.53±0.15)% SFAs. However, the content of MUFAs was significantly higher in camellia and olive oils than in soybean oil (22.36%), but the content of their PUFAs was significantly lower than in soybean oil (63.05%). In the PUFAs, the PUFA n-6/n-3 ratios were 33.33 for camellia oil, 7.69 for soybean oil, and 12.50 for olive oil.
Two minor fatty acids, arachidic acid (20:0) and gondoic acid (20:1n-9), were also identified in olive oil, but of these only gondoic acid (20:1n-9) was found in camellia oil, and neither in soybean oil.
3.2. Effects of different plant oils on body weight and liver index of rats
The body weights at the start of the experimental period and after 17 weeks are represented in Fig. 1a. The increase in body weight was significant in the RCDG and control diet group (CDG), but not significant in the HFDG, COFG, SOFG, or OOFG (P<0.05).
Fig. 1.
Effects of different diets on body weight, liver index, and serum lipids and lipoproteins in rats
(a) Changes of body weight at the start of the experimental period and after 17 weeks. (b) Liver index after 17 weeks. The liver index was calculated as liver weight/body weight×100%. (c) Effects on serum lipids and lipoproteins after 17 weeks. TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; RCDG, regular chow diet group; HFDG, high-fat diet group; CDG, control diet group; SOFG, soybean oil-fed group; COFG, camellia oil-fed group; OOFG, olive oil-fed group. All values are expressed as mean±standard deviation (SD), n=7 per group. Different letters on the bars indicate significant differences among groups (P<0.05) according to Student’s t-test or analysis of variance (ANOVA) test
The liver index (LI) of rats in each group after 17 weeks is shown in Fig. 1b. The LI was highest in the OOFG (7.35%), and the second highest in the COFG (7.06%), but there was no difference between the HFDG and COFG. The lowest LI was 2.36% in the RCDG.
3.3. Effects of different plant oils on serum lipids and lipoproteins
The effect of the diets among the different treatment groups on serum TC, TG, and lipoproteins, such as LDL-C and HDL-C, are presented in Fig. 1c. The TC concentrations varied greatly, ranging from 1.51 to 10.57 mmol/L among the different treatment groups. The highest concentration was (10.57±6.10) mmol/L in the OOFG, but there was no significant difference between the HFDG ((9.97±2.17) mmol/L) and the COFG ((8.70±3.20) mmol/L). The lowest concentration was (1.51±0.13) mmol/L in the RCDG, but there was no significant difference between the CDG ((2.04±0.36) mmol/L) and the SOFG ((4.41±1.23) mmol/L). The TG concentrations varied less among the different treatment groups: (1.03±0.18) mmol/L in the CDG, (0.82±0.15) mmol/L in the RCDG, (0.65±0.12) mmol/L in the HFDG, (0.65±0.23) mmol/L in the OOFG, (0.64±0.13) mmol/L in the COFG, and (0.44±0.06) mmol/L in the SOFG.
The LDL-C concentrations in the three oil diet groups were significantly higher than that in the RCDG ((0.10±0.02) mmol/L). The LDL-C concentration in the OOFG ((3.62±2.40) mmol/L) was significantly higher than that in the SOFG ((1.42±0.59) mmol/L) (P<0.05), but not significantly different from that of the HFDG ((3.88±1.06) mmol/L) or COFG ((2.99±1.10) mmol/L). The HDL-C concentrations in the CDG, RCDG, OOFG, and COFG were significantly lower than that in the HFDG ((1.15±0.20) mmol/L), but not significantly different among themselves.
3.4. Cytological observation of hepatocytes
The cytological characteristics of the hepatocytes from rats in the different diet groups are shown in Fig. 2. In the RCDG, no lipid droplets (LDs) were detectable in hepatic cells (Fig. 2a), but minimal microvesicular LDs were found in hepatic cells of the CDG (Fig. 2b). In the HFDG, more macrovesicular and minimal microvesicular LDs had accumulated in the hepatocytes (Fig. 2c).
Fig. 2.
Micrographs of the hepatocytes in liver tissues stained with hematoxylin and eosin in different diet groups of rats
(a) Normal hepatocytes in the regular chow diet group (RCDG); (b) Minimal microvesicular lipid droplets (LDs) in hepatic cells in the control diet group (CDG); (c) More macrovesicular LDs (arrowheads) and minimal microvesicular LDs in the high-fat diet group (HFDG); (d) More microvesicular LDs in the soybean oil-fed group (SOFG); (e) More microvesicular LDs and minimal macrovesicular LDs (arrowheads) in the camellia oil-fed group (COFG); (f) Most macrovesicular LDs (arrowheads) in the olive oil-fed group (OOFG). Scale bar=50 μm
By comparison, LD accumulation was a common characteristic of the three oil diet groups, but there were differences. More microvesicular LDs were observed in hepatic cells in the SOFG (Fig. 2d), more microvesicular and minimal macrovesicular LDs in the COFG (Fig. 2e), and a large LD or several large LDs almost filled hepatic cells in the OOFG (Fig. 2f).
3.5. Ultrastructure observation of hepatocytes
Ultrastructural micrographs of liver tissue in rats in the RCDG showed normal hepatic cells (Fig. 3a). Each cell was characterized by a round nucleus in the center and intact organelles, including a nucleolus in the nucleus (Fig. 3b), endoplasmic reticulum (ER), and mitochondria (Fig. 3c).
Fig. 3.
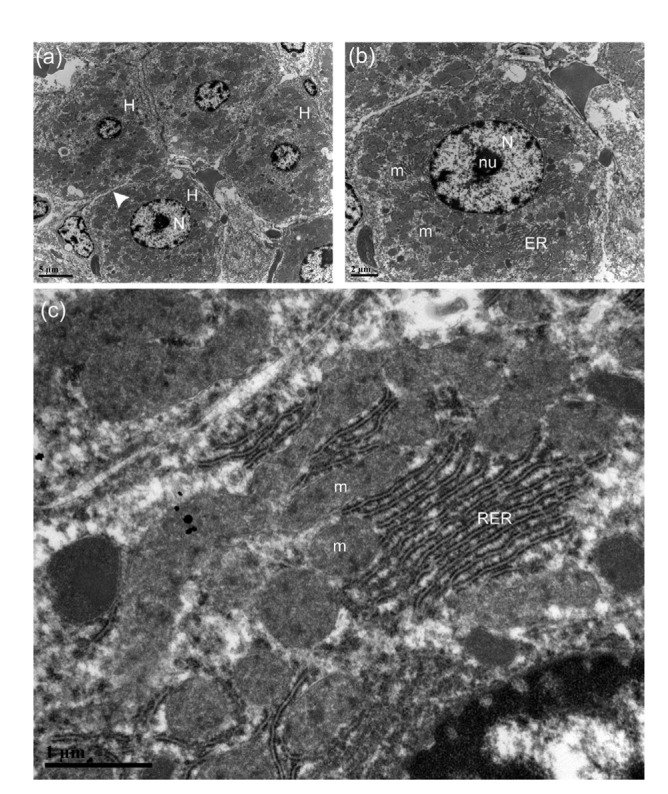
Transmission electron micrographs of hepatocytes (H) of rats in the regular chow diet group (RCDG)
(a) Normal hepatic cells with intact and clear plasma membranes (arrowhead). Note that the round nuclei (N) were centrally located in hepatic cells. Scale bar=5 μm. (b) The organelles were located at the periphery of the nucleus (N), which contained a nucleolus (nu). Note the abundance of mitochondria (m) and endoplasmic reticulum (ER). Scale bar=2 μm. (c) Intact mitochondria (m) and rough ER (RER). Scale bar=1 μm
No LDs were observed in hepatic cells. In the CDG, a few small LDs were seen in each hepatic cell (Fig. 4a). Although a round nucleus was centrally located in each hepatic cell, its nucleolus was obviously ruptured (Figs. 4a and 4b). A few lysosomes containing a granular substance appeared in the cytoplasm (Figs. 4b and 4c). Although the mitochondria appeared intact (Fig. 4c), the rough ER (RER) (Fig. 4d) was damaged compared to the RER in the RCDG (Fig. 3c). In the HFDG, LDs of variable sizes were distributed in each hepatic cell causing the nucleus, which contained an indistinguishable nucleolus, to deviate from the center (Figs. 5a and 5b). The plasma membrane was blebby (Fig. 5b) and mitochondria and RER had obviously disintegrated (Figs. 5c and 5d).
Fig. 4.
Transmission electron micrographs of hepatocytes (H) of rats in the control diet group (CDG)
(a) Normal hepatic cells with lipid droplets (LDs). Note that the LDs contained two types of lipid esters: those with low electron density (arrow) and those with high electron density (arrowhead). Scale bar=5 μm. (b) Organelles at the periphery of a nucleus (N) with a fractured nucleolus (nu). Note that lysosomes (Lys) contained a granular substance. Scale bar=2 μm. (c) Mitochondria (m), lysosomes (Lys), and LDs. Scale bar=1 μm. (d) Rough endoplasmic reticulum (RER). Note that the endoplasmic reticulum (ER) was not completely intact. Scale bar=1 μm
Fig. 5.
Transmission electron micrographs of hepatocytes (H) of rats in the high-fat diet group (HFDG)
(a) Hepatic cells with a variable size distribution of rich lipid droplets (LDs). Scale bar=10 μm. (b) A hepatic cell containing blebbing of plasma membranes and cytoplasmic accumulation of LDs. The nucleolus is indistinguishable within the nucleus (N). Scale bar=5 μm. (c) Damaged (*) mitochondria (m) and rough endoplasmic reticulum (RER). Scale bar=0.5 μm. (d) Damaged (*) RER. Scale bar=0.2 μm
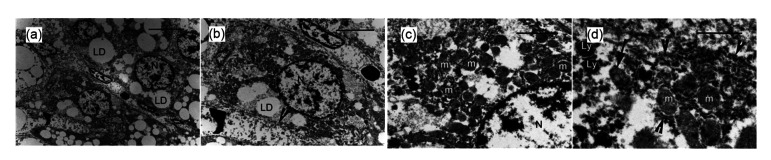
By comparison, the most striking common feature of hepatic cells among the three diet groups with low-to-very high fat diets was the accumulation of LDs of variable numbers and sizes, and the presence of affected organelles. In the OOFG, many large LDs or an enormous LD filled each hepatic cell causing the nucleus, which occasionally contained a distinguishable nucleolus, to seriously deviate from the center of the cell (Figs. 6a and 6b). A few lysosomes correlated with catabolism were observed (Fig. 6c). The degree of damage to the RER was similar to that in the HFDG, but there was damage to the mitochondria.
Fig. 6.
Transmission electron micrographs of hepatocytes (H) of rats in the olive oil-fed group (OOFG)
(a) Accumulations of large numbers of lipid droplets (LDs) in a hepatic cell. Note that the malformed nucleus is off-center and there is blebbing of the plasma membrane (arrowhead). Scale bar=10 μm. (b) A large LD in a hepatic cell. Scale bar=5 μm. Note that the large LD has caused the nucleus to be off-center. Scale bar=5 μm. (c) Lysosomes (Lys) and damaged (*) mitochondria (m). Scale bar=1 μm. (d) Damaged (arrowhead) rough endoplasmic reticulum (RER). Scale bar=0.5 μm
Compared with the OOFG, the LDs in the SOFG were significantly smaller (Figs. 7a and 7b), and there were almost no markedly off-center nuclei with indistinguishable nucleoli (Fig. 7b). Several lysosomes were seen, and even occasional multivesicular bodies correlated with catabolism (Fig. 7c). In most cases, although almost all mitochondria were intact (Fig. 7c), damage to the ER (Fig. 7d) was more severe than that in the HFDG and OOFG. In the COFG, there were significantly fewer and smaller LDs than in the OOFG, but more and larger LDs than in the SOFG. Nuclei with indistinguishable nucleoli were significantly off-center (Figs. 8a and 8b). The degree of damage to the RER was similar to that in the HFDG and OOFG, but there was a lower level of mitochondrial damage (Figs. 8c and 8d). Almost no lysosomes or multivesicular bodies were found.
Fig. 7.
Transmission electron micrographs of hepatocytes (H) of rats in the soybean oil-fed group (SOFG)
(a) Accumulation of lipid droplets (LDs) in hepatic cells. Note that the round nuclei (N) are centrally located in the cells. Scale bar=10 μm. (b) Several LDs in a hepatic cell. Note that the plasma membrane (arrowhead) is not intact. Scale bar=5 μm. (c) Intact mitochondria (m) and a clear nuclear envelope. Scale bar=2 μm. (d) Fractured rough endoplasmic reticulum (RER) (arrowhead). Note that there are several lysosomes (Lys) and a multivesicular body (arrow). Scale bar=1 μm
Fig. 8.
Transmission electron micrographs of hepatocytes (H) of rats in the camellia oil-fed group (COFG)
(a) Several large lipid droplets (LDs). Note that the nucleus (N) with an indistinguishable nucleolus is off-center. Scale bar=10 μm. (b) Distribution of lipid droplets (LDs) and endoplasmic reticulum (ER). Scale bar=5 μm. (c) Partially damaged mitochondria (m, *). Scale bar=5 μm. (d) Damaged rough ER (RER) (arrowhead) and mitochondria (*). Scale bar=0.5 μm
4. Discussion
NAFLD is associated with fat-rich nutrition. Previous studies have shown that increasing the PUFA n-6/n-3 ratio in the diet may favor lipid synthesis over oxidation and secretion, which is related to hepatic steatosis in patients with NAFLD (Araya et al., 2004). So, a lower n-6/n-3 ratio in the diet is desirable as a way of improving human health (Monteiro et al., 2014). However, our initial analysis of the composition of fatty acids in three kinds of oils in this study (Table 3) showed that the n-6/n-3 ratio was 33.33 in camellia oil, 7.69 in soybean oil, and 12.50 in olive oil, suggesting that camellia oil is less desirable than soybean or olive oil. This might explain why consumption of camellia oil has a higher risk of inducing NAFLD than consumption of soybean or olive oil, but there is no evidence to support this speculation.
Therefore, we further investigated the potential effect of camellia oil on NAFLD in rats. Determination of serum parameters showed that the level of total TGs in the SOFG ((0.44±0.06) mmol/L) was significantly lower than those in the OOFG ((0.65±0.23) mmol/L) and COFG ((0.64±0.13) mmol/L) (Fig. 1c). This is likely related to the lower n-6/n-3 ratio because dietary PUFA n-3 is able to limit triacylglycerol storage (Monteiro et al., 2014). However, the total TG levels in the COFG and OOFG were not closely associated with the n-6/n-3 ratio, suggesting that other nutritional factors might be involved.
There is a study showing that increased LD formation can contribute to hepatic steatosis, which is caused by a high-fat diet leading to the formation of massive amounts of LDs in hepatocytes (Wilfling et al., 2013). A close correlation has been found between the formation of large LDs in hepatocytes and hepatic steatosis (Gluchowski et al., 2017). Similarly, in this study, cytological analyses of the three different diet groups showed that hepatocytes exhibited no LDs in the RCDG with normal cellular architecture, few small LDs in the CDG still with preserved cellular architecture, and great numbers of LDs in the HFDG with abnormal cellular architecture (Fig. 2). These results confirm that a high-fat diet is associated with an increase in LDs and the degree of hepatic steatosis in hepatocytes. The LDs in the three oil diet groups were similar to those in the HFDG, but the number and size of the LDs were different (Fig. 2). There were more and larger LDs in the COFG than in the SOFG, but fewer in the COFG than in the OOFG.
The ultrastructural characteristics of hepatocytes are widely used to assess the relationship between different diets and LD formation and hepatic steatosis (Wei et al., 2008; Fujimoto and Parton, 2011; Wang et al., 2013; Ellatif et al., 2018). In this study, ultrastructural micrographs of the hepatocytes revealed the details of hepatic steatosis in hepatocytes of rats (Figs. 3–8). The degree of organelle damage in the hepatocytes was clearly associated with the number and size of the LDs: no LDs were found in the RCDG with normal cellular architecture (Fig. 3); a few small LDs appeared in the CDG, which had only slight ER damage (Fig. 4); and, many LDs of different sizes occurred in the HFDG accompanied by severe damage to the mitochondria and ER (Fig. 5). Similarly, although LD formation and organelle damage in hepatocytes were similar in the three oil diets, there were clear differences (Figs. 6–8). Markedly off-center nuclei caused by the LDs occurred in the OOFG and COFG, but not in the SOFG. Mitochondrial damage was most serious in the OOFG, relatively serious in the COFG and inconspicuous in the SOFG, while ER damage was more serious in the SOFG than in the OOFG and COFG. However, mitochondria play an important role in hepatocyte metabolism, being the primary site for the oxidation of fatty acids and oxidative phosphorylation, and hepatic mitochondrial dysfunction is crucial to the pathogenesis of NAFLD (Caldwell et al., 1999; Sanyal et al., 2001; Ibdah et al., 2005; Pessayre and Fromenty, 2005; Wei et al., 2008). So, the ultrastructural results revealed that the effect of camellia oil on NAFLD is intermediate between soybean oil and olive oil, and not entirely related to the n-6/n-3 ratio. These results provided novel insights into the effects of plant oils on NAFLD.
In addition, the accumulation of small LDs in hepatic nuclei of the OOFG (Fig. 6) was observed for the first time, and was identical to that observed after being fed a perfluorooctanoic acid diet (Wang et al., 2013), but the mechanism for transportation of LDs into the nucleus is unknown. Moreover, a few LDs containing two types of lipid esters with low or high electron density were observed in the CDG (Fig. 4a). This may be related to a change in the composition of lipid esters because the predominant cholesterol esters correspond to those with low electron density in an LD, while a high level of TG is related to a high electron density (Fujimoto and Parton, 2011).
Acknowledgments
We express our deepest thanks to all study participants. We are grateful to Prof. Jing-ze ZHANG (Zhejiang University, Hangzhou, China) for his helping and revising the manuscript. We would also like to thank the Bio-ultrastructure Analysis Lab of the Analysis Center of Agrobiology and Environmental Sciences, Zhejiang University, Hangzhou, China for its support.
Footnotes
Project supported by the Science and Technology Projects of Zhejiang Province (No. 2017C2003), China
Contributors: Chun-xue LI performed the experiments, analyzed the data, and wrote the manuscript. Li-rong SHEN supervised the project and revised the manuscript. Both authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Chun-xue LI and Li-rong SHEN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed. The experimental scheme was approved by the Ethics Committee of Zhejiang Chinese Medical University Laboratory Animal Research Center (Ethics No. 10849).
References
- 1.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci. 2004;106(6):635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 2.Boudour-Benrachou N, Plard J, Pinatel C, et al. Fatty acid compositions of olive oils from six cultivars from East and South-Western Algeria. Adv Food Technol Nutr Sci Open J. 2017;3:1–5. [Google Scholar]
- 3.Caldwell SH, Swerdlow RH, Khan EM, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31(3):430–434. doi: 10.1016/S0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 4.Cheng YT, Lu CC, Yen GC. Beneficial effects of camellia oil (Camellia oleifera Abel.) on hepatoprotective and gastroprotective activities. J Nutr Sci Vitaminol. 2015;61(S1):S100–S102. doi: 10.3177/jnsv.61.S100. [DOI] [PubMed] [Google Scholar]
- 5.Clemente TE, Cahoon EB. Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiol. 2009;151(3):1030–1040. doi: 10.1104/pp.109.146282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellatif MA, El-Karib AO, Dallak M, et al. Vitamin E protects against hepatocyte ultrastructural damage induced by high fat diet in a rat model of pre-diabetes. Int J Morphol. 2018;36(4):1350–1355. doi: 10.4067/S0717-95022018000401350. [DOI] [Google Scholar]
- 7.Ferramosca A, Zara V. Modulation of hepatic steatosis by dietary fatty acids. World J Gastroenterol. 2014;20(7):1746–1755. doi: 10.3748/wjg.v20.i7.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto T, Parton RG. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol. 2011;3(3):a004838. doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gluchowski NL, Becuwe M, Walther TC, et al. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14(6):343–355. doi: 10.1038/nrgastro.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han KH. Omega-3-fatty acid and triglyceride. Korean J Med. 2012;83(6):724–727. doi: 10.3904/kjm.2012.83.6.724. [DOI] [Google Scholar]
- 11.Ibdah JA, Perlegas P, Zhao YW, et al. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128(5):1381–1390. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Juárez-Hernández E, Chávez-Tapia NC, Uribe M, et al. Role of bioactive fatty acids in nonalcoholic fatty liver disease. Nutr J, 15:72. 2016 doi: 10.1186/s12937-016-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu XH, Jia LY, Gao Y, et al. Anti-inflammatory activity of total flavonoids from seeds of Camellia oleifera Abel. Acta Biochim Biophys Sin. 2014;46(10):920–922. doi: 10.1093/abbs/gmu071. [DOI] [PubMed] [Google Scholar]
- 14.Messina MJ. Soy foods: their role in disease prevention and treatment. In: Liu SK , editor. Soybeans. Springer, Boston, MA, USA; 1997. pp. 698–699. [DOI] [Google Scholar]
- 15.Monteiro J, Leslie M, Moghadasian MH, et al. The role of n-6 and n-3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct. 2014;5(3):426–435. doi: 10.1039/c3fo60551e. [DOI] [PubMed] [Google Scholar]
- 16.NHFPC CFDA. Determination of fatty acids in food, GB 5009. 168-2016. Food Safety National Standard of People’s Republic of China.2016. [Google Scholar]
- 17.Oğraş ŞŞ, Kaban G, Kaya M. The effects of geographic region, cultivar and harvest year on fatty acid composition of olive oil. J Oleo Sci. 2016;65(11):889–895. doi: 10.5650/jos.ess15270. [DOI] [PubMed] [Google Scholar]
- 18.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42(6):928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Prabakaran M, Lee KJ, An Y, et al. Changes in soybean (Glycine max L.) flour fatty-acid content based on storage temperature and duration. Molecules. 2018;23(10):2713. doi: 10.3390/molecules23102713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Wang Y, Liang Y, et al. Specific accumulation of lipid droplets in hepatocyte nuclei of PFOA-exposed BALB/c mice. Sci Rep, 3:2174. 2013 doi: 10.1038/srep02174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XQ, Zeng QM, Verardo V, et al. Fatty acid and sterol composition of tea seed oils: their comparison by the “FancyTiles” approach. Food Chem. 2017;233:302–310. doi: 10.1016/j.foodchem.2017.04.110. [DOI] [PubMed] [Google Scholar]
- 23.Wang XQ, Zeng QM, Del Mar Contreras M, et al. Profiling and quantification of phenolic compounds in Camellia seed oils: natural tea polyphenols in vegetable oil. Food Res Int. 2017;102:184–194. doi: 10.1016/j.foodres.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 24.Wei YZ, Rector RS, Thyfault JP, et al. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14(2):193–199. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilfling F, Wang HJ, Haas JT, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24(4):384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao XM, He LM, Chen YY, et al. Anti-inflammatory and antioxidative effects of Camellia oleifera Abel. components. Future Med Chem. 2017;9(17):2069–2079. doi: 10.4155/fmc-2017-0109. [DOI] [PubMed] [Google Scholar]
- 27.Yang CY, Liu XM, Chen ZY, et al. Comparison of oil content and fatty acid profile of ten new Camellia oleifera cultivars. J Lipids, 2016:3982486. 2016 doi: 10.1155/2016/3982486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng W, Endo Y. Lipid characteristics of camellia seed oil. J Oleo Sci. 2019;68(7):649–658. doi: 10.5650/jos.ess18234. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JZ, Chu FQ, Guo DP, et al. Cytology and ultrastructure of interactions between Ustilago esculenta and Zizania latifolia . Mycol Prog. 2012;11(2):499–508. doi: 10.1007/s11557-011-0765-y. [DOI] [Google Scholar]