Abstract
Aims
This study evaluated the impact of previous glycemic control and in-hospital use of antidiabetic/antihypertensive drugs on the prognosis of COVID-19 patients with diabetes.
Methods
In this retrospective cohort study, consecutive inpatients with laboratory confirmed COVID-19 were enrolled from Tongji Hospital (Wuhan, China). Patients without diabetes were matched to those with diabetes based on age, sex, and comorbidities. All patients were followed up to a clinical endpoint (discharge, worsening including transferring to ICU or immediate death). Data and outcomes were extracted from medical records and analyzed.
Results
64 patients with pre-existing diabetes were included in this study, with 128 matched patients without diabetes included as a control group. Patients with diabetes had a higher rate of worsening (18.8% versus 7.8%, p = 0.025). Multivariable regression showed increased odds of worsening associated with previous glycemic control reflected by HbA1c (odds ratio 3.29, 95% CI 1.19–9.13, p = 0.022) and receiver-operating characteristics (ROC) curve identified HbA1c of 8.6% (70 mmol/mol) as the optimal cut-off value. Univariate analysis demonstrated the in-hospital use of antidiabetic/antihypertensive drugs were not associated with a higher risk of worsening.
Conclusions
COVID-19 patients with diabetes had a higher risk of worsening, especially those with poorly-controlled HbA1c, with an optimal cut-off value of 8.6%. The in-hospital use of antidiabetic/antihypertensive drugs were not associated with increased odds of worsening in patients with diabetes.
Keywords: Antidiabetic drugs, Antihypertensive drugs, COVID-19, Diabetes, HbA1c
Abbreviations: ACE-2, Angiotensin-converting enzyme 2; ACEI, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin II receptor 1 blocker; BG, Blood glucose; CCB, Calcium channel blocker; cTnI, Cardiac troponin I; CRP, C-reactive protein; DM, Diabetes mellitus; HbA1c, Glycated hemoglobin A1c; ICU, Intensive care unit; COVID-19, Novel coronavirus disease 2019; NT-proBNP, N-terminal brain natriuretic peptide precursor; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
1. Introduction
Since Dec 2019, the novel coronavirus disease 2019 (COVID-19) has spread rapidly around the world and infected >10 million people hitherto [1]. The pathogen was identified as a novel, highly contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which can invade the human body through angiotensin-converting enzyme 2 (ACE-2) [2]. The clinical manifestations of COVID-19 include fever, dry cough, dyspnea, myalgia, fatigue etc, and severe cases can rapidly develop into acute respiratory distress syndrome, septic shock, and multiple organ dysfunction syndrome [3].
Recent studies have shown that diabetes mellitus (DM) is one of the most common comorbidities of COVID-19, with a prevalence ranging from 5.3% to 58.0% [4], [5], [6]. Diabetes has been associated with an increased mortality in previous viral epidemics, such as the outbreak of SARS-CoV-1 and Middle East respiratory syndrome coronavirus [7], [8]. Similar conclusions were reported in the pandemic of SARS-CoV-2. Some studies have suggested that diabetes is a risk factor for the poor prognosis of COVID-19 [4], [9], [10], while some studies reported that patients with diabetes seemed not to have a higher mortality risk [11], [12]. Data regarding the impact of diabetes on the prognosis of COVID-19 patients are scant and controversial.
Good glycemic control should be the most important for patients with diabetes during the COVID-19 infection as it has been shown to improve the innate immune system [13], [14]. Data from other viral epidemics such as SARS and influenza H1N1 has demonstrated that patients with poor glycemic control have a higher mortality risk [7], [15]. Unfortunately, the previous studies mostly focused on the impact of diabetes, as a comorbidity, on the prognosis of COVID-19. Despite a few studies reported that COVID-19 patients with poorly controlled fasting blood glucose in hospital were associated with higher mortality [16], there is still a lack of information on the impact of previous glycemic control on the prognosis of COVID-19 patients with diabetes. In addition, previous studies have reported that metformin could increase ACE-2 expression and improve its stability by impeding its ubiquitination and proteasomal degradation [17]. Angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor 1 blockers (ARB) also have been considered as a link between COVID-19 and diabetes because they are widely used in patients with diabetes [18]. However, there are still no solid evidences demonstrating the impact of these medications on the prognosis of this population.
In this report, we aimed to evaluate the prognosis of COVID-19 patients with diabetes and the impact of previous glycemic control on it. Besides, the influence of the commonly used antidiabetic and antihypertensive drugs on the prognosis of patients with diabetes was also explored.
2. Methods
2.1. Study design and population
This retrospective cohort study included consecutive confirmed COVID-19 inpatients from Guanggu branch of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), a designated hospital for the treatment of COVID-19 patients. Patients were admitted from February 3, 2020 to February 26, 2020. SARS-CoV-2 infection was confirmed in all patients based on reverse transcription-polymerase chain reaction according to the criteria of the New Coronavirus Pneumonia Prevention and Control Program (5th edition) published by the National Health Commission of China [19]. Patients with diabetes were designated according to the patient’s medical history. Then the patients without diabetes were matched (2:1 ratio) to the patients with diabetes based on age, sex, and comorbidities (hypertension, hyperlipemia, chronic renal diseases). The study was approved by the Ethics Committee of Tongji Hospital (TJ-IRB20200404). Written informed consent forms were waived in the urgency of pandemic.
2.2. Data collection
A trained team of physicians extracted the epidemiological, demographic, clinical, laboratory, treatment, and prognosis data from electronic medical records. The demographic characteristics, comorbidities, clinical symptoms, symptoms from onset to admission, vital signs, laboratory findings, antidiabetic and antihypertensive drugs used prior to admission and during hospitalization, and outcomes were collected. Most of the clinical data used in our study were collected from the first day of admission unless otherwise stated. Comorbidities were determined based on the patient’s self-report on admission. The glycated hemoglobin A1c (HbA1c) and the maximum of the blood glucose (BG) in-hospital were used to reflect the previous and in-hospital glycemic control, respectively. All data were double-checked by different researchers to guarantee the accuracy. All patients were followed up to a clinical endpoint (discharge, worsening including transferring to intensive care unit (ICU) or immediate death). Patients who transferred to ICU or died immediately before transferring to ICU were classified as the worsening group.
2.3. Statistical analysis
Continuous and categorical variables were described as median (interquartile range [IQR]) and number (%), respectively. Continuous variables between groups were compared by the Mann-Whitney U test, and categorical variables were compared by Chi-square or Fisher’s exact test where appropriate.
Binary logistic regression models were used to evaluate the impact of the previous and in-hospital glycemic control on the outcomes of the patients with diabetes, and three models were constructed to adjust potential confounding factors. Model 1 explored the risk factors associated with worsening by a univariate analysis, which included HbA1c, maximum of the BG in-hospital, age, sex, smoking, comorbidities other than diabetes, white cell count, the percentage of neutrophils, lymphopenia, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transferase, creatinine, C-reactive protein (CRP), D-dimer, N-terminal brain natriuretic peptide precursor (NT-proBNP), cardiac troponin I (cTnI), prothrombin time, total cholesterol, triglyceride, interleukin-6, tumor necrosis factor-α. Model 2 included HbA1c and the maximum of the BG in-hospital for a multivariate logistic regression analysis. On the basis of model 2, the variables with a P value < 0.05 in the univariate analysis were further added for a multivariate analysis in the model 3. Considering the limited number of the patients in the worsening group, three variables, which included lymphocyte, CRP and prothrombin time, were added in the model 3 to avoid over-fitting.
Receiver operating characteristic (ROC) curve was used to analyze the predictive value of the HbA1c level on the prognosis of the patients with diabetes. When we evaluated the association between the use of each antidiabetic or antihypertensive drug and the outcomes, due to the limited number of patients in each subset, logistic regression model was used and only glucose, HbA1c at admission or maximum of the glucose in-hospital were adjusted to avoid over-fitting. The results were shown in odds ratio (OR) and 95% confidence interval (CI). A two-sided P value < 0.05 was considered statistically different. All statistical analyses were performed with SPSS, version 24.0 (IBM SPSS).
3. Results
3.1. Demographics and characteristics
A total of 64 confirmed COVID-19 patients with pre-existing DM were included in this study (Table 1 ). 128 COVID-19 patients without diabetes were included as a control group, closely matched for age, sex and comorbidities. The median age of the patients with diabetes was 66.0 years (IQR, 59.0–71.0), and 35 (54.7%) were male. 39 (60.9%) patients with diabetes had at least one other comorbidities, with hypertension (37 [57.8%]) being the most common comorbidity. The median duration from the symptom onset to admission was 10.0 days (IQR, 7.0–15.0) for patients with diabetes and 14.0 days (IQR, 8.0–17.0) for patients without diabetes. The most common symptoms for both groups were fever and cough, followed by fatigue and diarrhea (Table 1). The patients with diabetes reported a significantly higher incidence of fever (76.6% versus 61.7%) compared to the group without diabetes.
Table 1.
Demographics and baseline characteristics of COVID-19 patients with or without a history of diabetes.
| Parameters | Without diabetes (n = 128) |
With diabetes (n = 64) |
P value |
|---|---|---|---|
| Age, years | 67.0 (59.3–71.0) | 66 (59.0–71.0) | 0.998 |
| Male gender | 69 (53.9%) | 35 (54.7%) | 0.830 |
| Female gender | 59 (46.1%) | 29 (45.3%) | —— |
| Comorbidities on Admission | 79 (61.7%) | 39 (60.9%) | 0.916 |
| Hypertension | 76 (59.4%) | 37 (57.8%) | 0.836 |
| Hyperlipemia | 9 (7.0%) | 5 (7.8%) | 1.000 |
| Chronic renal diseases | 1 (0.8%) | 0 (0.0%) | 1.000 |
| Smoking status | |||
| Never/unknown | 109 (85.2%) | 56 (87.5%) | 0.660 |
| Former/current | 19 (14.8%) | 8 (12.5%) | —— |
| Clinical characteristics on admission | |||
| Systolic pressure, mmHg | 132.0 (119.5–145.0) | 133.5 (120.0–145.8) | 0.695 |
| Diastolic pressure, mmHg | 78.0 (70.0–86.5) | 78.5 (70.0–87.8) | 0.873 |
| Pulse oxygen saturation, % | 95.0 (95.0–95.0) | 95.0 (95.0–95.0) | 0.529 |
| Duration from onset to admission, days | 14.0 (8.0–17.0) | 10.0 (7.0–15.0) | 0.145 |
| Fever | 79 (61.7%) | 49 (76.6%) | 0.040 |
| Highest temperature, ℃ | 38.3 (37.8–39.0) | 38.5 (38.0–38.9) | 0.573 |
| Fatigue | 30 (23.4%) | 15 (23.4%) | 1.000 |
| Cough | 88 (68.8%) | 41 (64.1%) | 0.514 |
| Snot | 0 (0.0%) | 0 (0.0%) | —— |
| Myalgia | 16 (12.5%) | 6 (9.4%) | 0.522 |
| Diarrhea | 21 (16.4%) | 11 (17.2%) | 0.891 |
Data are presented as numbers (%) or median [25th–75th percentile].
3.2. Laboratory indices and clinical outcomes
Laboratory findings on admission and clinical outcomes of both groups are summarized in Table 2 . The rate of disease worsening during hospitalization was significantly higher in the patients with pre-existing DM compared to the patients without diabetes (18.8% versus 7.8%, p = 0.025). Compared to the group without diabetes, patients with diabetes presented higher BG level as expected (8.6 mmol/L [6.5–15.6] versus 5.7 mmol/L [5.2–7.1]), with higher levels of HbA1c (8.1% [65 mmol/mol] versus 6.3% [45 mmol/mol]). The white cell count and the percentage of neutrophils were significantly higher in patients with diabetes. Moreover, patients with diabetes demonstrated a significantly higher incidence of lymphopenia (53.1% versus 33.6%). As for the inflammatory markers, the CRP level was significantly higher in the group with diabetes (39.3 mg/L [2.9–72.3] versus 7.6 mg/L [1.6–31.6]) while the procalcitonin level showed no difference between the two groups. Besides, patients with diabetes showed a higher level of the NT-proBNP.
Table 2.
Initial laboratory examination and outcomes of COVID-19 patients with or without a history of diabetes.
| Parameters | Without diabetes (n = 128) |
With diabetes (n = 64) |
P value |
|---|---|---|---|
| White cell count, × 109/L | 5.3 (4.3–6.8) | 6.1 (5.1–8.8) | 0.002 |
| < 4 | 24 (18.8%) | 6 (9.4%) | 0.092 |
| 4–10 | 95 (74.2%) | 45 (70.3%) | —— |
| > 10 | 7 (5.5%) | 13 (20.3%) | —— |
| Neutrophils, % | 64.0 (56.1–73.2) | 73.5 (60.7–82.8) | 0.001 |
| Lymphocyte, % | 24.2 (17.8–31.4) | 17.1 (10.2–26.2) | 0.001 |
| < 20 | 43 (33.6%) | 34 (53.1%) | 0.009 |
| ≥20 | 83 (64.8%) | 30 (46.9%) | —— |
| Monocytes, % | 9.4 (7.4–11.8) | 8.3 (6.6–10.2) | 0.002 |
| Hemoglobin, g/L | 125.5 (116.0–135.0) | 128.0 (115.0–138.0) | 0.257 |
| Platelet count, × 109/L | 258.0 (181.5–332.0) | 237.0 (188.0–323.0) | 0.849 |
| Alanine aminotransferase, U/L | 20.0 (13.0–33.0) | 23.0 (13.3–37.0) | 0.534 |
| Aspartate aminotransferase, U/L | 23.0 (18.0–33.0) | 25.0 (17.3–35.5) | 0.479 |
| Alkaline phosphatase, U/L | 66.5 (56.3–78.8) | 67.0 (57.3–84.0) | 0.614 |
| γ-glutamyl transferase, U/L | 23.5 (16.0–37.5) | 27.0 (17.3–64.0) | 0.089 |
| Total bilirubin, mmol/L | 7.8 (5.8–11.1) | 8.0 (5.9–11.8) | 0.522 |
| Creatinine, μmol/L | 71.0 (59.0–84.0) | 68.5 (53.8–87.5) | 0.618 |
| Urea nitrogen, mmol/L | 4.5 (3.6–5.6) | 5.3 (3.9–6.8) | 0.030 |
| Glomerular filtration rate, mL/min | 90.2 (74.5–97.3) | 90.5 (75.1–101.5) | 0.514 |
| C-reactive protein, mg/L | 7.6 (1.6–31.6) | 39.3 (2.9–72.3) | 0.006 |
| Procalcitonin, ng/mL | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 0.085 |
| Glucose, mmol/L | 5.7 (5.2–7.1) | 8.6 (6.5–15.6) | <0.001 |
| HbA1c, % | 6.3 (6.0–6.5) | 8.1 (6.6–9.7) | <0.001 |
| HbA1c, mmol/mol | 45 (42–48) | 65 (49–83) | <0.001 |
| Prothrombin time, second | 13.5 (13.0–14.2) | 13.7 (13.0–14.3) | 0.615 |
| Activated partial thromboplastin time, second | 38.2 (35.8–42.2) | 38.5 (34.6–44.1) | 0.836 |
| D-dimer, mg/L | 0.7 (0.3–1.5) | 0.8 (0.4–1.7) | 0.422 |
| N-terminal brain natriuretic peptide precursor, pg/mL | 113.5 (59.3–300.0) | 194.5 (87.8–423.0) | 0.032 |
| Cardiac troponin I, ng/mL | 4.3 (2.3–9.3) | 4.9 (2.2–17.4) | 0.457 |
| Interleukin-6, pg/mL | 4.5 (2.3–15.8) | 6.5 (1.8–27.3) | 0.451 |
| Tumor necrosis factor-α, pg/mL | 8.0 (6.0–10.7) | 7.8 (6.1–10.6) | 0.740 |
| ICU admission | 9 (7.0%) | 11 (17.2%) | 0.030 |
| Immediate death | 1 (0.8%) | 1 (1.6%) | 1.000 |
| Worsening | 10 (7.8%) | 12 (18.8%) | 0.025 |
Abbreviations: ICU, intensive care units.
Data are presented as numbers (%) or median [25th–75th percentile].
3.3. The association between glycemic control and the outcomes of the patients with diabetes
Complete data collected from 64 patients with diabetes (12 in the worsening group, and 52 in the discharge group) were included in the logistic regression models (Table 3 ). In the univariable analysis (Model 1), HbA1c, maximum of the BG in-hospital, lymphopenia, the percentage of neutrophils, CRP and prothrombin time were associated with worsening. In the model 2 included HbA1c and the maximum of the BG in-hospital, only HbA1c was associated with increased odds of worsening. When adjusting for lymphopenia, CRP and prothrombin time, HbA1c was still associated with increased odds of worsening.
Table 3.
Bivariate logistic regression of the association between glycemic control and the risk of disease worsening.
| Model 1a |
Model 2b |
Model 3c |
||||
|---|---|---|---|---|---|---|
| Parameters | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value |
| HbA1c, % | 2.73 (1.47–5.07) | 0.001 | 2.52 (1.31–4.87) | 0.006 | 3.29 (1.19–9.13) | 0.022 |
| Maximum of the blood glucose in-hospital, mmol/L | 1.20 (1.04–1.39) | 0.014 | 1.06 (0.88–1.28) | 0.546 | 0.97 (0.75–1.26) | 0.843 |
| Lymphocyte, %, < 20 | 13.87 (1.67–115.43) | 0.015 | 2.08 (0.14–31.93) | 0.600 | ||
| C-reactive protein, mg/L | 1.02 (1.01–1.03) | 0.007 | 1.02 (1.00–1.04) | 0.147 | ||
| Prothrombin time, second | 3.92 (1.55–9.89) | 0.004 | 2.96 (0.75–11.68) | 0.122 | ||
univariate analysis.
multivariate analysis included HbA1c and the maximum of the blood glucose in-hospital.
multivariate analysis included the variables with a P value < 0.05 in the univariate analysis.
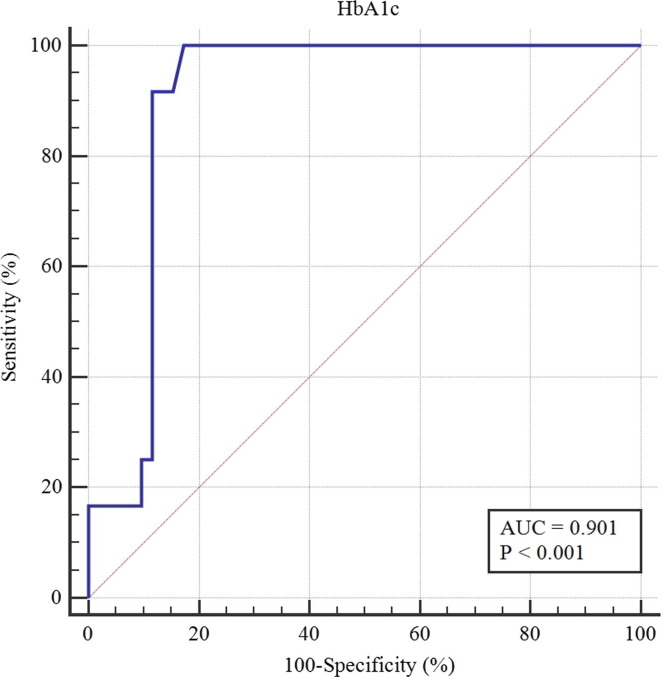
Using disease worsening as the end point, ROC curve analysis was used to identify the optimal HbA1c cutoff value, which was 8.6% (70 mmol/mol) (Fig. 1 ). With this value, the area under the ROC curve was 0.90 (95% CI, 0.83–0.98, p < 0.001), and the sensitivity and specificity for predicting disease worsening in the patients with diabetes were 100.0% and 82.7%, respectively. For each laboratory index, patients were divided into 2 groups for further analysis (Table 4 ). Compared to the HbA1c well-controlled patients, the poorly-controlled patients presented higher white cell count and the percentage of neutrophils. Also, the incidence of lymphopenia was higher in the poorly-controlled patients. Levels of the maximum of the BG in-hospital, hemoglobin, triglyceride, urea nitrogen, prothrombin time and cTnI were clearly elevated in the poorly-controlled patients compared with the well-controlled patients.
Fig. 1.
The predictive value of the HbA1c level for the worsening among COVID-19 patients with diabetes.
Table 4.
Demographics and clinical characteristics of COVID-19 patients with diabetes in the well-controlled and poorly-controlled HbA1c groups.
| Parameters | HbA1c ≤ 8.6% (n = 43) |
HbA1c > 8.6% (n = 21) |
P value |
|---|---|---|---|
| Age, years | 64.5 (58.8–71.0) | 67.0 (58.5–75.0) | 0.535 |
| Male gender | 21 (48.8%) | 15 (71.4%) | 0.073 |
| Female gender | 22 (51.2%) | 6 (28.6%) | —— |
| Comorbidities on Admission | |||
| Hypertension | 28 (65.1%) | 9 (42.9%) | 0.090 |
| Hyperlipemia | 4 (9.3%) | 1 (4.8%) | 1.000 |
| Chronic renal diseases | 0 (0.0%) | 0 (0.0%) | —— |
| White cell count, × 109/L | 5.7 (5.0–8.0) | 7.7 (6.1–14.1) | 0.010 |
| < 4 | 5 (11.6%) | 1 (4.8%) | 0.654 |
| 4–10 | 31 (72.1%) | 14 (66.7%) | —— |
| > 10 | 7 (16.3%) | 6 (28.6%) | —— |
| Neutrophils, % | 69.1 (58.7–78.1) | 82.4 (67.3–88.9) | 0.002 |
| Lymphocyte, % | 20.5 (13.6–30.0) | 11.5 (5.9–21.8) | 0.011 |
| < 20 | 19 (44.2%) | 15 (71.4%) | 0.040 |
| ≥20 | 24 (55.8%) | 6 (28.6%) | —— |
| Monocytes, % | 8.4 (7.2–10.3) | 7.1 (3.4–8.8) | 0.030 |
| Hemoglobin, g/L | 126.0 (112.0–134.0) | 137.0 (126.5–145.0) | 0.005 |
| Platelet count, × 109/L | 237.0 (196.0–340.0) | 214.5 (166.3–312.5) | 0.305 |
| Alanine aminotransferase, U/L | 23.0 (13.0–35.0) | 24.0 (16.0–39.5) | 0.582 |
| Aspartate aminotransferase, U/L | 25.0 (16.0–33.0) | 30.0 (20.5–37.0) | 0.331 |
| Total bilirubin, mmol/L | 7.9 (5.7–10.3) | 8.6 (6.1–17.7) | 0.131 |
| Alkaline phosphatase, U/L | 67.0 (54.0–84.0) | 69.0 (58.0–85.5) | 0.602 |
| γ-glutamyl transferase, U/L | 27.0 (16.0–66.0) | 28.0 (19.0–65.5) | 0.932 |
| Total cholesterol, mmol/L | 3.7 (3.2–4.3) | 3.8 (3.1–5.0) | 0.516 |
| Triglyceride, mmol/L | 1.1 (0.9–1.7) | 1.8 (1.6–2.6) | 0.010 |
| Creatinine, μmol/L | 67.0 (52.0–81.0) | 74.0 (61.0–112.5) | 0.141 |
| Urea nitrogen, mmol/L | 5.0 (3.6–5.9) | 6.5 (4.5–9.7) | 0.016 |
| C-reactive protein, mg/L | 22.9 (2.0–64.4) | 46.9 (15.7–125.7) | 0.110 |
| Procalcitonin, ng/mL | 0.1 (0.1–0.1) | 0.1 (0.1–0.4) | 0.306 |
| Glucose, mmol/L | 7.6 (6.1–11.7) | 12.6 (8.1–19.3) | 0.010 |
| HbA1c, % | 7.3 (6.8–7.6) | 9.4 (9.1–10.2) | <0.001 |
| HbA1c, mmol/mol | 56 (51–60) | 79 (76–88) | <0.001 |
| Maximum of the glucose in-hospital, mmol/L | 8.3 (6.6–12.3) | 12.0 (8.8–16.5) | 0.002 |
| Prothrombin time, second | 13.4 (12.8–14.0) | 13.9 (13.4–15.5) | 0.015 |
| Activated partial thromboplastin time, second | 38.2 (34.3–41.9) | 40.3 (35.3–48.0) | 0.153 |
| D-dimer, mg/L | 0.7 (0.4–1.6) | 1.2 (0.6–2.2) | 0.217 |
| N-terminal brain natriuretic peptide precursor, pg/mL | 166.5 (82.0–396.8) | 388.5 (97.0–983.5) | 0.230 |
| Cardiac troponin I, ng/mL | 3.8 (1.9–7.6) | 13.8 (3.0–36.8) | 0.011 |
| Interleukin-6, pg/mL | 4.8 (1.6–27.5) | 12.0 (2.3–24.3) | 0.605 |
| Tumor necrosis factor-α, pg/mL | 7.3 (4.5–10.0) | 8.9 (6.9–10.9) | 0.083 |
| ICU admission | 0 (0.0%) | 11 (52.4%) | <0.001 |
| Immediate death | 0 (0.0%) | 1 (4.8%) | 0.328 |
| Worsening | 0 (0.0%) | 12 (57.1%) | <0.001 |
Abbreviations: ICU, intensive care units.
Data are presented as numbers (%) or median [25th–75th percentile].
3.4. The association between the use of antidiabetic/antihypertensive drugs and the outcomes
Of the 64 patients with diabetes, 52 (81.3%) patients received antidiabetic treatment during hospitalization, including metformin (18/52 [34.6%]), insulin (22/52 [42.3%]), α-glucosidase inhibitors (26/52 [50.0%]), pioglitazone (6/52 [11.5%]), sulfonylureas (6/52 [11.5%]), dipeptidyl peptidase-4 inhibitors (6/52 [11.5%]). 36 (56.3%) patients received antihypertensive treatment during hospitalization, including ACEIs/ARBs (14/36 [38.9%]), calcium channel blockers (CCBs) (27/36 [75.0%]), β-receptor blockers(9/36 [25.0%]), spironolactone (4/36 [11.1%]). Moreover, 10 (10/64 [15.6%]) patients received statins therapy. The association between the use of antidiabetic/antihypertensive drugs and disease worsening was shown in Supplementary Table 1. Drugs with fewer users were not analyzed. Results demonstrated that α-glucosidase inhibitors and CCBs use were significantly associated with decreased odds of disease worsening. After adjusting for glucose, HbA1c at admission and maximum of the glucose in-hospital, there were no significant associations between the use of metformin, insulin, ACEIs/ARBs, statins and disease worsening.
4. Discussion
This retrospective cohort study have 3 novel findings with important implications for the COVID-19 patients with diabetes. First of all, not only did we find that COVID-19 patients with pre-existing DM had an increased risk of disease worsening, but poor previous glycemic control, which was reflected by HbA1c, was associated with higher odds of disease worsening. Second, to our knowledge, this was the first study investigating the prognostic value of HbA1c in the COVID-19 patients with diabetes, and a HbA1c level of 8.6% (70 mmol/mol) was the optimal cutoff value. Furthermore, we found that α-glucosidase inhibitors and CCBs use were associated with reduced odds of disease worsening. Metformin, insulin, ACEIs/ARBs and statins were not associated with the worsening of COVID-19 in the patients with diabetes. These findings could help frontline medical doctors predict the prognosis of COVID-19 patients according to their previous history of DM and the level of HbA1c, and choose appropriate antidiabetic/antihypertensive therapy for the patients with diabetes.
Previously, the prognosis of COVID-19 patients with pre-existing DM has been reported. The majority of the studies demonstrated that diabetes was an important risk factor for the progression or mortality in COVID-19 [4], [9], [10], while some reported that COVID-19 patients with diabetes seemed not to have a higher mortality risk [11], [12]. A recent meta-analysis involving 4 studies suggested that COVID-19 patients with diabetes had an increased risk of ICU admission and mortality [20], which was consistent with the results in our study. It is worth noting that the judgement of pre-existing diabetes was based on the patient’s self-report at admission, which could lead to the underdiagnosis of diabetes in the control group. In fact, the median level of HbA1c in the control group was 6.3%, which was above the normal upper limit of HbA1c (6.0%). Thus, although our results showed an increased risk of disease worsening in COVID-19 patients with diabetes, the risk might still be underestimated.
HbA1c is considered to be a good indicator reflecting long-term glycemia [21]. Previous studies have reported that patients with poor HbA1c control were more susceptible to infections, such as the infections of Chlamydia pneumoniae, hemolytic streptococci groups B, G, Klebsiella pneumoniae, Mycobacterium tuberculosis, and exhibited worse prognosis compared to the patients with well HbA1c control [22], [23], [24], [25]. Besides, chronic hyperglycemia was thought to downregulate ACE-2 expression, making cells susceptible to the damage of SARS-CoV-2 [26]. However, to our knowledge, the association between HbA1c level and the prognosis of COVID-19 patients with diabetes has not been explored. Our study found that among COVID-19 patients, those with poor HbA1c control had a higher risk of disease worsening, with an optimal cut-off value of 8.6% (70 mmol/mol), demonstrating the importance of previous glycemic control. Furthermore, we compared the clinical characteristics of the HbA1c well-controlled group with the poorly-controlled group. The HbA1c poorly-controlled patients presented a higher incidence of lymphopenia (71.4% versus 44.2%) compared to the well-controlled patients. Also, the levels of the percentage of neutrophils, prothrombin time and cTnI were significantly elevated in the poorly-controlled group. The changes in these clinical indicators were consistent with those in the COVID-19 severe cases that have been reported previously [4], [11], [27], suggesting that the higher risk of disease worsening in the poorly-controlled group might be associated with the changes in these clinical indicators in addition to the poor glycemic control.
The association between the in-hospital use of common antidiabetic/antihypertensive drugs and the prognosis of the COVID-19 patients with diabetes was also explored in this study for the first time. The results demonstrated that α-glucosidase inhibitors and CCBs use were associated with a lower risk of disease worsening, and no significant associations were observed between the use of metformin, insulin, ACEIs/ARBs, statins and disease worsening after adjusting for glucose, HbA1c at admission and maximum of the glucose in-hospital. Previous studies demonstrated that ACE-2 Ser680 residue could be phosphorylated to improve the stability and increase the expression of ACE-2 in human umbilical vein endothelial cells by the 5′-AMP-activated protein kinase, which was the molecular effector of the majority of metformin’s pharmacological actions [17]. Special considerations were also recommended on the use of metformin due to the lactic acidosis associated with it [26]. In addition, previous studies reported that ACEIs/ARBs could upregulate the ACE-2 expression, which might facilitate the entry and proliferation of SARS-CoV-2 [13], [28]. Thus, there are concerns about the safety of using ACEIs/ARBs in patients with diabetes. Our results demonstrated that there were no significant associations between the use of metformin, insulin, α-glucosidase inhibitors, ACEIs/ARBs, CCBs, statins and increased odds of disease worsening. Even the use of α-glucosidase inhibitors and CCBs were significantly associated with decreased odds of disease worsening. Although the potential bias of reverse causation cannot be ruled out, our results at least suggested that the use of these drugs were not associated with a higher risk of disease worsening. However, considering the risk of acute metabolic decompensation caused by metformin in COVID-19 patients with severe symptoms as well as the fact that the maximum of the in-hospital BG alone cannot fully reflect the in-hospital glycemic control, the safety of the in-hospital use of metformin needs more studies to explore.
There were some limitations of our study. First, the interpretation of our results might be limited by the sample size. Second, owing to the retrospective design of the study, the lack of data didn’t allow us to analyze the type of DM, disease course and mean in-hospital BG. Third, due to the lack of mean in-hospital BG resulting from the urgency of pandemic, only the maximum of the in-hospital BG could be used to reflect the in-hospital glycemic control, which could reduce the convictive power of the conclusions about the safety of antidiabetic/antihypertensive drugs. In the urgent conditions, medical history was not taken in detail and some laboratory examinations were not performed in all patients.
5. Conclusions
COVID-19 patients with pre-existing DM had an increased risk of disease worsening, especially those with poorly-controlled HbA1c, with an optimal cut-off value of 8.6% (70 mmol/mol). The in-hospital use of common antidiabetic/antihypertensive drugs were not associated with a higher risk of disease worsening in COVID-19 patients with diabetes.
6. Ethics approval and consent to participate
Ethical approval for this study was granted by the Ethics Committee of Tongji Hospital (TJ-IRB20200404). Written informed consent was waived due to the urgency of pandemic.
7. Data availability
The dataset used during the current study are available from the corresponding author on reasonable request.
8. Contribution statement
All authors read and approved the final manuscript. SC and XY designed the study. ZL and XH collected data. XB conducted the analysis and wrote the first draft. WJ and LQ finalized the manuscript. The corresponding authors attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding
This study was supported by the following funding: National Major Scientific and Technological Special Project for “Significant New Drugs Development” (grant number 2017ZX09304022); China Ying-cai Young Scientific Talent Research Project (grant number 2017-N-07).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108386.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Organization, W.H.: Coronavirus disease (COVID-19) Situation dashboard. https://who.sprinklr.com/ (2020). Accessed 2 July 2020
- 2.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabet Metabolic Syndrome. 2020;14(4):303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med J British Diabet Assoc. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 8.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A., et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis Off Publicat Infect. Dis Soc America. 2014;59(2):160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo, W., Li, M., Dong, Y., Zhou, H., Zhang, Z., Tian, C., Qin, R., Wang, H., Shen, Y., Du, K., Zhao, L., Fan, H., Luo, S., Hu, D.: Diabetes is a risk factor for the progression and prognosis of COVID-1Diabetes/metabolism research and reviews, e3319 (2020). doi:10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed]
- 10.Huang R., Zhu L., Xue L., Liu L., Yan X., Wang J., et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi-center study. PLoS NeglTrop Dis. 2020;14(5) doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan China. JAMA Internal Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respirat Med. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabet Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabet Metabolic Syndrome. 2020;14(3):211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badawi A., Ryoo S.G. Prevalence of Diabetes in the 2009 Influenza A (H1N1) and the Middle East Respiratory Syndrome Coronavirus: A Systematic Review and Meta-Analysis. J Public Health Res. 2016;5(3):733. doi: 10.4081/jphr.2016.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31(6):1068–1077.e1063. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Dong J., Martin M., He M., Gongol B., Marin T.L., et al. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am J Respir Crit Care Med. 2018;198(4):509–520. doi: 10.1164/rccm.201712-2570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal R., Bhadada S.K. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes Res Clin Pract. 2020;163 doi: 10.1016/j.diabres.2020.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.China, N.H.C.o.: New coronavirus pneumonia prevention and control program (5th edition) (in Chinese). (2020). Accessed 26 February 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf.
- 20.Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol Off Publicat Pan American Soc Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowley K.G., Daniel M., O'Dea K. Screening for diabetes in Indigenous populations using glycated haemoglobin: sensitivity, specificity, post-test likelihood and risk of disease. Diabet Med J British Diabet Assoc. 2005;22(7):833–839. doi: 10.1111/j.1464-5491.2005.01447.x. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo A., Paolillo R., Iafusco D., Prisco F., Romano Carratelli C. Chlamydia pneumoniae infection in adolescents with type 1 diabetes mellitus. J Med Microbiol. 2012;61(Pt 11):1584–1590. doi: 10.1099/jmm.0.048512-0. [DOI] [PubMed] [Google Scholar]
- 23.Thomsen R.W., Riis A.H., Kjeldsen S., Schønheyder H.C. Impact of diabetes and poor glycaemic control on risk of bacteraemia with haemolytic streptococci groups A, B, and G. J Infect. 2011;63(1):8–16. doi: 10.1016/j.jinf.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Lee C.H., Chen I.L., Chuah S.K., Tai W.C., Chang C.C., Chen F.J., et al. Impact of glycemic control on capsular polysaccharide biosynthesis and opsonophagocytosis of Klebsiella pneumoniae: Implications for invasive syndrome in patients with diabetes mellitus. Virulence. 2016;7(7):770–778. doi: 10.1080/21505594.2016.1186315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia L.L., Li S.F., Shao K., Zhang X., Huang S. The correlation between CT features and glycosylated hemoglobin level in patients with T2DM complicated with primary pulmonary tuberculosis. Infect Drug Resistance. 2018;11:187–193. doi: 10.2147/idr.S146741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabet Endocrinol. 2020;8(6):546–550. doi: 10.1016/s2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cure E., Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabet Metabolic Syndrome. 2020;14(4):349–350. doi: 10.1016/j.dsx.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used during the current study are available from the corresponding author on reasonable request.