Highlights
-
•
FBG is J-shaped associated with COVID-19 severity in non-diabetic patients.
-
•
Optimal Fasting glycemic target for COVID-19 patients is 4.74–5.78 mmol/L.
-
•
Both low and high FBG levels are increase COVID-19 severe/critical rate.
Keywords: Coronavirus disease 2019 (COVID-19), Hyperglycemia, Hypoglycemia, Fasting blood glucose, Glycemic target
Abstract
Aims
Coronavirus disease 2019 (COVID-19) has become a recognized worldwide pandemic. Researchers now know that mortality from COVID-19 can be reduced through early prevention measures. This retrospective, multi-centered study of 293 COVID-19 patients without diabetes explores the association between fasting blood glucose (FBG) levels and the risk of COVID-19 disease progression, with the goal of providing clinical evidence for glycemic targets in patients.
Methods
The multivariate stepwise binary logistic regression analysis was used to test the dose–response effects of FBG levels on the risk of severe and critical condition in COVID-19 patients.
Results
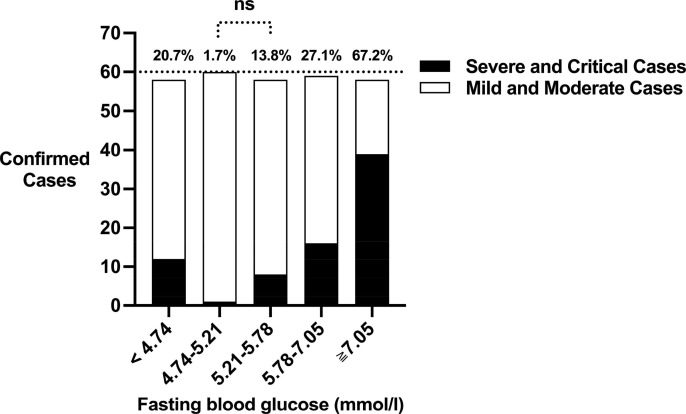
FBG levels were plotted in quintiles with set at <4.74, 4.74–5.21, 5.21–5.78, 5.78–7.05, and ≧7.05 mmol/L. The constituent ratio of severe or critical cases in each FBG quintile was 20.7%, 1.7%, 13.8%, 27.1%, and 67.2%, respectively (P < 0.0001). When the second quintile was used as the reference, the adjusted odds ratios (AORs) (95%CI) for the risk of severe/critical condition in COVID-19 was 25.33 (2.77, 231.64), 1.00 (Reference), 3.13 (0.33, 29.67), 10.59 (1.23, 91.24), 38.93 (4.36, 347.48) per FBG quintile respectively (P < 0.001).
Conclusions
We provide evidence of J-shaped associations between FBG and risk of severe and critical condition in non-diabetes patients with COVID-19, with nadir at 4.74–5.78 mmol/L.
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is becoming a pandemic worldwide. By the middle of June 2020, the disease has reached 216 countries, areas or territories, with over 8.24 million cases of infection and over 445 thousand confirmed deaths (WHO, https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Generally, many patients who had severe infection progressed rapidly to critical condition including multi-organ failure and sometimes death [1]. Better early warning signs of probable rapid progression of COVID-19 could help doctors anticipate and more rapidly act to prevent escalation in patients with severe symptoms of COVID-19.
Researchers are beginning to reach a consensus that COVID-19 patients with hyperglycemia or diabetes are associated with a higher risk of severely ill and mortality [2], [3], [4], [5], [6], [7]. Wang and his colleagues have reported that COVID-19 patients with hyperglycemia or diabetes are more likely to need ICU care [8]. Wang et al. also demonstrated that fasting blood glucose (FBG) ≥ 7 mmol/L at admission was an independent risk factor for 28-day mortality in COVID-19 patients without pre-existing diabetes [7]. These studies suggest that early management of blood glucose levels in COVID-19 may serve as a useful tool in managing the disease and saving lives. Interestingly, we noticed that Zhang et al. reported an appropriately 1% confirmed patients with COVID-19 presenting decreased glucose (<3.9 mmol/L) [9] suggesting that hyperglycemia may not be the exclusive relationship between blood glucose and COVID-19. The American Diabetes Association (ADA)’s criteria notes that hypoglycemia is generally defined as a blood glucose level <3.9 mmol/L (70 mg/dL) and that for nondiabetic individuals <2.8 mmol/L (50 mg/dL) is threshold for impairment of cognitive function [10], [11], levels associated with a range of adverse clinical outcomes including death [10], [12]. Studies have supported that both low and high levels of glycemic control have been associated with an increased mortality risk in diabetes patients [13]. However, few studies have looked at lower limit of blood glucose control other than hyperglycemia in COVID-19 patients without pre-existing diabetes. Current research does not provide sufficient evidence for doctors to judge the potential value of knowing an optimal glycemic target as a diagnostic tool in treating severe COVID-19 patients who do not have pre-existing diabetes.
The purpose of this study was to explore the association between fasting blood glucose (FBG) levels and risk of COVID-19 progressing into severe or critical condition in patients without pre-existing diabetes, thus providing clinical evidence for determining optimal glycemic targets.
2. Materials and methods
2.1. Subjects
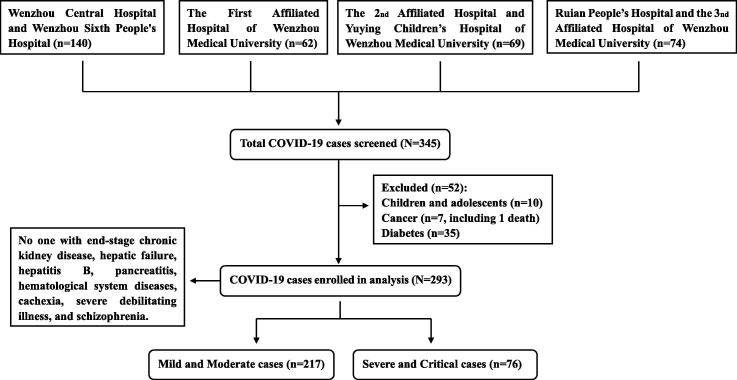
This retrospective, multi-centered study drew data from five hospitals in Wenzhou, China. Records from 345 COVID-19 patients were initially included in the study, but 52 were excluded including children and adolescents, as well as patients with cancer, cachexia or pre-existing diabetes (see Fig. 1 ). This left 293 patients records for the analysis, including 217 mild and moderate cases and 76 severe and critical cases. No one with end-stage chronic kidney disease, hepatic failure, hepatitis B, pancreatitis, hematological system diseases, cachexia, severe debilitating illness, and schizophrenia (Fig. 1).
Fig. 1.
Flow diagram presenting inclusion and exclusion criteria for subjects in this study.
2.2. Ethical statements
The study was approved by the Research Ethics Review Committee of Tongji University and Department of Infectious Diseases, Wenzhou Central Hospital and Sixth People's Hospital of Wenzhou, Wenzhou, Zhejiang, China (No. K2020-01-005(5)). The study was conducted in accordance with the provisions of the Declaration of Helsinki as revised in 2013. Given the urgency of the COVID-19 pandemic, obtaining the informed consent forms was waived by the Ethics Boards of the hospitals participating in this study.
2.3. Data collection
All data used in this study come from patients with confirmed COVID-19 from Wenzhou, the city outside of Hubei Province that has been most affected by COVID-19 infections. From January 10th to 23rd, 2020, approximately 48,800 people traveled from Wuhan to Wenzhou [14] and as of May 3, 2020, the Municipal Health Commission of Wenzhou reported 504 confirmed cases of COVID-19 with one death. The most recent available numbers of confirmed cases came from five hospitals including:
-
•
Wenzhou Central Hospital
-
•
Wenzhou Sixth People's Hospital
-
•
The First Affiliated Hospital of Wenzhou Medical University
-
•
The 2nd Affiliated Hospital of Wenzhou Medical University
-
•
Ruian People’s Hospital (The 3nd Affiliated Hospital of Wenzhou Medical University)
Patients in this study were admitted to hospital between Jan 17 to Feb 22, 2020. Demographic, clinical, laboratory, and outcome data were pulled from the electronic hospital information system using a standardized form. Patients’ biochemical indexes obtained from the fasting blood sample at time of admission were included in analysis to avoid the influence of medical treatment. All medical data were checked by two medical doctors (B.Z. and C.H.) and the leader author (S.Q. and D.C.) adjudicated any different interpretation between the two medical doctors.
2.4. Diagnostic and classification criteria
The diagnosis and classification criteria for COVID-19 in this study were based on guidelines from the Diagnosis and Treatment Program of New Coronavirus Pneumonia (seventh trial version, China) (http://www.nhc.gov.cn/). We confirmed SARS-CoV-2 infection by RT-PCR of samples taken from upper nasopharyngeal swabs. Two sets of primers were used for two target genes according to the protocol issued by the National Institute for Viral Disease Control and Prevention in China as previously described. [15]. The classification of severity included: (1) Mild, with mild symptoms, no pneumonia in imaging diagnosis, (2) Moderate, with fever, respiratory tract symptoms, and pneumonia in imaging diagnosis, (3) Severe, meeting criteria of either anhelation (respiratory rate ≥30 beats/min), or finger oxygen saturation (≤93% at resting, or arterial blood oxygen partial pressure at (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa), (4) Critical, where patients had either respiratory failure requiring mechanical ventilation, shock, or required ICU care for organ failure. We classified mild and moderate patients as the milder category, otherwise severe and critical ill patients as the severer category.
2.5. Statistical methods
Continuous data were presented as medians (interquartile ranges, IQR) or means ± standard deviations (SD) based on the data distribution. Categorical variables were presented as percentages (%). Student’s t-test or Mann-Whitney U test were used to compare continuous data between groups based on the homogeneity of variance test. Chi-squared (χ2) tests were used to compare categorical data between the groups. Z-tests (p-value adjusted by a Bonferroni method for multiple testing) were used to pairwise comparison. Spearman’s bivariate simple correlation analysis was conducted to explore the associations between FBG level and variables that demonstrated significant associations with the COVID-19 severity in the univariate binary logistic regression analysis. We excluded variables with 10% missing values. The multiple potential confounders were used as independent variables in the multivariate step-wise binary logistic regression analysis to test the combined effect of these factors on the adjusted odds ratios (AORs) for severe or critical condition in COVID-19. Each continuous variable was converted into a categorical variable before entered into models. All statistical analyses were performed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA) and Graphpad prism 8.0 software. Results were considered to be statistically significant at two-tailed P value of <0.05.
3. Results
3.1. Patient characteristics
COVID-19 patients in this study were mostly mid-aged men. Most patients with mild and moderate symptoms had low grade fever, while patients classified as severe and critical had a medium or high body temperature. In comparison to mild and moderate cases, severe and critical patients had significantly higher FBG (5.30, IQR 4.80–5.90 vs. 7.35, IQR 5.60–9.58 mmol/L, P < 0.0001), BMI, C-reactive protein (CRP), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GTT), and triglycerides (TG) levels. Conversely, COVID-19 patients in severe or critical condition had significantly lower HDL levels (1.15, IQR 0.97–1.41 vs. 1.02, IQR 0.83–1.25 mmol/L, P < 0.0001) (Table 1 ).
Table 1.
Characteristics of COVID-19 patients in this study.
| Characteristics | ALL (N = 293) | Mild and Moderate (N = 217) | Severe and Critical (N = 76) | P-Value |
|---|---|---|---|---|
| Age (years) | 47.0(37.0, 55.5) | 44.0(34.0, 53.0) | 54.0(46.5, 66.75) | <0.0001 |
| Gender (male%) | 151(51.5%) | 107(49.3%) | 44(57.9%) | 0.197 |
| BMI (kg/m2) | 23.80 ± 3.39 | 23.43 ± 3.13 | 25.11 ± 3.91 | 0.001 |
| Body temperature (℃) | 37.70(37.0, 38.40) | 37.60(37.0,38.0) | 38.40(37.58, 38.85) | <0.0001 |
| FBG (mmol/L) | 5.50(4.88, 6.60) | 5.30(4.80, 5.90) | 7.35(5.60, 9.58) | <0.0001 |
| FBG quintile (median) | <0.0001 | |||
| <4.74 (4.50 mmol/L) | 58(100%) | 46(79.3%) | 12(20.7%) | a* |
| 4.74–5.21 (5.02 mmol/L) | 60(100%) | 59(98.3%) | 1(1.7%) | b |
| 5.21–5.78 (5.50 mmol/L) | 58(100%) | 50(86.2%) | 8(13.8%) | a, b |
| 5.78–7.05 (6.21 mmol/L) | 59(100%) | 43(72.9%) | 16(27.1%) | a |
| >7.05 (9.05 mmol/L) | 58(100%) | 19(32.8%) | 39(67.2%) | c |
| C-reactive protein (mg/L) | 9.30(2.28, 24.13) | 7.90(2.30, 20.68) | 24.10(1.25, 44.93) | 0.048 |
| Creatine kinase (U/L) | 71.00(47.00, 105.0) | 71.80(46.50, 105.0) | 69.65(55.00, 104.0) | 0.746 |
| Lactate dehydrogenase (U/L) | 210.5(174.0, 274.0) | 198.0(165.50, 241.75) | 272.0(208.0, 389.25) | <0.0001 |
| Lymphocyte count (×109/L) | 1.20(0.90, 1.70) | 1.30(0.90, 1.70) | 1.0(0.78, 1.50) | 0.226 |
| Creatinine (μmol/L) | 62.0(55.25, 73.0) | 63.0(56.0, 75.0) | 62.0(55.0, 71.25) | 0.816 |
| Alanine aminotransferase (U/L) | 22.0(15.0, 35.0) | 22.0(13.0,33.0) | 24.0(17.25, 41.75) | 0.130 |
| Aspartate aminotransferase (U/L) | 25.0(20.0, 35.0) | 23.0(18.0, 32.0) | 30.0(23.0, 43.0) | <0.0001 |
| Alkaline phosphatase (U/L) | 55.0(42.25, 68.0) | 55.0(45.0, 68.0) | 52.0(34.75, 79.0) | 0.367 |
| Gamma-glutamyl transpeptidase (U/L) | 22.5(15.0, 47.50) | 21.0(15.0, 42.0) | 43.5(19.25, 91.0) | 0.007 |
| Cholesterol (mmol/L) | 3.97 ± 0.86 | 3.95 ± 0.86 | 4.02 ± 0.87 | 0.563 |
| Triglycerides (mmol/L) | 1.17(0.91, 1.62) | 1.11(0.88, 1.58) | 1.40(1.01, 1.69) | 0.006 |
| High density lipoprotein(mmol/L) | 1.10(0.93, 1.33) | 1.15(0.97, 1.41) | 1.02(0.83, 1.25) | 0.012 |
| Low density lipoprotein (mmol/L) | 2.17 ± 0.79 | 2.20 ± 0.82 | 2.13 ± 0.73 | 0.534 |
Continuous data are presented as means ± standard deviations (SD) or medians (interquartile ranges, IQR) based on the data distribution. Categorical variables are presented as percentages (%). *abc: there is no statistically significant difference between FBG quintiles with the same small letter. BMI, body mass index; FBG, fasting blood glucose.
3.2. The severe and critical rate across FBG quintiles
FBG levels were plotted in quintiles with levels set at less than 4.74 mmol/L, 4.74–5.21 mmol/L, 5.21–5.78 mmol/L, 5.78–7.05 mmol/L, and greater than or equal to 7.05 mmol/L. The median value for each quintile was 4.50, 5.02, 5.50, 6.21, and 9.05 mmol/L, respectively. In the χ2 tests, the expected frequency in each cell was greater than 5 and the minimum was 14.8. These results suggest that the sample size and cell distribution fully met χ2 test’s requirements. As shown in Fig. 2 , associations between FBG levels and the risk of severe and critical condition were non-linear for COVID-19 patients. The constituent ratio of severe and critical cases in each FBG quintile was 20.7%, 1.7%, 13.8%, 27.1%, and 67.2%, respectively (P-trend < 0.0001). For pairwise comparison, Z-tests showed that significant differences of constituent ratio of severe and critical cases were exhibited between “4.74–5.21 mmol/L” quintile and the other quintiles except for “5.21–5.78 mmol/L” quintile (Table 1).
Fig. 2.
The constituent ratio of severe and critical cases in confirmed COVID-19 patients across fasting blood glucose (FBG) quintiles. The constituent ratio of severe and critical cases in each FBG quintile (<4.74, 4.74–5.21, 5.21–5.78, 5.78–7.05, and ≧ 7.05 mmol/L) was 20.7%, 1.7%, 13.8%, 27.1%, and 67.2%, respectively. White bar = cumulatively mild and moderate cases. Black bar = cumulatively severe and critical cases. ns: no significant difference.
3.3. Indicators for the risk of severe and critical condition in COVID-19 patients
The factors associated with the risk of severe or critical condition in COVID-19 are presented in Supplementary Table 1. Among those factors, age categories were set at <30, 30–39, 40–49, 50–59, 60–69, 70–79, ≧80 years old. BMI, body temperature, CRP, LDH, AST, GTT, TG, and HDL were fitted in quartile categories. The category boundaries of each variable were described in the footnote of Supplementary Table 1. The univariate logistic regression analysis demonstrated a significantly higher odds ratio (OR) of severe or critical condition in COVID-19 patients with older age (OR 1.718, 95%CI 1.393–2.118, P < 0.0001) and elevated BMI (OR 1.570, 95%CI 1.199–2.056, P = 0.001), body temperature (OR 1.996, 95%CI 1.299–3.069, P = 0.002), FBG (OR 1.909, 95%CI 1.579–2.309, P < 0.0001), CRP (OR 1.512, 95%CI 1.019–2.243, P = 0.040), LDH (OR 2.250, 95%CI 1.671–3.030, P < 0.0001), AST (OR 1.662, 95%CI 1.291–2.140, P < 0.0001), and GTT (OR 1.640, 95%CI 1.102–2.441, P = 0.015) levels. Conversely, HDL (OR 0.643, 95%CI 0.497–0.833, P = 0.001) was found to be negatively associated with the risks of patients progressing into severe or critical condition.
Spearman’s bivariate simple correlation analysis demonstrated that FBG levels were positively associated with age (r = 0.425, P < 0.0001), BMI (r = 0.160, P = 0.007), LDH (r = 0.373, P < 0.0001), AST (r = 0.324, P < 0.0001) and GTT (r = 0.233, P = 0.001) levels. Furthermore, FBG levels were significantly negatively associated with HDL levels (r = −0.214, P = 0.001). However, the relationship between FBG levels and body temperature (r = 0.040, P = 0.595), and CRP (r = 0.108, P = 0.149) levels were weak. In addition, we decided to choose the largest contributing variable of liver function test, AST, to enter into each model in the multivariate logistic regression analysis. Together, age, gender, BMI, FBG, HDL, LDH, and AST were used as independent variables in the multivariate step-wise binary logistic regression analysis.
3.4. Dose-response relationship of FBG and risk of severe and critical condition in COVID-19
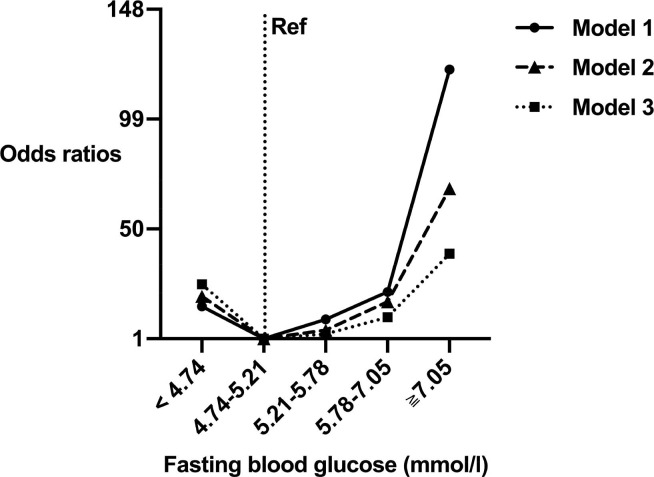
In multivariate logistic regression Model 1, which included FBG quintiles, we observed a significant, J-shaped association between FBG levels across all FBG quintiles and the risk of patients progressing into severe or critical condition. The second quintile was used as the reference, OR (95%CI) of the risk of COVID-19 progressing into severe or critical condition was 15.39 (1.93, 122.72), 1.00 (Reference), 9.63 (1.16, 79.70), 21.95 (2.80,171.93), 121.11 (15.57,941.82) in each FBG quintile, respectively (P-trend < 0.001, Table 2 ). This association was also significant in Model 2 with age, gender, and BMI as additional co-variables (the second quintile was used as the reference, AOR (95%CI) = 19.96 (2.41,165.36), 1.00 (Ref), 4.89 (0.54, 44.24), 17.49 (2.18, 140.04), 68.11 (8.54, 543.05) per FBG quintile respectively; P-trend < 0.001). Model 3 were fully adjusted for age, gender, BMI, FBG quintile, HDL, LDH, and AST. In Model 3, the second quintile was used as the reference, AOR (95%CI) was 25.33 (2.77, 231.64), 1.00 (Reference), 3.13 (0.33, 29.67), 10.59 (1.23, 91.24), 38.93 (4.36, 347.48) per FBG quintile respectively (P-trend <0.001). We performed dose–response curves demonstrating visualized evidence of a J-shaped association between FBG levels and risk of severe and critical state in COVID-19 (Fig. 3 ). In addition, Model 3 shown that age (OR 1.504, 95%CI 1.103–2.052, P = 0.010) and BMI (OR 1.477, 95%CI 1.008–2.163, P = 0.045) were independent indicators for severe or critical risk in COVID-19, with little evidence of association between HDL, LDH, and AST with severe or critical risk.
Table 2.
Multivariable-Adjusted Association of FBG Level and Risk of Severe and Critical Condition for COVID-19 Patients (N = 293).
| FBG quintile (mmol/L) | Median, mmol/L | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| AOR (95% CI) | P | AOR (95% CI) | P | AOR (95% CI) | P | ||
| <4.74 | 4.50 | 15.39(1.93,122.72) | 0.010 | 19.96(2.41,165.36) | 0.006 | 25.33(2.77, 231.64) | 0.004 |
| 4.74–5.21 | 5.02 | 1.00 (Ref) | ND | 1.00 (Ref) | ND | 1.00 (Ref) | ND |
| 5.21–5.78 | 5.50 | 9.63(1.16, 79.70) | 0.036 | 4.89(0.54, 44.24) | 0.158 | 3.13(0.33, 29.67) | 0.320 |
| 5.78–7.05 | 6.21 | 21.95(2.80,171.93) | 0.003 | 17.49(2.18, 140.04) | 0.007 | 10.59(1.23, 91.24) | 0.032 |
| ≧7.05 | 9.05 | 121.11 (15.57,941.82) | <0.0001 | 68.11(8.54, 543.05) | <0.0001 | 38.93(4.36, 347.48) | 0.001 |
FBG data were plotted in quintiles. Model 1 included FBG quintile. Model 2 included FBG quintile, age, gender, and BMI. Model 3 was additionally adjusted for HDL, LDH, and AST levels. FBG, fasting blood glucose; BMI, body mass index; HDL, high-density lipoprotein; LDH, lactate dehydrogenase; AST aspartate aminotransferase; AOR, adjusted odds ratio; ND, not determined; CI, confidence interval; Ref, reference.
Fig. 3.
Adjusted dose–response curves by fasting blood glucose (FBG) quintiles for severe and critical condition of COVID-19 patients. Odds ratios of severe and critical condition in COVID-19 patients according to FBG quintiles (<4.74, 4.74–5.21, 5.21–5.78, 5.78–7.05, and ≧ 7.05 mmol/L) were estimated using the median value for each quintile (4.50, 5.02, 5.50, 6.21, and 9.05 mmol/L, respectively). Model 1 (solid black curve) included FBG quintile. Model 2 (black dashed curve) included FBG quintile, age, gender, and body mass index (BMI). Model 3 (black dotted curve) was additionally adjusted for lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and high-density lipoprotein (HDL) levels.
4. Discussion
To our knowledge, our study provides the first evidence that associations between FBG and risk of severe or critical condition were J-shaped for adult, symptomatic COVID-19 patients without diabetes, with a nadir at 4.74–5.78 mmol/L, and varying magnitudes of association. For FBG levels <4.74 mmol/L, FBG was inversely associated with risk of severe and critical condition up to 4.74–5.78 mmol/L with positive association above this point. It is worth noting that there was a substantially higher risk of patients developing severe or critical condition for those with FBG less than 4.74 mmol/L but above the ADA’s hypoglycemia category (the minimum value of FBG in this study was 3.47 mmol/L) [10], [11]. This suggests that relatively decreased glucose levels in patients should be a cause for concern. In addition, our AORs for FBG levels ≧7.05 mmol/L suggested highest risk of severe or critical case in this category. This finding aligns with another recent study that claimed that FBG ≥ 7 mmol/L was an independent risk factor for 28-day mortality in COVID-19 patients without pre-existing diabetes [7]. Similarly, other research recommends that urine glucose levels be used to the differentiate COVID-19 severity [16]. However, studies into the effects of glucose levels on COVID-19 severity generally found linearity [7], [16]. It is possible that the relatively small size among COVID-19 populations who with decreased glucose obscures the risk of hypoglycemia for COVID-19 patients.
Many studies have demonstrated that hyperglycemia or diabetes is independent risk factor for the progression and mortality in patients with many infectious diseases such as SARS and COVID-19 [2], [3], [4], [5], [6], [7], [17]. Immune system dysregulation, rather than actual elevated glucose levels, may be the contributing factor of susceptibility to pathogen infection and severe conditions in diabetes patients [18]. Hyperglycemia, especially in patients without diabetes, may have exacerbated COVID-19 symptoms through separate mechanisms. Early studies have shown that increased levels of serum proinflammatory cytokines are associated with Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus-1 (SARS-CoV-1) infection [19], [20]. More recent studies have shown that patients with COVID-19 also had high amounts of IFNγ, IL1β, IL6, MCP1, and IP10 [9], [21]. In addition, patients requiring ICU admission had higher concentrations of proinflammatory cytokines than did those who did not require going to the ICU, suggesting that the sustained inflammatory response followed by cytokine storm may be associated with COVID-19 severity [21]. Hyperglycemia has been demonstrated to increase inflammatory cytokine levels, oxidative stress and potentially alters the balance between inflammatory and anti-inflammatory cytokines [22]. In addition, innate immune responses to infection have been demonstrated to be altered by acute hyperglycemia, which may in part explain the poor outcomes in COVID-19 patients who develop hyperglycemia [23], [24]. Furthermore, elevated glucose concentrations in airway epithelial secretion may damage the defensive capacity of airway epithelia [25].
Unfortunately, we had no data about pancreas islet function in this study, otherwise we could have further explored the mechanisms underlying hyperglycemia. Previous studies reported that acute viral respiratory infection has been related to transiently decreased insulin sensitivity [26]. Moreover, angiotensin-converting enzyme-2 (ACE-2) receptors are expressed in pancreatic islets. It has been reported that individuals infection with SARS-CoV-1 developed hyperglycemia [27]. Further studies with large patient cohorts will be required to properly evaluate pancreas islet function in patients with COVID-19.
Hypoglycemia can cause acute harm to patients and in severe cases can lead to loss of consciousness, seizure, coma, or death [10], [12]. It has been demonstrated that the replication of SARS-CoV-2 viruses consumes substantial amount of ATP in the body [28] so is logical to imagine that patients with decreased blood glucose may have fewer energetic resources to provide for the energy needed at cellular level work to fight acute levels of viral infection. In addition, glucose can be oxidized through a pentose phosphate pathway, yielding nicotinamide adenine dinucleotide phosphate (NADPH), thus maintaining the reducing state of glutathione (GSH). Reduced glutathione works for the anti-oxidant defense system and works with the immune system to fight invasive pathogenic microorganisms [28], [29]. On the cellular level, blood glucose is largely required for activated immune cells to mount a robust response [29]. These processes together indicate that comparatively low blood glucose levels result in enhanced oxidative stress and impaired immune responses. Collectively, until it is proven not to be causal, it is prudent to avoid hypoglycemia regardless of the cause of the condition in COVID-19 patients. Collectively, it is prudent to avoid hypoglycemia regardless of its cause in COVID-19 patients.
Various studies agreed with our finding patients increased levels of CRP, and LDH were associated with the severity of COVID-19 patients, particular in middle-aged men [8], [9], [14], [21], [30]. In addition, we found that BMI had an inverse effect on severe and critical condition in COVID-19 patients. Indeed, obesity has been demonstrated as a risk factor for increasing severity of SARS-CoV-2-related illness [31]. Here, we demonstrated for the first time that HDL can be used as a protective factor for preventing COVID-19 exacerbation. Consistent with this finding, there are studies supporting the anti-inflammatory effects of HDL [32].
We acknowledge that our sample size limited the glycemic target research for COVID-19 patients with diabetes. More solid estimates of optimal glycemic target of COVID-19 will be determined through analyses of larger sample size cohorts and related quality data. Indeed, Li et al. provided clinical evidence for glycemic target within 3.9–10.0 mmol/L for COVID-19 patients with diabetes [33]. However, our study did show a relevant effect of appropriate glycemic target on the risk of confirmed severity and criticality of COVID-19 in non-diabetes patients supporting the need to continue this area of study to provide more robust guidance for managing the COVID-19 pandemic. Ideally, continuous glucose monitoring (CGM) should be used to identify characteristics of glycaemia that are associated with severity of COVID-19. However, we had insufficient data about CGM in this study due to the different management plan in multiple hospitals. In addition, the research design could have resulted in selection bias as all subjects were Han Chinese and enrolled from one city, which weakens the generalizability of the results to other races. Furthermore, being a cross-sectional study, cause–causality relationships and mechanisms underlying the association between FBG and risk of COVID-19 exacerbation is difficult to elucidate.
In conclusion, the associations between FBG and risk of severe and critical condition were J-shaped for non-diabetes adult patients with COVID-19, with nadir at 4.74–5.78 mmol/L.
Author contribution statement
All authors have met the requirements for authorship. All authors have read and approved the final manuscript. SQ and DC oversaw the study design, data analysis, results interpretation, and wrote the manuscript. BZ and SWJ designed the study, performed all programming and data analysis, and wrote and reviewed the manuscript, LPW and CCH planned and directed the statistical analysis and interpretation of the results. ZW reviewed and edited the manuscript. LB, HS, and XCW contributed clinical expertise during the review and editing of the manuscript. SQ and DC are the guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding information
This work was supported by the National Key R&D Program of China (No. 2018YFC1314101; 2016YFC1305600), the National Natural Science Foundation of China (No. 81970677), and the Key Scientific and Technological Innovation Projects of Wenzhou (ZY202004). The funders had no role in the design and conduct of the study, the completion of the analysis, the interpretation of the data, or the content and preparation of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge Li Ming Wen (School of Public Health, University of Sydney, Australia) for his kind support for editing and proofreading this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108381.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Weiss P. Murdoch DR Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A.K. Khunti K Assessment of risk, severity, mortality, glycemic control and antidiabetic agents in patients with diabetes and COVID-19: a narrative review. Diabetes Res Clin Pract. 2020;165 doi: 10.1016/j.diabres.2020.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo W., Li M., Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu L., She Z.G., Cheng X. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(1068–1077) doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A.K., Gupta R., Ghosh A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sardu C., D'Onofrio N., Balestrieri M.L. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43:1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S., Ma P., Zhang S. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020 doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes A 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018; 41: S55–S64. Doi: 10.2337/dc18-S006. [DOI] [PubMed]
- 11.International Hypoglycaemia Study G Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017; 40: 155-157. Doi: 10.2337/dc16-2215. [DOI] [PubMed]
- 12.Zoungas S., Patel A., Chalmers J. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 13.Forbes A., Murrells T., Mulnier H. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:476–486. doi: 10.1016/S2213-8587(18)30048-2. [DOI] [PubMed] [Google Scholar]
- 14.Han Y., Liu Y., Zhou L. Epidemiological assessment of imported coronavirus disease 2019 (COVID-19) cases in the most affected city outside of Hubei Province, Wenzhou, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu H., Wu J., Hong L. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R., Ma Q., Han H. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0220. [DOI] [PubMed] [Google Scholar]
- 17.Yang J.K., Feng Y., Yuan M.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson K., Morris J., Bridson T. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falzarano D., de Wit E., Rasmussen A.L. Treatment with interferon-alpha2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong C.K., Lam C.W., Wu A.K. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito K., Nappo F., Marfella R. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 23.Dasu M.R., Devaraj S., Zhao L. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jafar N., Edriss H. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. 2016;351:201–211. doi: 10.1016/j.amjms.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Philips B.J., Meguer J.X., Redman J. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29:2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 26.Sestan M., Marinovic S., Kavazovic I. Virus-induced interferon-gamma causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49(164–177) doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Yang J.K., Lin S.S., Ji X.J. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z., Liu G., Wang L. From the insight of glucose metabolism disorder: oxygen therapy and blood glucose monitoring are crucial for quarantined COVID-19 patients. Ecotoxicol Environ Saf. 2020;197 doi: 10.1016/j.ecoenv.2020.110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang A., Luan H.H. An evolutionary perspective on immunometabolism. Science. 2019;363 doi: 10.1126/science.aar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J.-K., Jin J.-M., Liu S. Blood glucose is a representative of the clustered indicators of multi-organ injury for predicting mortality of COVID-19 in Wuhan, China. medRXiv. 2020 doi: 10.1101/2020.04.08.20058040. [DOI] [Google Scholar]
- 31.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Nardo D., Labzin L.I., Kono H. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lihua Zhu Z.-G.S., Cheng Xu. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1–10. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.