Abstract
Dynamic nuclear polarization (DNP) has shown great promise as a tool to enhance the nuclear magnetic resonance (NMR) signals of proteins in the cellular environment. As the sensitivity increases, the ability to select and efficiently polarize a specific macromolecule over the cellular background has become desirable. Here, we address this need and present a tetrazine-based DNP polarization agent that can be targeted selectively to proteins containing the unnatural amino acid (UAA) norbornene-lysine. The UAA can be introduced efficiently by genetic means in the cellular milieu. Our approach is bio-orthogonal and easily adaptable to any protein of interest. We illustrate the scope of our methodology and investigate the DNP polarization transfer mechanisms in several biological systems. Our results shed light on the complex polarization transfer pathways in targeted DNP and ultimately pave the way to selective DNP-enhanced NMR spectroscopy in both bacterial and mammalian cells.
Keywords: amber suppression, bioconjugation, NMR spectroscopy, protein chemistry, structural biology
Graphical abstract:
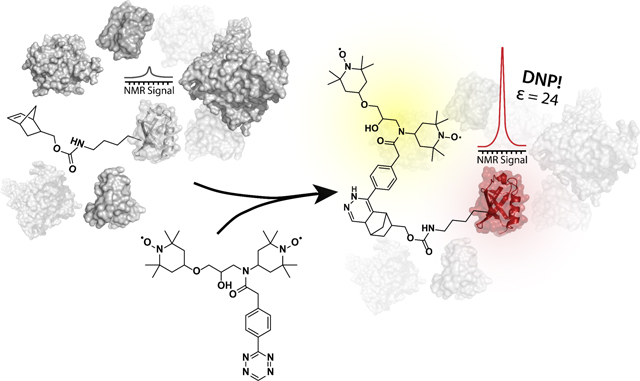
Target enhancement:
A combined tetrazine and amber suppression approach allows the selective NMR signal enhancement of proteins in complex mixtures. The achieved enhancements enable NMR data collection for just 5 μg of isotopically labeled protein samples.
Dynamic nuclear polarization (DNP) has made significant impact on the solid-state nuclear magnetic resonance (NMR) investigations of complex biological systems.[1, 2] With now routine signal enhancements in the 100-fold range, DNP has aided the in vitro structural studies of amyloid polymers,[3, 4] membrane proteins[5, 6] and biological material such as collagen, bones and tissues[7, 8]. Improvements in DNP instrumentation, sample preparation and polarization agent design have also allowed NMR spectroscopists to turn their attention to the cellular environment where the structural analysis of endogenous concentrations of biological macromolecules has come within reach.[3, 9–11] In a typical DNP experiment, the sample is doped with millimolar amounts of polarization agents, small molecules that contain two stable unpaired electron spins. The sample is cryoprotected, frozen to ~ 100 K and subjected to continuous microwave irradiation during magic angle spinning (MAS). The generated electron spin polarization is transferred to the nuclear spins, resulting in significant enhancement of the NMR signals. Concurrently, multidimensional NMR experiments are performed and structural constraints obtained with much improved sensitivity. While this strategy has been applied to most in vitro and cellular DNP experiments to date, it suffers from one major limitation: the polarization transfer is non-selective and results in enhancements for all molecules in the sample, including the cellular background.[10, 11] To overcome this shortcoming, several biochemical strategies can be employed including overexpression of the protein of interest[12, 13] or the introduction of recombinant isotopically labeled protein into cells by electroporation[11]. These approaches, however, require extensive perturbation of the cellular milieu and are not applicable to the structural studies of endogenous concentrations of cellular components. Alternatively, the design of targeted DNP polarization agents has been explored with selectivity achieved either through cysteine chemistry or protein-ligand interactions.[14–20] The applications of these strategies, however, are limited due to the reducing cellular environment or the requirement for a known synthetically accessible ligand of the target protein. We, therefore, sought to develop a general targeting strategy that can be applied to any protein of interest in a cysteine-independent manner, and that is compatible with DNP-enhanced NMR studies of proteins in the cellular interior.
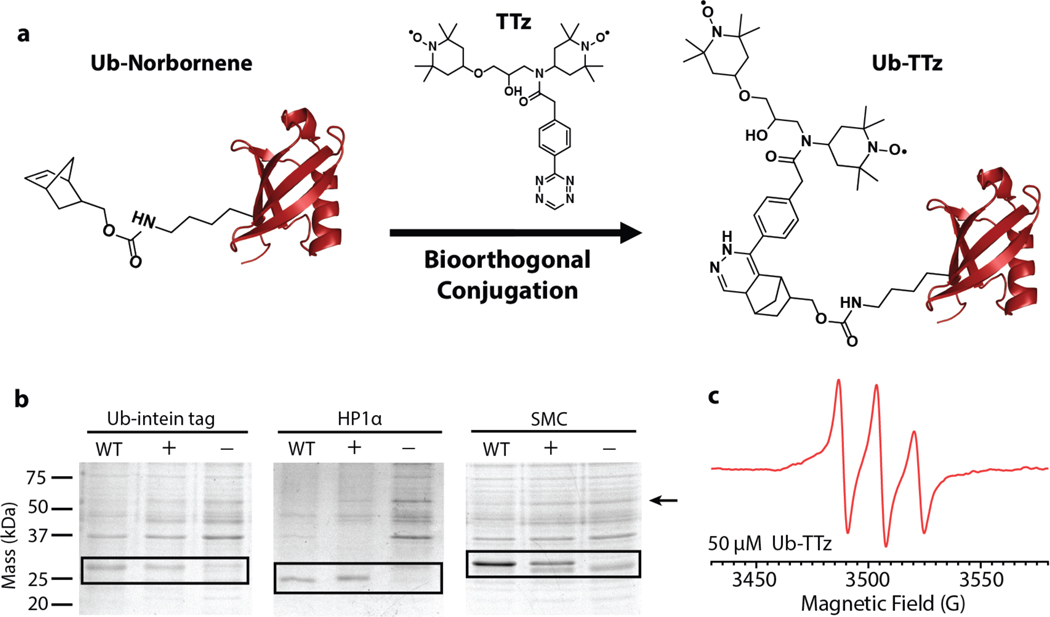
Our targeting approach is based on the bio-orthogonal chemical reactions between tetrazines and strained cycloalkenes.[21, 22] These reactions display superb efficiency and selectivity in the cellular milieu and are routinely used to label cellular proteins with optical probes.[23–26] Here, we harness their beneficial properties to attach DNP polarization agents to a variety of proteins (Fig. 1a). To this end, we started by coupling a tetrazine moiety to TOTAPOL, a stable, nitroxide-based DNP polarization agent[27] (SI Fig.1). The TOTAPOL-tetrazine biradical (TTz) displays the characteristic nitroxide hyperfine splitting in its EPR spectra and can be efficiently conjugated to strained cycloalkenes such as norbornene. Next, we synthesized the unnatural amino acid (UAA) norbornene-lysine[25] and introduced it at position 6 in the sequence of the model protein ubiquitin through amber suppression (Fig. 1b; SI Fig. 2). Amber suppression is a molecular biology technique that enables the introduction of UAAs in living cells by genetic means through the reassignment of the amber (TAG) stop codon.[28] The UAA is incorporated at the desired TAG position through an orthogonal tRNA and aminoacyl-tRNA synthetase (aaRS) pair introduced into the cell via a separate plasmid construct. While amber suppression is often used for optical and EPR experiments,[29–31] our application required the concurrent 13C,15N-labeling of the targeted protein and thus presented additional challenges for protein expression. After testing several plasmid vectors and constructs, we chose the pULTRA vector where the expression of the tRNA/aaRS pair is under the control of a lacI promoter[32] and we combined it with a tRNA/aaRS sequence optimized for the incorporation of norbornene-lysine[24]. Using this strategy, we observed high yields of isotopically labeled and modified proteins, not only for ubiquitin but also for several other systems of varying size and complexity including heterochromatin protein 1 (HP1α) and the structural maintenance of chromosomes (SMC) protein (Fig. 1b). Armed with these reagents, we next performed the biorthogonal reaction to produce protein-TTz conjugates. The reaction between tetrazine and norbornene was robust, with high efficiency under both denaturing and native conditions. After successful protein-TTz conjugation, excess TTz was removed by dialysis and the expected 1:1 ratio of protein to TTz was confirmed by LC-MS and EPR (Fig. 1c, SI Fig. 3 & 4).
Figure 1.
a) A general polarization agent targeting strategy based on the bio-orthogonal reaction between tetrazine and norbornene. b) Incorporation of norbornene-lysine into three different proteins by amber suppression. The bands compare the expression of unmodified 13C,15N-labeled proteins (WT), the expression of 13C,15N-labeled proteins containing the UAA (+) and a control sample where the UAA was not added to the media and no protein expression is expected (−). c) 9 GHz EPR spectrum of 50 μM ubiquitin-TTz.
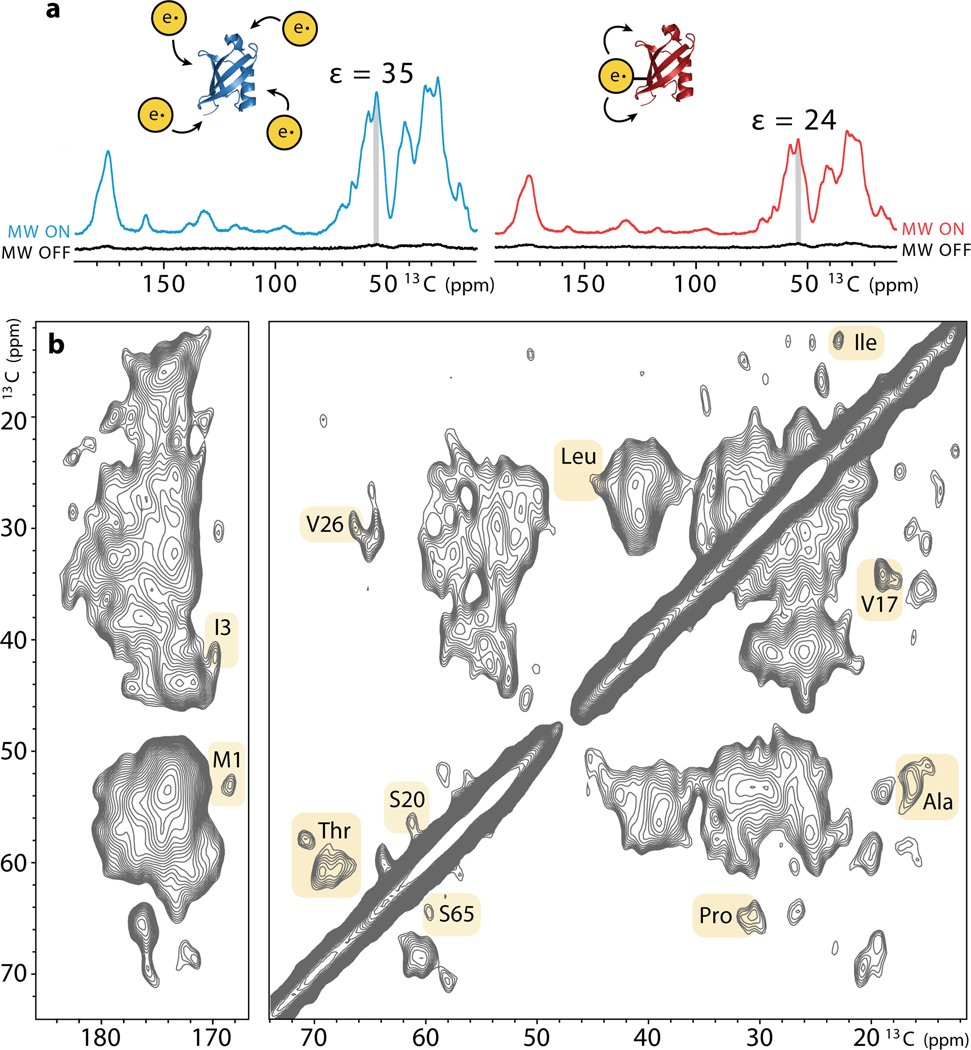
We next proceeded to determine the efficiency of TTz as a polarization source in comparison to the standard DNP sample preparation where the excess polarization agent is evenly distributed throughout a glassy matrix. As a direct measure of the enhancement, we used the signal in the presence of microwaves divided by the signal in the absence of irradiation. Under these conditions, we measured an enhancement of 24 for 1 mM ubiquitin-TTz which is 68% of the enhancement provided by dispersed TOTAPOL despite the 15-fold difference in radical concentration (Fig. 2a). The enhancement observed in our conjugated system is comparable or higher than the enhancements reported for other targeted-DNP approaches[14] and it was sufficient to record a 2D 13C-13C spectrum of just 100 ug of ubiquitin (1 mM) in less than a day (Fig. 2b). Comparison to a ubiquitin spectrum obtained with 15 mM dispersed TOTAPOL did not reveal any significant differences in the linewidths of the two samples (SI Fig. 5). Overall, our linewidths (1.5 – 2 ppm for resolved peaks) appear similar to the linewidths obtained in other frozen protein solutions where significant surface exposure to the solvent can lead to inhomogeneous line broadening.[11, 33–35] Many of the correlations in our spectrum could be assigned based on the recently published low temperature assignments of ubiquitin samples enhanced with dispersed AMUPol.[11]
Figure 2.
a) Comparison of the DNP enhancements measured in a sample containing 1 mM wildtype ubiquitin with 15 mM dispersed TOTAPOL (left) and 1 mM ubiquitin-TTz (right). Spectra were acquired at 600 MHz 1H Larmor frequency, 12 kHz MAS, 100 K. b) DNP-enhanced 2D 13C-13C CORD correlation[36] spectrum of 1 mM ubiquitin-TTz acquired at 24 kHz.
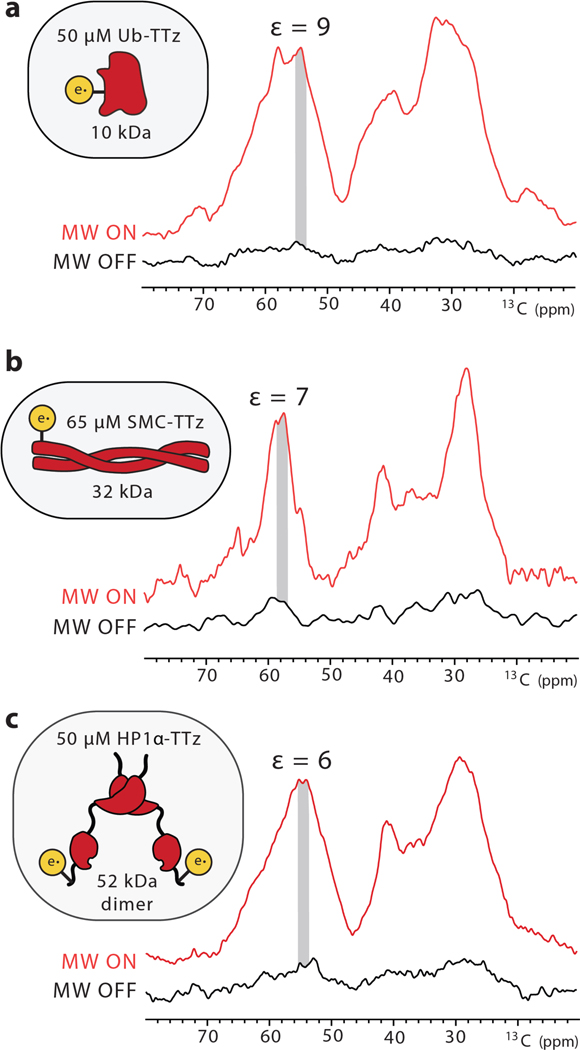
DNP enhancements depend on many experimental parameters including polarization agent concentration, interscan delay and MAS frequency.[1, 27, 37, 38] Since these parameters can provide valuable information regarding the DNP polarization transfer pathways, we proceeded to investigate them in more detail. First, we compared conjugated samples at 1 mM protein (i.e. 1 mM TTz) and dilute samples at ~ 50 μM protein and TTz (Fig. 3). Similarly to previous reports,[19] the measured enhancements at lower concentration were smaller, in the 6 – 10 range for different proteins. It should be noted, however, that dispersed TOTAPOL or TTz samples with similar concentration of biradicals yielded no enhancement. Therefore, conjugation significantly improves DNP polarization transfers by bringing the polarization source and target in close proximity to each other. Under these conditions, we did not observe evidence for significant depolarization or bleaching due to the attached radical. (SI Fig. 6).[39, 40] The amount of protein in these samples is in the 5 – 10 μg range and is close to the endogenous amounts of protein present in 106 mammalian cells that can easily fit into an NMR rotor.[11, 41]
Figure 3.
DNP enhancements for low concentrations of a) Ub-TTz; b) SMC-TTz; and c) HP1α-TTz.
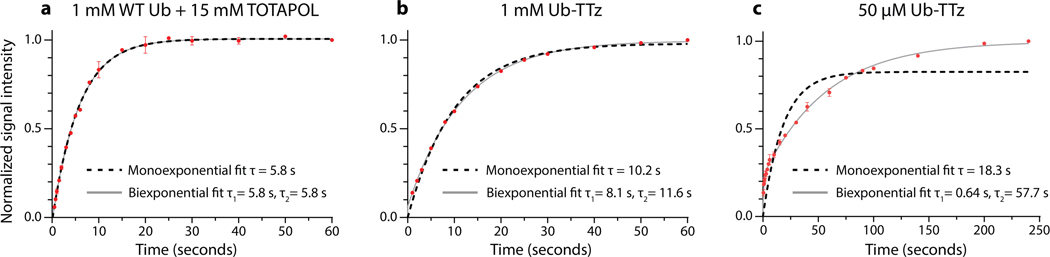
Intrigued by the concentration dependence of the enhancements in our targeted systems, we also measured polarization buildup curves that report on the distance dynamics of polarization transfers (Fig. 4, SI Fig.7).[42, 43] To this end, we compared the buildup behavior of a sample prepared with 15 mM dispersed TOTAPOL and samples containing 1 mM or 50 μM UbTTz. While the dispersed sample displayed a monoexponential buildup curve with a 5.8 s time constant, the behavior of the Ub-TTz samples appeared more complex. In particular, the buildup curve for the 50 μM Ub-TTz sample is consistent with two events, one with a short buildup time, and another event characterized with a much longer time constant. Considering the low concentration of Ub-TTz in this sample, we interpret the first buildup event as the fast polarization transfer from TTz to the directly conjugated protein (~ 3 nm in size), while the second step reflects long distance transfer from one conjugate to another (~ 50 nm distance). Furthermore, our observations also suggest that at ~ 1 mM Ub-TTz where the distance between biradicals is ~ 15 nm, the buildup curve is at the turning point between mono- and bi-exponential behavior. In this case, each protein is polarized by its own source as well as TTz moieties on neighboring proteins, resulting in larger enhancements and buildup times on the order of 8–12 s. As noted before,[19] the majority of published targeted DNP studies so far have been performed at concentrations of 1 mM or above, and, thus, most likely, the reported enhancements reflect a significant intermolecular polarization transfer component.
Figure 4.
Polarization buildup curves for a) 1 mM wild-type ubiquitin with 15 mM dispersed TOTAPOL; b) 1 mM Ub-TTz; and c) 50 μM Ub-TTz.
Finally, we explored the effects of MAS frequency (SI Fig. 8 & 9). Previous studies have reported a complicated relationship between MAS frequency and DNP enhancements due to a time dependent modulation of the electron and nuclear dipolar couplings.[37, 44–46] In our case, increasing the MAS frequency from 12 kHz to 24 kHz reduced the enhancements in both dispersed and conjugated samples although the reduction was much less pronounced for Ub-TTz at low concentration. For example, the signal intensities for the Cα region decreased by 44% for the dispersed sample, 49% for the 1 mM Ub-TTz sample and 15% for the 50 μM Ub-TTz sample. These results suggest that tethering the biradical to the protein of interest suppresses the unfavorable MAS relationship, presumably due to a less pronounced dependence on long-distance polarization transfers that may be affected by MAS as predicted by ab initio simulations of MAS DNP enhancements.[47]
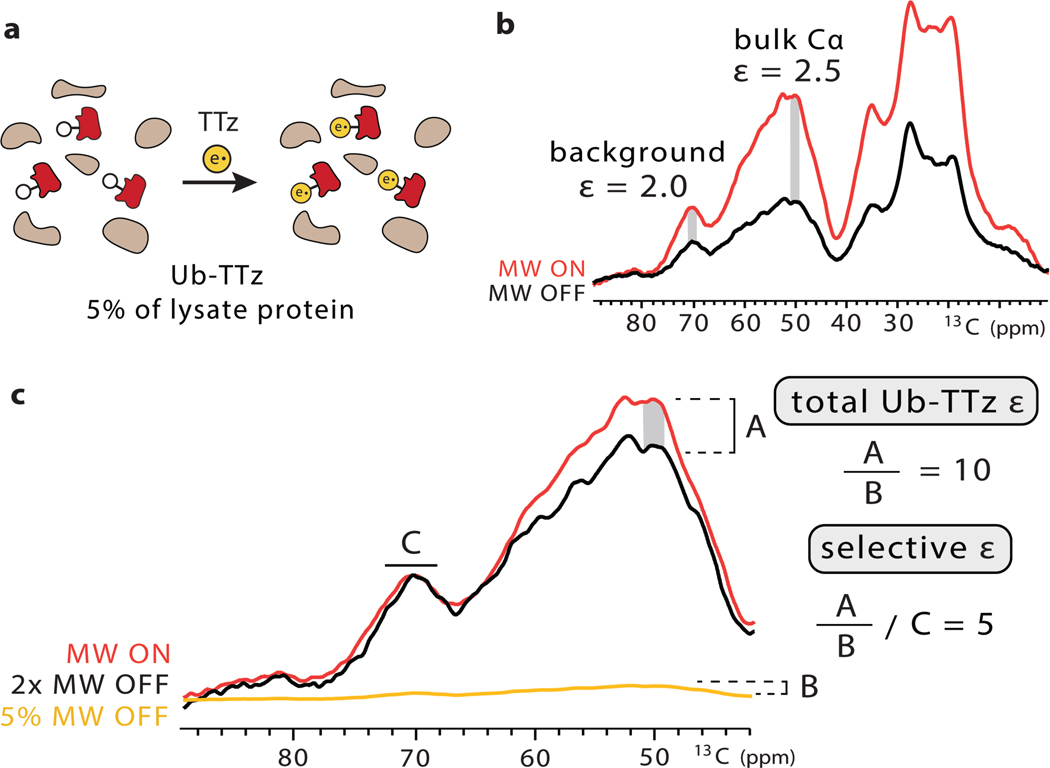
To further demonstrate the scope of our targeted bio-orthogonal approach, we extended our studies to a more complex setting. In particular, we targeted isotopically labeled bacterial lysates containing overexpressed ubiquitin with the norbornene-lysine UAA. Since TOTAPOL is susceptible to reduction,[48] we used dialysis to remove any endogenous thiols (Fig. 5). After incubation with TTz, we again dialyzed the lysate to remove unreacted TTz and confirmed complete TTz to protein conjugation by LC-MS (SI Fig. 10). We performed biochemical assays to estimate the ubiquitin amount in this sample, which was ~ 5% of the total protein and was present at ~ 40 μM in the DNP rotor (SI Fig. 10). Using the background subtraction approach introduced by Viennet et al.[18], we estimate that the selective enhancement for conjugated ubiquitin is 5-fold greater than the enhancement of the lysate background. Our overall ubiquitin enhancement in this setting is ~10, which is on par with the enhancements we measured for similar concentrations of purified protein-TTz conjugates. The ratio of specific to background enhancement in lysates can be tuned by the interscan delay, where shorter delays favor the targeted protein due to the close proximity of the paramagnetic species (SI Fig. 11). The specificity can be improved even further by coupling targeted DNP with selective isotopic labeling strategies and direct protein expression where only the protein of interest is isotopically labeled and enhanced.[49]
Figure 5.
a) Targeted DNP strategy for bacterial lysates. b) Overall signal enhancement of the 13Clabeled lysate. c) Strategy to determine the selective enhancement of Ub-TTz in the lysate. The microwave (MW) off signal was scaled to the microwave on signal so that the glycerol solvent peaks matched in intensity. The leftover enhancement was denoted as A. Then, the microwave off signal was scaled down to reflect the 5% abundance of Ub-TTz in the sample and the intensity of this rescaled spectrum was denoted as B. The ratio of A to B produced the total enhancement on ubiquitin. The selective enhancement is equal to the total ubiquitin enhancement divided by the background enhancement.
In summary, our tetrazine-based agents represent a general and efficient strategy to target DNP polarization to a variety of protein systems both in vitro and in complex biological settings. Since tools for amber suppression exist for different organisms, our approach is applicable to virtually any protein in bacterial or mammalian cells where tetrazine-based reagents have exhibited good permeability and superb reactivity and selectivity.[23, 26, 50] In this study, we chose to couple tetrazine to TOTAPOL due to the ease of synthesis of the biradical, however, our strategy is compatible with other popular DNP polarization agents such as AMUPol[51] or future biradicals that might be more stable in the cellular milieu. Our results also demonstrate that much is still to be learned about the polarization transfer mechanisms in targeted DNP. For example, we show that at low protein concentrations, the intramolecular short-range polarization transfer can be decoupled from the intermolecular long-range transfer mechanisms. We also explore the relationship between enhancement and MAS frequency and expect that targeted DNP will outperform conventional DNP strategies as improvements in instrumentation bring DNP into the fast MAS regime. A thorough understanding of the DNP polarization transfer mechanisms in conjugated systems will be essential in the selective signal enhancement of proteins of various sizes and shapes and on preventing polarization “leakage” into the cellular background. We are also excited at the prospect of combining our targeted DNP approach with other developments in the field such as higher magnetic fields,[52, 53] time domain DNP experiments[54] and optical approaches[10] for the comprehensive description of protein structures in cells.
Supplementary Material
Acknowledgements
We would like to thank Dr. Ivan Sergeyev, Justin Lee, and Dr. Andy Borovik for help with EPR data acquisition, Dr. Peter Schultz, Dr. Minseob Koh, Dr. Carsten Schultz and Dr. Jan-Erik Hoffmann for help with amber suppression, Brysa Alvarado and Melinda Serrato for assistance with TOTAPOL synthesis. B. A. was supported by NIH Molecular Biophysics Training Grant T32 GM008326. This work utilized the Biotechnology Research Center for NMR Molecular Imaging of Proteins at UCSD, supported by NIH grant P41 EB002031.
References
- [1].Lilly Thankamony AS, Wittmann JJ, Kaushik M, Corzilius B, Prog. Nucl. Magn. Reson. Spectrosc. 2017, 102–103, 120–195. [DOI] [PubMed] [Google Scholar]
- [2].Jaudzems K, Polenova T, Pintacuda G, Oschkinat H, Lesage A, J. Struct. Biol. 2019, 206, 90–98. [DOI] [PubMed] [Google Scholar]
- [3].Frederick KK, Michaelis VK, Corzilius B, Ong TC, Jacavone AC, Griffin RG, Lindquist S, Cell. 2015, 163, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Debelouchina GT, Bayro MJ, Fitzpatrick AW, Ladizhansky V, Colvin MT, Caporini MA, Jaroniec CP, Bajaj VS, Rosay M, Macphee CE, Vendruscolo M, Maas WE, Dobson CM, Griffin RG, J. Am. Chem. Soc. 2013, 135, 19237–19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Andreas LB, Barnes AB, Corzilius B, Chou JJ, Miller EA, Caporini M, Rosay M, Griffin RG, Biochemistry. 2013, 52, 2774–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kaplan M, Narasimhan S, de Heus C, Mance D, van Doorn S, Houben K, Popov-Celeketic D, Damman R, Katrukha EA, Jain P, Geerts WJC, Heck AJR, Folkers GE, Kapitein LC, Lemeer S, van PMP Bergen En Henegouwen, M. Baldus, Cell. 2016, 167, 1241–1251 e1211. [DOI] [PubMed] [Google Scholar]
- [7].Chow WY, Li R, Goldberga I, Reid DG, Rajan R, Clark J, Oschkinat H, Duer MJ, Hayward R, Shanahan CM, Chem. Commun. (Cambridge, U.K.). 2018, 54, 12570–12573. [DOI] [PubMed] [Google Scholar]
- [8].Azais T, Von Euw S, Ajili W, Auzoux-Bordenave S, Bertani P, Gajan D, Emsley L, Nassif N, Lesage A, Solid State Nucl. Magn. Reson. 2019, 102, 2–11. [DOI] [PubMed] [Google Scholar]
- [9].Costello WN, Xiao Y, Frederick KK, Methods Enzymol. 2019, 615, 373–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Albert BJ, Gao C, Sesti EL, Saliba EP, Alaniva N, Scott FJ, Sigurdsson ST, Barnes AB, Biochemistry. 2018, 57, 4741–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Narasimhan S, Scherpe S, Lucini Paioni A, van der Zwan J, Folkers GE, Ovaa H, Baldus M, Angew. Chem., Int. Ed. Engl. 2019, 58, 12969–12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Renault M, Pawsey S, Bos MP, Koers EJ, Nand D, Tommassen-van Boxtel R, Rosay M, Tommassen J, Maas WE, Baldus M, Angew. Chem., Int. Ed. Engl. 2012, 51, 2998–3001. [DOI] [PubMed] [Google Scholar]
- [13].Yamamoto K, Caporini MA, Im SC, Waskell L, Ramamoorthy A, Biochim. Biophys. Acta, Biomembr. 2015, 1848, 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rogawski R, McDermott AE, Arch. Biochem. Biophys. 2017, 628, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kaushik M, Bahrenberg T, Can TV, Caporini MA, Silvers R, Heiliger J, Smith AA, Schwalbe H, Griffin RG, Corzilius B, Phys. Chem. Chem. Phys. 2016, 18, 27205–27218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Voinov MA, Good DB, Ward ME, Milikisiyants S, Marek A, Caporini MA, Rosay M, Munro RA, Ljumovic M, Brown LS, Ladizhansky V, Smirnov AI, J. Phys. Chem. B. 2015, 119, 10180–10190. [DOI] [PubMed] [Google Scholar]
- [17].van der Cruijsen EA, Koers EJ, Sauvee C, Hulse RE, Weingarth M, Ouari O, Perozo E, Tordo P, Baldus M, Chem. - Eur. J. 2015, 21, 12971–12977. [DOI] [PubMed] [Google Scholar]
- [18].Viennet T, Viegas A, Kuepper A, Arens S, Gelev V, Petrov O, Grossmann TN, Heise H, Etzkorn M, Angew. Chem., Int. Ed. Engl. 2016, 55, 10746–10750. [DOI] [PubMed] [Google Scholar]
- [19].Rogawski R, Sergeyev IV, Li Y, Ottaviani MF, Cornish V, McDermott AE, J. Phys. Chem. B. 2017, 121, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marin-Montesinos I, Goyard D, Gillon E, Renaudet O, Imberty A, Hediger S, De Paepe G, Chem. Sci. 2019, 10, 3366–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Devaraj NK, Weissleder R, Hilderbrand SA, Bioconjugate Chem. 2008, 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Blackman ML, Royzen M, Fox JM, J. Am. Chem. Soc. 2008, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu H, Devaraj NK, Acc. Chem. Res. 2018, 51, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hoffmann JE, Plass T, Nikic I, Aramburu IV, Koehler C, Gillandt H, Lemke EA, Schultz C, Chem. - Eur. J. 2015, 21, 12266–12270. [DOI] [PubMed] [Google Scholar]
- [25].Lang K, Davis L, Torres-Kolbus J, Chou CJ, Deiters A, Chin JW, Nat. Chem. 2012, 4, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Plass T, Milles S, Koehler C, Szymanski J, Mueller R, Wiessler M, Schultz C, Lemke EA, Angew. Chem., Int. Ed. Engl. 2012, 51, 4166–4170. [DOI] [PubMed] [Google Scholar]
- [27].Song CS, Hu KN, Joo CG, Swager TM, Griffin RG, J. Am. Chem. Soc. 2006, 128, 11385–11390. [DOI] [PubMed] [Google Scholar]
- [28].Wang L, Brock A, Herberich B, Schultz PG, Science. 2001, 292, 498–500. [DOI] [PubMed] [Google Scholar]
- [29].Evans EG, Millhauser GL, Methods Enzymol. 2015, 563, 503–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schmidt MJ, Fedoseev A, Bucker D, Borbas J, Peter C, Drescher M, Summerer D, ACS Chem. Biol. 2015, 10, 2764–2771. [DOI] [PubMed] [Google Scholar]
- [31].Tyagi S, Lemke EA, Curr. Opin. Struct. Biol. 2015, 32, 66–73. [DOI] [PubMed] [Google Scholar]
- [32].Chatterjee A, Sun SB, Furman JL, Xiao H, Schultz PG, Biochemistry. 2013, 52, 18281837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Debelouchina GT, Bayro MJ, van der Wel PC, Caporini MA, Barnes AB, Rosay M, Maas WE, Griffin RG, Phys. Chem. Chem. Phys. 2010, 12, 5911–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Linden AH, Franks WT, Akbey U, Lange S, van Rossum BJ, Oschkinat H, J. Biomol. NMR. 2011, 51, 283–292. [DOI] [PubMed] [Google Scholar]
- [35].Siemer AB, Huang KY, McDermott AE, PLoS One. 2012, 7, e47242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hou G, Yan S, Trebosc J, Amoureux JP, Polenova T, J Magn Reson. 2013, 232, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mentink-Vigier F, Akbey U, Hovav Y, Vega S, Oschkinat H, Feintuch A, J Magn Reson. 2012, 224, 13–21. [DOI] [PubMed] [Google Scholar]
- [38].Takahashi H, Fernandez-de-Alba C, Lee D, Maurel V, Gambarelli S, Bardet M, Hediger S, Barra AL, De Paepe G, J Magn. Reson. 2014, 239, 91–99. [DOI] [PubMed] [Google Scholar]
- [39].Mentink-Vigier F, Paul S, Lee D, Feintuch A, Hediger S, Vega S, De Paepe G, Phys. Chem. Chem. Phys. 2015, 17, 21824–21836. [DOI] [PubMed] [Google Scholar]
- [40].Thurber KR, Tycko R, J. Chem. Phys. 2014, 140, 184201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wisniewski JR, Hein MY, Cox J, Mann M, Mol. Cell. Proteomics. 2014, 13, 34973506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].van der Wel PC, Hu KN, Lewandowski J, Griffin RG, J. Am. Chem. Soc. 2006, 128, 10840–10846. [DOI] [PubMed] [Google Scholar]
- [43].Pinon AC, Schlagnitweit J, Berruyer P, Rossini AJ, Lelli M, Socie E, Tang MX, Pham T, Lesage A, Schantz S, Emsley L, J. Phys. Chem. C. 2017, 121, 15993–16005. [Google Scholar]
- [44].Mentink-Vigier F, Akbey U, Oschkinat H, Vega S, Feintuch A, J. Magn. Reson. 2015, 258, 102–120. [DOI] [PubMed] [Google Scholar]
- [45].Corzilius B, Andreas LB, Smith AA, Ni QZ, Griffin RG, J. Magn. Reson. 2014, 240, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rosay M, Tometich L, Pawsey S, Bader R, Schauwecker R, Blank M, Borchard PM, Cauffman SR, Felch KL, Weber RT, Temkin RJ, Griffin RG, Maas WE, Phys. Chem. Chem. Phys. 2010, 12, 5850–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Perras FA, Pruski M, J. Chem. Phys. 2018, 149, 154202. [DOI] [PubMed] [Google Scholar]
- [48].McCoy KM, Rogawski R, Stovicek O, McDermott AE, J. Magn. Reson. 2019, 303, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Luchinat E, Banci L, Acc. Chem. Res. 2018, 51, 1550–1557. [DOI] [PubMed] [Google Scholar]
- [50].Chin JW, Nature. 2017, 550, 53–60. [DOI] [PubMed] [Google Scholar]
- [51].Sauvee C, Rosay M, Casano G, Aussenac F, Weber RT, Ouari O, Tordo P, Angew. Chem., Int. Ed. Engl. 2013, 52, 10858–10861. [DOI] [PubMed] [Google Scholar]
- [52].Fricke P, Mance D, Chevelkov V, Giller K, Becker S, Baldus M, Lange A, J. Biomol. NMR. 2016, 65, 121–126. [DOI] [PubMed] [Google Scholar]
- [53].Barnes AB, Markhasin E, Daviso E, Michaelis VK, Nanni EA, Jawla SK, Mena EL, DeRocher R, Thakkar A, Woskov PP, Herzfeld J, Temkin RJ, Griffin RG, J. Magn. Reson. 2012, 224, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tan KO, Yang C, Weber RT, Mathies G, Griffin RG, Sci Adv. 2019, 5, eaav6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.