Abstract
As no specific pharmacological treatment has been validated for use in coronavirus disease 2019 (COVID-19), we aimed to assess the effectiveness of azithromycin (AZM) in these patients at a referral centre in Iran. An open-label, randomised controlled trial was conducted on patients with laboratory-confirmed COVID-19. A total of 55 patients in the control group receiving hydroxychloroquine (HCQ) and lopinavir/ritonavir (LPV/r) were compared with 56 patients in the case group who in addition to the same regimen also received AZM. Patients with prior cardiac disease were excluded from the study. Furthermore, patients from the case group were assessed for cardiac arrythmia risk based on the American College of Cardiology (ACC) risk assessment for use of AZM and HCQ. The main outcome measures were vital signs, SpO2 levels, duration of hospitalisation, need for and length of intensive care unit admission, mortality rate and results of 30-day follow-up after discharge. Initially, there was no significant difference between the general conditions and vital signs of the two groups. The SpO2 levels at discharge were significantly higher, the respiratory rate was lower and the duration of admission was shorter in the case group. There was no significant difference in the mortality rate between the two groups. Patients who received AZM in addition to HCQ and LPV/r had a better general condition. HCQ+AZM combination may be beneficial for individuals who are known to have a very low underlying risk for cardiac arrhythmia based on the ACC criteria.
Keywords: COVID-19, SARS-CoV-2, Azithromycin, Hydroxychloroquine, Lopinavir, Ritonavir
1. Introduction
In late December 2019, an outbreak of an emerging disease with a remarkably high virulence in Wuhan, China, soon became a global concern. Disease symptoms resemble a viral pneumonia and genetic analysis of lower respiratory tract samples of early infected patients showed an infection caused by the novel coronavirus 2019-nCoV, subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19). The disease rapidly spread throughout China and infected multiple other countries [1,2]. On 12 March 2020, the World Health Organization (WHO) declared the epidemic of COVID-19 as a global pandemic. In addition to primary respiratory involvement, reports show other organ systems, including the gastrointestinal, neurological and haematopoietic systems, can also be considerably affected by this virus [3,4]. Coronavirus infections in humans are mostly mild; a meta-analysis of epidemiologic studies conducted in China showed that 12.6–23.5% of patients experience a severe form of COVID-19 with an overall mortality rate of 2.0–4.4% [5]. There are no specific pharmacological treatments for the novel coronavirus as yet [6]. Repositioning of well-known medications as antiviral treatment is preferred in circumstances where there is little time for standard randomised controlled trials and preliminary laboratory investigations into a new medication. Complete knowledge of the possible side effects and safety profile of old medications lays the groundwork for better monitoring of the treatment effect and outcome [7].
Multiple medications have been used in clinical trials against COVID-19. Chloroquine (CQ), an immunomodulatory drug, is widely used as an antimalarial agent and was discovered to have broad-spectrum antiviral effects in 2006 [8]. Hydroxychloroquine (HCQ), an analogue of CQ, has a better clinical safety profile and allows for a higher daily dose compared with CQ [9,10]. The combination lopinavir/ritonavir (LPV/r) has been approved and used for human immunodeficiency virus (HIV) infection across the world; both substances are protease inhibitors, but ritonavir also enhances the pharmacokinetic and pharmacodynamic properties of lopinavir [11]. These antiviral agents have been used in the treatment of Middle East respiratory syndrome (MERS) [12]. Lopinavir has also been proven to have in vitro activity against SARS-CoV infection in humans [13], [14], [15]. Azithromycin (AZM), a macrolide antibiotic, has shown efficacy in preventing severe respiratory infections in patients suffering from viral pneumonia [16]. In vitro studies have demonstrated that it is active against Zika and Ebola viruses [17] and it has a high affinity for the binding interaction site of the SARS-CoV-2 spike protein and angiotensin-converting enzyme 2 (ACE2) [18], which is the critical human cell receptor for the SARS-CoV-2 virus, and it is believed that blocking this interaction can potentially prevent the infection [19].
The combination of HCQ and AZM has been very well received among physicians and, according to an online international survey of 5500 physicians over 13–15 April 2020, these medications are the most commonly used medication in the treatment of COVID-19 [20]. However, there are a considerable cardiac risks associated with the concomitant use of AZM and HCQ, and cardiac arrhythmias caused by QT interval prolongation can potentially increase the mortality rate in patients who are treated with this combination [21,22].
In this clinical trial, we evaluated the potential treatment benefits of this combination in patients with low risk for QT prolongation and arrhythmia. We used the scoring system proposed by the American College of Cardiology (ACC) [23] to exclude patients with a moderate to high risk of cardiac arrhythmias from the study.
2. Materials and methods
2.1. Participants
Between 24 April and 8 May 2020, a total of 202 patients with compelling clinical symptoms for a diagnosis of COVID-19 were admitted to Ziaeian Hospital in Tehran (Iran). All patients underwent reverse transcriptase PCR (RT-PCR) testing and a lung computed tomography (CT) scan. Inclusion criteria were a positive RT-PCR test and significant findings compatible with radiographic imaging of COVID-19 pulmonary involvement. Exclusion criteria were age <18 years, pregnancy or nursing during the time of admission, past history or concurrent cardiac disease, recent history of antiviral therapy, and contraindications for use of HCQ, AZM or LPV/r (KaletraⓇ; AbbVie Inc., Chicago, IL, USA), such as retinopathy or glucose-6-phosphate dehydrogenase deficiency, or a history of allergic reactions to these medicines.
2.2. Study arms and treatment plans
Patients were randomly divided into two treatment groups, a case group comprising 56 patients and a control group comprising 55 patients. On the first day of admission, laboratory studies including complete blood count and erythrocyte sedimentation rate (ESR) were performed. The case group received oral AZM 500 mg daily, oral LPV/r 400/100 mg twice daily and oral HCQ 400 mg daily. The control group received oral LPV/r 400/100 mg twice daily and oral HCQ 400 mg daily; for both treatment groups, all medications were administered for 5 days.
On the first day of admission, for patients assigned to the case treatment group, the risk for ventricular arrhythmia in concurrent treatment with HCQ and AZM was calculated based on the proposed guideline by the ACC [23], and patients with a score of ≥7 were excluded from the study.
Patients were assessed by daily measurements of core body temperature, respiratory rate, heart rate and peripheral capillary oxygen saturation (SpO2). Daily electrocardiogram (ECG) studies were also conducted to monitor possible evolution of heart rate-corrected QT (QTc) interval prolongation, in which case, treatment with AZM and HCQ would have been stopped. For correction of the QT interval, Bazett's formula (QTc = QT/√RR) was used [24]. In case of deterioration in general and/or pulmonary condition, methylprednisolone was prescribed [25]. Patients were discharged when they achieved a stable SpO2 > 92%, had no respiratory distress and were afebrile for 3 consecutive days. The primary endpoints in this trial were a decrease in mortality, duration of hospitalisation and need for intensive care unit (ICU) admission. Secondary endpoints were determined as improvements in SpO2 and vital signs as well as the general wellbeing of the patient.
Sample size calculation was performed for non-inferiority tests of difference between two group proportions. We assumed an effectiveness of 65% for the intervention group and effectiveness of 50% for the control group. We also assumed a margin of non-inferiority of at least 10% between the two groups. With these assumptions, a sample size of 48 cases in each group was calculated. After consideration of a dropout rate of 10%, the total sample size of 110 cases was calculated. The power of the study was determined as 90% (G*Power, Erdfelder, Faul, & Buchner, 1996).
2.3. Ethical considerations
In accordance with the Declaration of Helsinki, written informed consent was obtained from all participants before inclusion in the study. Patients were assured that declining to participate or leaving the study at any point would not affect the quality of their treatment and that they would thereafter receive standard care. The study protocol was approved by the Institutional Review Board of Tehran University of Medical Sciences.
2.4. Measurements and statistical analysis
Distribution of age, sex, initial clinical symptoms and vital signs measured on the first day of admission were compared between the two groups. The vital signs including core body temperature, respiratory rate, heart rate and SpO2 were also compared on the third and last days of treatment between the two groups as an outcome measure. Differences in duration of hospitalisation, number of patients whose condition deteriorated and required ICU admission, length of ICU stay, mortality rate and results of 30-day follow-up after discharge were also evaluated as outcome measures.
Analysis was performed using IBM SPSS Statistics for Windows v.22.0 (IBM Corp., Armonk, NY, USA). Quantitative variables were reported as the mean ± standard deviation and qualitative variables as the frequency and percentage. Because of the normal distribution of the data, the independent t-test was used to assess the difference in means. The χ2 test and Fisher's exact test were used to assess the statistical relationships between categorical variables. The level of significance was set at a P-value of <0.05 for all analyses. The number needed to treat with a confidence interval (CI) of 95% was reported for requirement for ICU admission, need for intubation and mortality rate. We also evaluated the effect size based on Hedges’ g because of the difference in the number of participants in each group.
2.5. Safety
Since a stepwise plan was practiced in our study, patients with any prior cardiac disease were excluded from the study. Furthermore, the ACC criteria for risk assessment of simultaneous use of AZM and HCQ were assessed for each person to make sure no patient has an increased risk of ventricular arrythmia. All patients were also monitored closely for any signs of ECG rhythm abnormality or clinical features of cardiac arrhythmia.
3. Results
3.1. Demographic characteristics
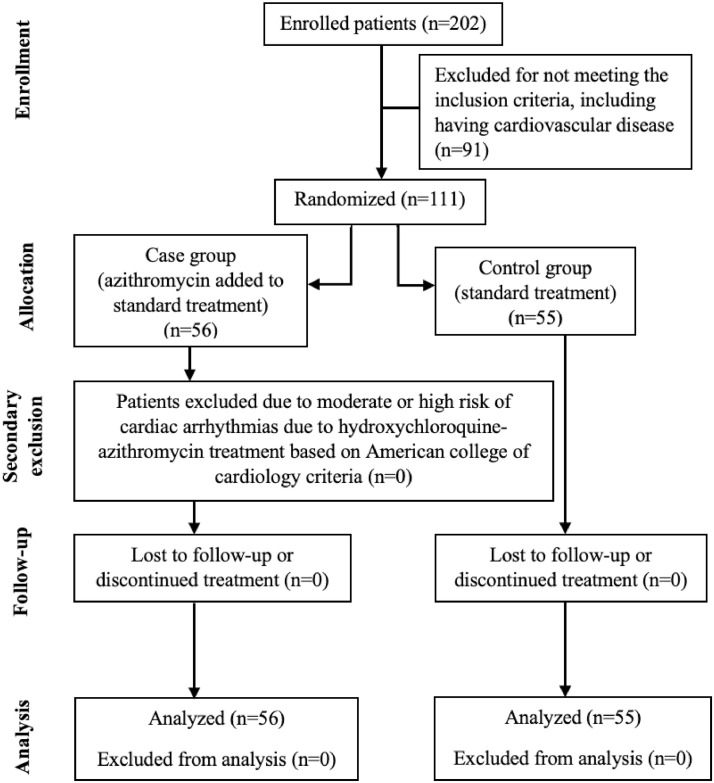
Based on the inclusion criteria and after excluding 91 cases, 111 cases were included in the study, which were randomised and allocated between the two treatment groups of case (n = 56) and control (n = 55) patients (Fig. 1 ). All patients completed their 5-day required treatment duration.
Fig. 1.
Randomisation and treatment protocols of the patients.
In the case treatment group, the ventricular arrhythmia risk score was three in 12 patients (21.4%), four in 32 patients (57.1%) and five in 12 patients (21.4%). The mean age and demographic factors such as sex were not significantly different between the two treatment arms (P = 0.700 and 0.387, respectively) (Table 1 ).
Table 1.
Demographic, clinical and laboratory findings in the two treatment groupsa.
| Variable | Case group (n = 56) | Control group (n = 55) | P-value |
|---|---|---|---|
| Age (years) | 54.38 ± 15.92 | 59.89 ± 15.55 | 0.700 |
| Body temperature on admission (°C) | 38.07 ± 0.69 | 37.72 ± 0.91 | 0.020 |
| White blood cell count (× 109/L) | 6.94 ± 2.65 | 6.28 ± 2.30 | 0.160 |
| Haemoglobin (g/dL) | 13.65 ± 1.97 | 12.80 ± 1.94 | 0.200 |
| Platelet count (× 109/L) | 230.45 ± 111.77 | 238.46 ± 99.56 | 0.690 |
| ESR (mm/h) | 64.86 ± 29.12 | 70.71 ± 32.05 | 0.320 |
| RR on admission (breaths/min) | 23.75 ± 5.19 | 22.62 ± 5.72 | 0.280 |
| SpO2 on admission (%) | 89.61 ± 2.98 | 89.51 ± 6.84 | 0.920 |
| Day 3 SpO2 (%) | 89.36 ± 4.59 | 88.75 ± 7.67 | 0.610 |
| Sex | |||
| Female | 28 (50.00) | 32 (58.18) | 0.387 |
| Male | 28 (50.00) | 23 (41.82) | |
| Fever | 38 (67.86) | 33 (60.00) | 0.389 |
| Dyspnoea | 41 (73.21) | 43 (78.18) | 0.542 |
| Myalgia | 18 (32.14) | 22 (74.55) | 0.000 |
| Chills | 18 (32.14) | 25 (45.45) | 0.150 |
| Weakness | 10 (17.86) | 3 (5.45) | 0.042 |
| Cough | 34 (60.71) | 41 (74.55) | 0.120 |
| Sputum production | 3 (5.36) | 8 (14.55) | 0.105 |
| Haemoptysis | 3 (5.36) | 0 (0.00) | 0.243 |
| Headache | 6 (10.71) | 18 (32.7) | 0.005 |
| Vomiting | 7 (12.50) | 16 (29.09) | 0.031 |
| Chest pain | 10 (17.86) | 12 (21.82) | 0.601 |
| Primary endpoints | |||
| Hospital stay (days) | 4.61 ± 2.59 | 5.96 ± 3.21 | 0.020 |
| Need for ICU admission | 2 (3.57) | 7 (12.73) | 0.070 |
| Death | 0 (0.00) | 1 (1.82) | 0.495 |
| Secondary endpoints | |||
| Discharge body temperature (°C) | 36.88 ± 0.33 | 36.77 ± 0.53 | 0.190 |
| ICU length of stay (days) | 5.00 ± 0.01 | 4.43 ± 2.99 | 0.157 |
| RR at discharge (breaths/min) | 15.85 ± 1.99 | 17.42 ± 2.42 | 0.010 |
| SpO2 at discharge (%) | 93.95 ± 2.14 | 92.40 ± 4.58 | 0.030 |
| Need for intubation | 0 (0.00) | 3 (5.45) | 0.118 |
ESR, erythrocyte sedimentation rate; RR, respiratory rate; SpO2, peripheral capillary oxygen saturation; ICU, intensive care unit.
NOTE: The control group received oral lopinavir/ritonavir 400/100 mg twice daily and oral hydroxychloroquine 400 mg daily; the case group in addition to the same regimen also received oral azithromycin 500 mg daily.
Data are the mean ± standard deviation or n (%).
3.2. Clinical and laboratory findings
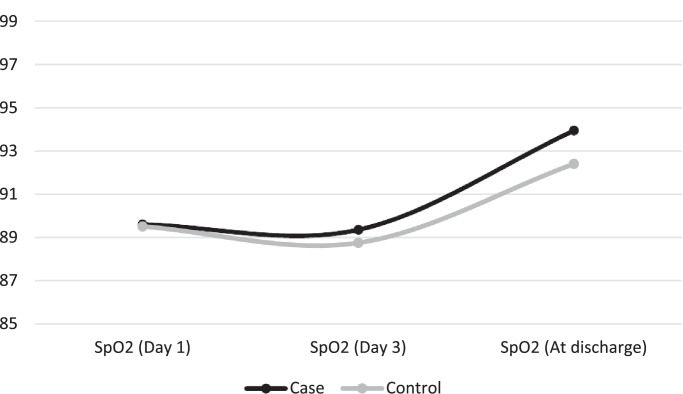
Table 1 gives the clinical features and laboratory test results of patients in both groups. Fever, dyspnoea, chills, cough, production of sputum, haemoptysis and chest pain were not significantly different between the two groups (P > 0.05). Myalgia, headache and vomiting were initially reported more by control patients (P = 0.000, 0.005 and 0.031, respectively). Weakness was found significantly more frequently in patients in the case group (P = 0.042) . The mean SpO2 levels upon admission and on the third day of admission were not significantly different between the two groups (P = 0.920 and 0.610, respectively) (Fig. 2 ). Laboratory test results did not show a significant difference between the two groups (P > 0.05).
Fig. 2.
Comparison of SpO2 (%) changes between the two treatment groups. The control group received oral lopinavir/ritonavir 400/100 mg twice daily and oral hydroxychloroquine 400 mg daily; the case group in addition to the same regimen also received oral azithromycin 500 mg daily.
3.3. Treatment outcomes
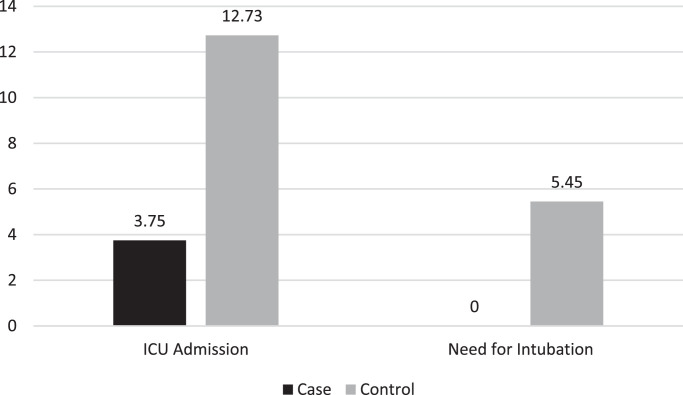
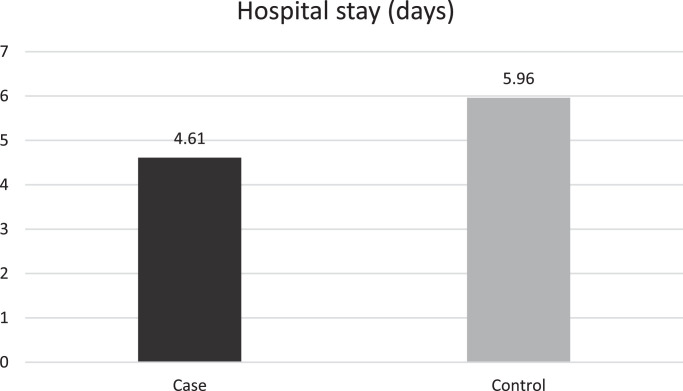
Table 1 shows treatment outcomes in both treatment groups. Core temperature was not significantly different between the case and control groups (36.88 °C vs. 36.77 °C, respectively; P = 0.190). At discharge, SpO2 levels were significantly higher in the case group (93.95% vs. 92.40%; P = 0.030) and the respiratory rate was significantly lower (15.85 breaths/min vs. 17.42 breaths/min; P = 0.010). The duration of hospitalisation in the case group was significantly shorter than the control group (4.61 days vs. 5.96 days; P = 0.02) (Fig. 3 ). The calculated effect size for SpO2 levels, respiratory rate at discharge and duration of hospitalisation were –0.461, 0.721 and 0.618 (all medium effect sizes), respectively (Table 2 ). Two patients in the case group and seven patients in the control group required ICU admission, which did not show statistical significance (3.6% vs. 12.7%, respectively; P = 0.070). Three patients in the control group were intubated during the course of admission versus no patients in the case group, which was statistically insignificant (P = 0.118) (Fig. 4 ). The difference between the mean duration of ICU admission was not significant between the groups (5.00 days vs. 4.43 days; P = 0.157). There was one death in the control group and none in the case group; this difference was insignificant (P = 0.495). No patient in either group experienced cardiac arrhythmia or QTc prolongation.
Fig. 3.
Comparison of the mean duration of hospitalisation (days) between the two treatment groups. The control group received oral lopinavir/ritonavir 400/100 mg twice daily and oral hydroxychloroquine 400 mg daily; the case group in addition to the same regimen also received oral azithromycin 500 mg daily.
Table 2.
Effect sizes for outcome of patients in the case and control treatment groups.
| Variable | Hedges’ g | 95% CI |
|---|---|---|
| SpO2 at discharge | –0.461 | –0.838, –0.084 |
| RR at discharge | 0.721 | 0.337, 1.105 |
| Length of hospital stay | 0.618 | 0.103, 0.858 |
CI, confidence interval; SpO2, peripheral capillary oxygen saturation; RR, respiratory rate.
NOTE: The control group received oral lopinavir/ritonavir 400/100 mg twice daily and oral hydroxychloroquine 400 mg daily; the case group in addition to the same regimen also received oral azithromycin 500 mg daily.
Fig. 4.
Requirement for intensive care unit (ICU) admission and intubation in the two treatment group. The control group received oral lopinavir/ritonavir 400/100 mg twice daily and oral hydroxychloroquine 400 mg daily; the case group in addition to the same regimen also received oral azithromycin 500 mg daily. The number of patients is given on the y-axis, with percentage above the bars.
4. Discussion
During the rapidly spreading global pandemic of COVID-19, it is important to have an effective and safe treatment plan. There are several reports on the effectiveness of various medications, but as yet none of them have been proven to be significantly effective. Recently, combination therapy with HCQ and AZM has become one of the most favoured treatment regimens among medical professionals [26], [27], [28]. Studies have shown that the combination of HCQ and AZM can reinforce the efficacy of HCQ [28,29]. Gautret et al. conducted a non-randomised open-label clinical trial that has shown promising results for the combination of HCQ and AZM [27]. In their study, they reported that the HCQ+AZM combination had a significant effect on viral load reduction within only 3–6 days of treatment in COVID-19 patients [27], but a number of reviews have questioned the randomisation technique used in this study and pointed out that the small study patient population and other methodological pitfalls question the certainty with which the results of this study are to be received [30], [31], [32], [33]. Million et al. published an article that was an extension to the previous study by Gautret et al. in which they conducted a retrospective analysis of 1061 cases in France and reported that HCQ+AZM combination therapy is beneficial and significantly lowers the mortality rate of COVID-19 [34].
In the current study, which was an open-label, blocked, randomised clinical trial, we found that patients who received AZM in addition to HCQ had a shorter duration of hospitalisation in comparison with the control group, with a medium effect size (P = 0.020, Hedges’ g = 0.618). In their multicentre, randomised, open-label, three-group, controlled trial, Calvalcanti et al. evaluated the safety and efficacy of HCQ and AZM in hospitalised patients with suspected or confirmed COVID-19 [35]. They reported that the duration of hospital stay was longer in patients who were treated with both HCQ and AZM compared with those who were only treated with HCQ, but this difference was non-significant [10.3 days vs. 9.6 days; odds ratio (OR) = 0.7, 95% CI –0.6 to 1.9] [35].
In our study, there were two patients in the case group (3.6%) and seven patients in the control group (12.7%) who required ICU admission, which does not show statistical significance. In their retrospective multicentre cohort study, Rosenberg et al. evaluated 1438 patients with a confirmed diagnosis of COVID-19. They reported a higher frequency of ICU admission in patients receiving HCQ and AZM (30.7%) or HCQ alone (19.2%) compared with those receiving only AZM (10.9%) [36].
In our study, there was one death in the control group and none in the case group; this difference was insignificant (P = 0.495). However, in their multicentre retrospective observational study in hospitalised patients positive for COVID-19, Arshad et al. reported that treatment with AZM alone significantly decreased the mortality hazard ratio by 66% and combination therapy with HCQ+AZM decreased the mortality hazard ratio by 71% [37]. They also performed a multivariate Cox regression model and found that combination therapy had no significant effect on the mortality rate [37]. Rosenberg et al. reported that the probability of death for patients who were receiving treatment with HCQ+AZM was 25.7% (95% CI 22.3–28.9%) [36]. They compared patients who were treated with HCQ, AZM and HCQ+AZM with patients who received no treatment and reported that there was no significant association between treatment with these medications—alone or in combination—and the in-hospital mortality rate [36]. However, the observational design of their study may limit definite interpretations of their findings. Despite the results of other studies that report an effective virucidal potency for the combination of HCQ and AZM [34,36,38], Molina et al. did not find any evidence to support the efficacy of this combination in viral clearance or improvement of clinical status of their patients [33].
Our study also showed that patients in the case treatment group who were treated with AZM in addition to the main treatment regimen had significantly higher SpO2 levels (P = 0.030) and a lower respiratory rate at the time of discharge (P = 0.010). The effect sizes showed the differences between two groups in these variables were considerable (Hedges’ g = –0.461 and 0.721, respectively). Calvalcanti et al. reported that patients receiving HCQ+AZM compared with patients who received AZM alone had a non-significant lower rate of need for oxygenation with a high-flow nasal cannula or non-invasive ventilation during treatment (9.3% vs. 10.7%; OR = 0.92, 95% CI 0.5–1.7) [35].
Possible side effects of a treatment are determining factors in evaluating the suitability of a medication regimen. CQ or HCQ as monotherapy have some common adverse effects such as pruritus, nausea and headache as well as some uncommon but serious adverse effects such as arrhythmias due to QT interval prolongation, hypoglycaemia, idiosyncratic hypersensitivity reactions and neuropsychiatric effects [39]. Prolongation of the QTc interval and torsade de pointes are the most important side effects of separate and, especially, concomitant treatment with HCQ and macrolides such as AZM that can negatively affect the survival rate [35,37]. Lane et al. evaluated the safety of HCQ alone and in combination with AZM. They studied 323 122 patients who were treated with this combination and concluded that a short-term treatment with HCQ is safe, but long-term treatment or addition of AZM to the treatment (even in the short-term) may increase the risk of heart failure or cardiovascular mortality rate, which can be caused by their synergetic effects on the QTc interval, leading to a lethal arrhythmia [40]. Considering the possible side effects of this combination therapy, clinicians should consider having a baseline QTc interval and monitoring of QTc interval, heart rate and serum electrolytes during administration of these drugs [38]. A scoring system to predict the risk of QT interval prolongation in hospitalised patients has been designed (Table 3 ). Scores of <7, 7–10 and ≥11, respectively, correlate with a low, medium and high risk of QT interval prolongation in hospitalised patients [23]. In our study, all patients had a risk score of <6, all patients were monitored during treatment and none of them experienced QTc interval prolongation, which would have warranted a halt in treatment with HCQ and AZM.
Table 3.
Calculation of risk score for QTc interval prolongation.
| Risk factor | Score |
|---|---|
| Age ≥68 years | 1 |
| Female sex | 1 |
| Loop diuretic | 1 |
| Serum K+ ≤3.5 mEq/L | 2 |
| Admission QTc ≥ 450 ms | 2 |
| Acute MI | 2 |
| ≥2 QTc-prolonging drugs | 3 |
| Sepsis | 3 |
| Heart failure | 3 |
| One QTc-prolonging drug | 3 |
| Maximum risk score | 21 |
K+, potassium; MI, myocardial infarction.
The small sample size and open-label design are limitations of our study. Because of the shortage in our resources, we could not test the viral loads of the patients at daily intervals.
5. Conclusion
Patients in the group receiving the experimental treatment regimen that included AZM had a significantly shorter hospital stay as well as significantly higher SpO2 and lower respiratory rate at discharge. However, a risk scoring system should be utilised before initiating treatment to prevent QTc prolongation, especially for high-risk patients.
Funding: This study was supported by the Tehran University of Medical Sciences research centre [grant no. 47493].
Competing interests: None declared.
Ethical approval: This project was approved by the Institutional Review Board of Tehran University of Medical Sciences [IR.TUMS.VCR.REC.1399.165].
References
- 1.Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadr S, SeyedAlinaghi S, Ghiasvand F, Hassan Nezhad M, Javadian N, Hossienzade R, et al. Isolated severe thrombocytopenia in a patient with COVID-19: a case report. IDCases. 2020;21:e00820. doi: 10.1016/j.idcr.2020.e00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behzad S, Aghaghazvini L, Radmard AR, Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson P, Rolain J-M, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377–1382. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 10.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okubo K, Isono M, Asano T, Sato A. Lopinavir–ritonavir combination induces endoplasmic reticulum stress and kills urological cancer cells. Anticancer Res. 2019;39:5891–5901. doi: 10.21873/anticanres.13793. [DOI] [PubMed] [Google Scholar]
- 12.Arabi YM, Alothman A, Balkhy HH, Al-Dawood A, AlJohani S, Al Harbi S, et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir–ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, Chan KH, Jiang Y, Kao RYT, Lu HT, Fan KW, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C-Y, Jan J-T, Ma S-H, Kuo C-J, Juan H-F, Cheng Y-SE, et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci U S A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosseboeuf E, Aubry M, Nhan T, Pina JJ, Rolain JM, Raoult D, et al. Azithromycin inhibits the replication of Zika virus. J Antivir Antiretrovir. 2018;10:6–11. [Google Scholar]
- 18.Sandeep S, McGregor K. Energetics based modeling of hydroxychloroquine and azithromycin binding to the SARS-CoV-2 spike (S) protein–ACE2 complex. ChemRxiv. 2020 Mar 24 doi: 10.26434/chemrxiv.12015792. [DOI] [Google Scholar]
- 19.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.004. 905–13.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sermo . April 2020. Sermo's COVID-19 real time barometer study.https://public-cdn.sermo.com/covid19/c7/cf1f/1fcad8/cad8234b3d9288a1ed3ae51afc/w4-sermo-covid-19-barometer.pdf [accessed 15. [Google Scholar]
- 21.Chorin E, Dai M, Shulman E, Wadhwani L, Bar-Cohen R, Barbhaiya C, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26:808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 22.Morgan ND, Patel S V, Dvorkina O. Suspected hydroxychloroquine-associated QT-interval prolongation in a patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:286–288. doi: 10.1097/RHU.0b013e31829d5e50. [DOI] [PubMed] [Google Scholar]
- 23.Simpson TF. Ventricular arrhythmia risk due to hydroxychloroquine–azithromycin treatment for COVID-19. Cardiology Magazine. 2020 https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 [accessed 29 March 2020] [Google Scholar]
- 24.Rabkin SW. Nomenclature, categorization and usage of formulae to adjust QT interval for heart rate. World J Cardiol. 2015;7:315. doi: 10.4330/wjc.v7.i6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 26.Choudhary R, Sharma AK. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. New Microbes New Infect. 2020;35 doi: 10.1016/j.nmni.2020.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautret P, Lagier J-C, Parola P, Meddeb L, Mailhe M, Doudier B, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Jean S-S, Lee P-I, Hsueh P-R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53:436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanin A, Calegari J, Beverina A, Tiraboschi S. Hydroxychloroquine and azithromycin as a treatment of COVID-19. Intern Emerg Med. 2020;23 doi: 10.1007/s11739-020-02388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulme OJ, Wagenmakers E-J, Damkier P, Madelung CF, Siebner HR, Helweg-Larsen J, et al. A Bayesian reanalysis of the effects of hydroxychloroquine and azithromycin on viral carriage in patients with COVID-19. MedRxiv. 2020 Apr 28 doi: 10.1101/2020.03.31.20048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negida A, Bahbah EI. 2020. Comments on the hydroxychloroquine/azithromycin study and lessons for future clinical trials of COVID-19 treatments. [DOI] [Google Scholar]
- 32.Dahly D, Gates S, Morris T. Statistical review of hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label nonrandomized clinical trial. Preprint posted online. 2020 Mar 23 doi: 10.5281/zenodo.3724167. [DOI] [Google Scholar]
- 33.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Million M, Lagier J-C, Gautret P, Colson P, Fournier P-E, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N Engl J Med. 2020 Jul 23 doi: 10.1056/nejmoa2019014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitra RL, Greenstein SA, Epstein LM. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: possible benefits of intravenous lidocaine. HeartRhythm Case Rep. 2020;6:244–248. doi: 10.1016/j.hrcr.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. Can Med Assoc J. 2020;192:E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane JCE, Weaver J, Kostka K, Duarte-Salles T, Abrahao MTF, Alghoul H, et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. MedRxiv. 2020 May 31 doi: 10.1101/2020.04.08.20054551. [DOI] [Google Scholar]