Abstract
Fibrous tissue and/or new bone are often found surrounding a cochlear implant in the cochlear scalae. This new intrascalar tissue could potentially limit cochlear implant function by increasing impedance and altering signaling pathways between the implant and the auditory nerve. In this study, we investigated the relationship between intrascalar tissue and 5 measures of implant function in guinea pigs. Variation in both spiral ganglion neuron (SGN) survival and intrascalar tissue was produced by implanting hearing ears, ears deafened with neomycin, and neomycin-deafened ears treated with a neurotrophin. We found significant effects of SGN density on 4 functional measures but adding intrascalar tissue level to the analysis did not explain more variation in any measure than was explained by SGN density alone. These results suggest that effects of intrascalar tissue on electrical hearing are relatively unimportant in comparison to degeneration of the auditory nerve, although additional studies in human implant recipients are still needed to assess the effects of this tissue on complex hearing tasks like speech perception. The results also suggest that efforts to minimize the trauma that aggravates both tissue development and SGN loss could be beneficial.
Keywords: auditory prosthesis, fibrosis, spiral ganglion neurons, psychophysical thresholds, electrically evoked compound action potentials
INTRODUCTION
Many recipients of cochlear implants (CIs) demonstrate remarkable recovery of hearing, but others do not (Blamey et al. 1992; Moberly et al. 2016). Patients also exhibit differences in hearing between stimulation sites (electrodes) within ears (Zwolan et al. 1997; Garadat et al. 2012; Schvartz-Leyzac and Pfingst 2016). Because the purpose of the CI is to stimulate the auditory nerve (Clark et al. 1984; Hochmair and Hochmair-Desoyer 1981), variation in survival of spiral ganglion neurons (SGNs) has been proposed to explain variation in patients’ hearing with a CI (Parkins 1985; Blamey et al. 1992). Some studies have supported this hypothesis (Zhou and Pfingst 2014; Seyyedi et al. 2014; Kamakura and Nadol 2016; Pfingst et al. 2017; Schvartz-Leyzac and Pfingst 2018) but others have not (Khan et al. 2005; Fayad and Linthicum 2006). Furthermore, the studies showing that variation in SGN density explains some of the variation in CI function also show that much of the variation in function remains unexplained (Kamakura and Nadol 2016; Pfingst et al. 2011, 2017). Other factors proposed to contribute to the unexplained variability in CI function include patient age and cognitive function at implantation, duration of deafness prior to implantation, etiology of hearing loss, residual hearing and formation of fibrotic tissue and bone in the cochlea after implantation (Blamey et al. 1992; Choi and Oghalai 2005; Green et al. 2007; Heydebrand et al. 2007; Budenz et al. 2011; Holden et al. 2013).
In this study, we focused on the fibrotic tissue and bone (intrascalar tissue) that forms in the cochlea and can fill the space between implant and nerve. This tissue is a common finding in human CI recipients (Eshraghi et al. 2003; Seyyedi and Nadol 2014), where it can be an impediment to reimplantation after device failure (Côté et al. 2007; Reis et al. 2017); it is also a common finding in animal studies (Shepherd et al. 1983; Honeder et al. 2015; Ryu et al. 2015). The formation of this tissue appears to be triggered by trauma incurred during implant surgery, which leads to activation of pro-inflammatory cascades (Bas et al. 2012a, 2012b; Eshraghi et al. 2013; Kel et al. 2013; Zhang et al. 2015). The fibrous tissue that forms in the cochlea is similar to that seen during wound healing in other tissues (Kel et al. 2013; Zhang et al. 2015), but is not restricted to the site of injury and may grow to fill the fluid spaces of the inner ear and even be replaced with bone (Li et al. 2007; Fayad et al. 2009). The deleterious effect of this tissue on residual acoustic hearing has been studied extensively (Choi and Oghalai 2005; O'Leary et al. 2013; Burghard et al. 2014; Yamahara et al. 2018).
Intrascalar tissue is expected to affect electrical hearing with a CI because the proteins and mineral that comprise the tissue have intrinsically higher impedances than the perilymph it replaces (Geddes and Baker 1967; Gabriel et al. 1996). Still, it is not clear that intrascalar tissue necessarily affects CI function. Some studies have found a correlation between intrascalar tissue and changes in impedance (Richardson et al. 2009; Wilk et al. 2016), but others reported no correlation between intrascalar tissue and CI function (Honeder et al. 2015; Ishai et al. 2017). One complication hindering studies of this relationship is that SGN density also may be reduced by the immune response to trauma (Ryan et al. 2002; Fayad et al. 2009; Souter et al. 2012; Bas et al. 2015); which could produce a correlation between intrascalar tissue and CI function even if intrascalar tissue does not interfere with communication between the implant and the neurons. Another complication is that the position of the implant within the inner ear can affect electrical hearing (Shepherd et al. 1993; Schvartz-Leyzac et al. 2020), so differences in function could reflect differences in the original position or changes in position due to tissue growth or other factors. In addition, there may be changes in SGN physiology that are not reflected in the number or morphology of cell bodies in Rosenthal’s canal (Ramekers et al. 2014; Fransson et al. 2015; Jahn and Arenberg 2019). Those changes could account for variation in CI function that is independent of SGN number or density but is correlated with intrascalar tissue, providing a link between intrascalar tissue and CI function that is independent of SGN density and impedance.
The aim of this study was to test whether the fraction of variation in CI function that is independent of SGN function is correlated with intrascalar tissue. Guinea pigs were treated to produce variation in both SGN survival and intrascalar tissue by implanting hearing ears, ears deafened with neomycin, and neomycin-deafened ears treated with a neurotrophin. Implant function was measured over several months, then SGN density was quantified and intrascalar tissue was categorized. Treatment groups exhibited similar ranges of intrascalar tissue levels, and within treatment groups, different intrascalar tissue levels spanned similar broad ranges of SGN density, permitting tests for independent effects of these factors on CI function. SGN density was significantly correlated with 2 of 3 psychophysical measures and 2 electrophysiological measures. Intrascalar tissue level did not have a significant effect on variation that was left unexplained by SGN variation. However, these results provide further motivation to minimize surgical trauma and explore other methods of reducing the activity of the immune system to reduce both intrascalar tissue growth and SGN loss.
METHODS
Overview
We used data from 52 guinea pigs in this study; all were specific pathogen-free, pigmented males bred and maintained by the Unit for Laboratory Animal Medicine at the University of Michigan. The animal-use protocol was reviewed and approved by the Institutional Animal Care and Use Committee. After training and implantation, these animals were used in multiple psychophysical and electrophysiological studies. For most of the animals, a previous report compared psychophysical and electrophysiological functional data to spiral ganglion neuron (SGN) survival (Pfingst et al. 2017). In the current study, these and additional psychophysical and electrophysiological data are compared to the density of fibrous and bone tissue near the stimulating electrode in the scala tympani. In choosing the psychophysical and electrophysiological measures to examine, we selected some measures shown in a previous study to be significantly correlated with SGN density (Pfingst et al. 2017) and some related measures that were not included in that study. Details of the training, treatment, and testing procedures were presented in previous publications (Kang et al. 2010; Pfingst et al. 2017) and are summarized below.
Prior to deafening or any other cochlear treatments, guinea pigs were trained using a positive-reinforcement, operant conditioning paradigm to perform a stimulus-detection task. They were trained to report detection of pure-tone stimuli between 50 Hz and 24 kHz presented in a sound-attenuating booth while harnessed facing a food dispenser and water spout and they were tested under the same conditions. After stable acoustic behavioral thresholds were obtained, each animal was deafened with neomycin in one ear to facilitate free-field acoustic testing of the experimentally treated ear, which would receive a cochlear implant. Animals were then assigned to one of 3 groups differing in treatment of the implanted ear (described below). After implant surgery, animals were tested over a period of 4 to 21 months using electrical stimuli delivered to a primary test electrode on the implant to obtain the electrophysiological and psychophysical data. Because it can take 120 days or more for psychophysical and electrophysiological data to stabilize after implant insertion and other treatments (Pfingst et al. 2015), only data obtained after stabilization are reported in this study. When the electrical testing was completed, the animals were euthanized and the implanted ear was prepared for histological examination. Survival times from implantation to euthanasia varied for several reasons, including differences in (A) time to stabilization, (B) animals’ work habits, and (C) the number of experiments in which animals participated. One animal was euthanized earlier due to health issues.
Treatment Groups
The 52 guinea pigs included in this study were divided into 3 treatment groups. The objective of these various treatments was to obtain a population of animals with a wide range of cochlear health (nerve survival, hair cell survival, fibrosis, etc.). Also, there was variability across animals in response to each treatment type, which further contributed to the goal of obtaining a large range of cochlear health across subjects. The three treatment groups were as follows. (1) An Implanted-Hearing (IH) group, N = 21, received an implant in a normal-hearing ear that received no other treatment. All of the IH animals had some surviving inner hair cells and some residual acoustic hearing at multiple frequencies post-implantation. (2) A Neomycin + Implant (NI) group, N = 12, was composed of 9 animals that were given a cochlear injection of 5 % neomycin in the treatment ear prior to the implant and 3 that received both neomycin and an adeno-associated virus vector lacking a gene insert (AAV.empty) before the implant. These ears were treated as a single group because a previous study found that implanted ears treated with neomycin and those treated with both neomycin and AAV.empty were not significantly different in SGN density (Pfingst et al. 2017). None of the NI animals had measurable acoustic hearing. (3) A Neomycin + Neurotrophin + Implant (NNI) group, N = 19, was given neomycin and an AAV vector with a neurotrophin gene insert (neurotrophin 3—AAV. Ntf3, N = 17, or brain-derived neurotrophic factor—AAV. BDNF, N = 2) prior to implantation. The AAV vectors were serotype 2, which infects cells in the membranous labyrinth, both in the area of the deafened auditory epithelium and in other areas including the lateral wall marginal cells (Pfingst et al. 2017, appendix). Three of the NNI group animals had surviving hair cells and residual hearing, which could be due to incomplete deafening or preservation of hair cells by the neurotrophin (suggested by gene transfer of GDNF—Suzuki et al. 2000; Kawamoto et al. 2003; Liu et al. 2008). Several studies suggest neurotrophin treatment also may have improved survival of synapses on the few surviving inner hair cells (Wang and Green 2011; Sly et al. 2016; Suzuki et al. 2016), including studies using AAV vectors (Chen et al. 2018; Hashimoto et al. 2019). The other 16 NNI animals with no surviving hair cells also had no measurable acoustic hearing.
Cochlear Implants
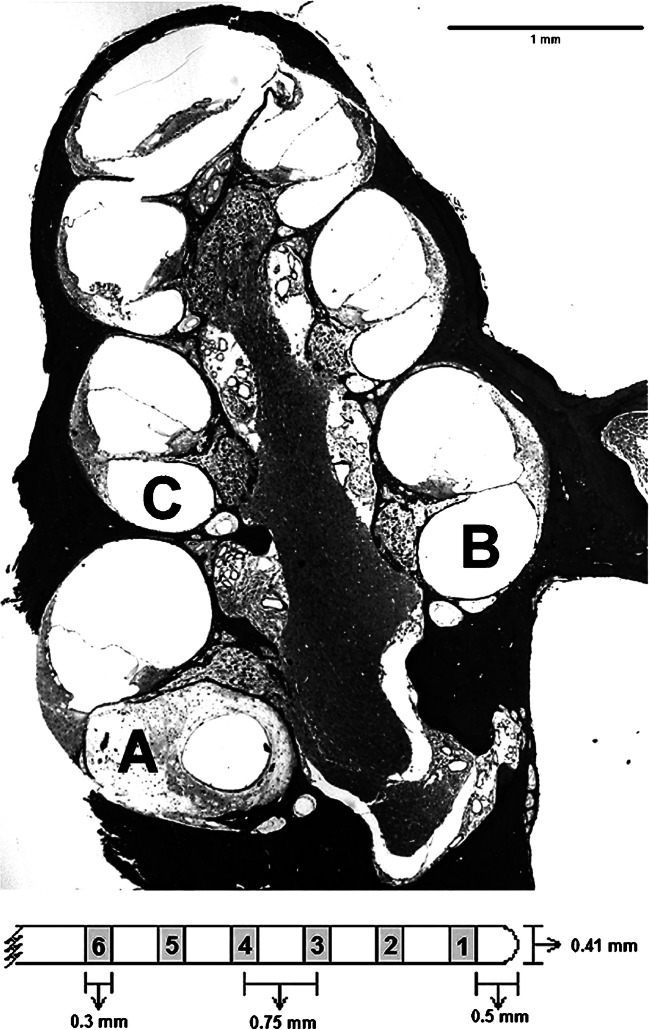
All animals were implanted with banded cochlear implants (purchased from Cochlear Corporation, Englewood, CO). Figure 1 (top portion) illustrates the implant location in the scala tympani and the profiles of interest inside the cochlea that were used for SGN density and tissue density analysis. Each implant consisted of 8 band-type electrodes surrounding a silicone rubber carrier, with dimensions as illustrated in the bottom of Fig. 1 and a ground ball (~ 1 mm) tucked into the muscle dorsal to the bulla. The primary stimulation site for the psychophysical and electrophysiological measures was the second most apical electrode (Electrode #2). In the 2 ears where Electrode #2 failed, Electrode #3 was used, with no noticeable change in the data. Stimulation was monopolar and went from the primary electrode to a remote ground. For psychophysical measures, current went from the primary electrode to the ground ball. For electrophysiological measures, current went from the primary electrode to the “anchor bolt” (described below) because that configuration gave stronger electrophysiological responses compared to using the ground ball. Electrophysiological recordings were made from an electrode adjacent to the primary electrode (either apical or basal) ground to a screw on the vertex.
Fig. 1.
Mid-modiolar section of a guinea pig cochlea (micrograph) illustrates the implant location in the scala tympani (profile A) and the profiles of interest inside the cochlea that were used for SGN density and tissue density analysis (profiles A, B, and C in bold). Also shown is a schematic of an eight-electrode scala tympani cochlear implant, (below the micrograph); only the first 6 potentially intracochlear electrodes are shown
Deafening, Inoculation, and Implantation Procedures
All surgical procedures were performed by the same surgeon. The procedures have been described previously (Pfingst et al. 2017) and are briefly summarized, below. For all surgical procedures, guinea pigs were anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg), placed on a heating pad and prepared for surgery.
All animals received neomycin in the ear that was not to be implanted. The cochlea was accessed through a post-auricular incision, and neomycin solution (60 μl of 10 % solution (w/v) or 10 μl of 5 % solution) was injected by syringe through the round window. This procedure was performed after training was completed and before any procedures were performed on the ear that would be implanted.
There were 3 main protocols for the implanted ear; in the simplest protocol, the animal received only the implant and hardware for mounting electrical connectors. An area of the skull surrounding bregma was exposed and a small bolt was anchored to the skull (specified as the “anchor bolt”) for mounting the connector that interfaced with a stimulator/recorder (described below) and as a reference for the primary electrode stimulated during ECAP measures. An additional screw was placed at the vertex for the ECAP recording ground. Then, the cochlea was accessed through a post-auricular incision and a cochleostomy was performed. An eight-electrode scala tympani implant was inserted 4 to 5 mm resulting in 5 to 6 intracochlear electrodes. The implant was secured in place with a silk suture and Durelon at the bulla; the ground ball tucked into the muscle dorsal to the bulla; and the incision was closed.
In the second protocol, the 9 NI animals that did not also receive AAV.empty were given neomycin in the ear to be implanted, using the same procedure that was used for the contralateral ears. Eight ears received 60 μl of 10 % neomycin and 1 received 10 μl of 5 % neomycin. After neomycin injection, the animal underwent surgery for implant insertion and attachment of associated hardware as described above. Differences in neomycin amount and concentration in the NI group stem from animals being combined from multiple studies with varying treatment paradigms. Regardless of the different dosages, neomycin treatment resulted in severe depletion of SGNs, which was the goal for this group.
In the third protocol, for the experimentally treated ear of all 21 animals that received neomycin and an AAV vector before the implant (3 NI animals and all 19 NNI animals), a small cochleostomy sufficient to admit the inoculation cannula was performed and 10 μl of 5 % neomycin solution was delivered through that opening into the scala tympani. After a 20-min wait time, the cannula was removed and a drop of HEALON (hyaluronic acid; 10 mg/mL; Abbott Medical Optics, Inc., Santa Ana, CA) was placed on the cochleostomy to promote penetration of administered substances through membranes. A second cannula was inserted and 5 μl of the vector was infused into the scala tympani. When the infusion was complete, the cannula was removed and either the cochleostomy was temporarily covered with a piece of muscle and left alone for 30 min before proceeding with the implantation or the cochleostomy and the bulla were sealed with Durelon and left for 2 weeks before implantation. Twelve animals were deafened, inoculated, and then implanted 30 min after AAV inoculation; 10 animals were deafened, inoculated, and then implanted 2 weeks later in a second surgery. The use of the 2-week delay between inoculation and implantation was based on a concern that placing a cochlear implant in the cochlea shortly after inoculation might reduce the effectiveness of the inoculation; however, SGN densities were not significantly different (t(15) = 0.941, p = 0.362) and all NNI animals were treated as a single group. After the waiting period, the animal underwent surgery for implant insertion and attachment of associated hardware as described above, except that the previous cochleostomy was exposed and enlarged to accommodate the implant.
Functional Measures
Five measures of CI performance were evaluated on a standardized schedule, described previously (Pfingst et al. 2017); all data reported here were collected after electrical detection thresholds were considered stable according to previously established criteria (Pfingst et al. 2017) until termination. Some measures were obtained for only a subset of animals due to scheduling constraints; the numbers of animals in each treatment group tested on each measure are listed in Table 1. All stimulation and all electrophysiological recordings used monopolar electrode configurations. For animals with residual hearing, measurement of acoustic thresholds alternated with the electrical threshold testing.
Table 1.
Numbers of animals in each treatment group tested for each implant function measure
| Measure | Treatment group | Total | ||
|---|---|---|---|---|
| IH | NI | NNI | ||
| Psychophysical | ||||
| MPI slope (156 to 625 pps) | 21 | 12 | 19 | 52 |
| Threshold for pulse train (156 pps) | 21 | 12 | 19 | 52 |
| Threshold for single pulse (25 μs/phase biphasic) | 11 | 9 | 19 | 39 |
| Electrophysiological | ||||
| ECAP AGF slope (45 μs pulse with 2.1 μs IPG) | 6 | 4 | 18 | 28 |
| IPG Effect (2.1 μs vs. 30 μs IPG) | 6 | 2 | 16 | 24 |
Three psychophysical measures were analyzed in this study: (1) multipulse integration (MPI) slope (the change in thresholds in dB per doubling of pulse rate); (2) thresholds for 200 ms trains of 25 μs/ph biphasic pulses at 156 pps; and (3) thresholds for single 25 μs/ph biphasic pulses. Psychophysical thresholds used for comparison to histological measures were the average of three to five thresholds for each condition. MPI slope was determined by fitting a linear function to the threshold vs. pulse rate data. Stimuli were 200 ms trains of 25 μs/ph biphasic pulses with pulse rates ranging from 156 to 625 pps in steps of doubling (31 to 125 pulses/200 ms stimulus).
The two electrophysiological measures used in this study were: (1) electrically evoked compound action potential (ECAP) amplitude growth functions (AGFs) (ECAP amplitude vs. stimulus level); and (2) the IPG Effect (effect of interphase gap (IPG) on the ECAP AGF slope). For ECAP AGF slope recordings, a biphasic pulse with a 45-μs phase duration and 2.1 μs or 3.0 μs interphase gap (IPG) presented at 50 pps for 20 iterations. The IPG Effect was calculated as the difference between AGF slopes obtained using the 2.1 μs and 30 μs IPG durations.
ECAPs were recorded in awake guinea pigs while the animals were standing in the test cage. Electrophysiological stimulation and recording utilized a MED-EL “Pulsar” CI100 receiver/stimulator connected to the implant through a percutaneous electrical connector, and to a standard PC via a Research Interface Box (RIB II; University of Innsbruck). Measurement parameters were controlled using custom software. For each session, the maximum stimulus level (MSL) was determined using criteria described previously (Pfingst et al. 2017), and responses were obtained for a set of 15 stimulus levels evenly spaced from zero to the MSL. This series of responses was used to compute AGFs as described previously (Pfingst et al. 2017). In brief, the N1 and P2 peaks of the response waveform were identified with the aid of a custom-made Matlab program, and the difference between their magnitudes (in μV) were plotted against stimulus current (in μA). Then, the linear portion of the AGF from 100 μV to the MSL was fit by regression. All recordings obtained during the stable period were used to compute the average AGF slope during that period.
Histological Analyses
Animals were anesthetized and perfused transcardially with either 4 % paraformaldehyde or 2 % glutaraldehyde with 0.15 M cacodylate buffer as described previously (Pfingst et al. 2017). Temporal bones were removed and decalcified for 3 to 6 months. Once the implant was visible through the decalcified bone, a mark was made in the lateral wall to indicate the position of the primary stimulating electrode and the implant was then removed. When decalcification was complete, specimens were embedded in JB-4 and 3-μm sections were cut starting just basal to the labeled mark. Approximately 45 sections were collected per cochlea and histological analysis began with the section closest to the mark indicating the position of the primary stimulating electrode. Four other sections were chosen consecutively at least 6 μm apart to avoid counting a cell twice. If a section was unusable due preparation artifacts (including breaks or tears in Rosenthal’s canal), the next usable section was evaluated. Sections were stained with toluidine blue and observed with a Leica DMRB microscope (Leica, Eaton, PA, USA) and photographed with a CCD Cooled SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA). These photographs, which spanned at least 39 μm, were used to determine SGN density and the type and level of intrascalar tissue near the primary stimulating electrode.
For each selected slide, the cochlear profile occupied by the primary stimulating electrode (usually the most basal profile that was visible) was designated profile A, and the next two more apical profiles were designated B and C (Fig. 1). For each of those three profiles, SGNs in Rosenthal’s canal were counted and the cross-sectional area of the canal was measured using ImageJ (U. S. National Institutes of Health, Bethesda, Maryland, USA). Only cells with diameters between 12 and 25 μm and a visible nuclear envelope of 5 to 9 μm in diameter were counted. Cells showing signs of severe pathology (shrunken or irregularly shaped soma) were not counted. SGN density was calculated as the sum of the number of cells counted divided by the sum of the areas of those profiles. The region of interest for SGN density calculations depended on the nature of the functional measure. For analyses of psychophysical detection threshold measures, only profile A SGN densities (nearest the stimulating electrode) were used because the current spread for stimuli at threshold levels was assumed to be relatively small. For analyses of supra-threshold (electrophysiological) measures, in which higher stimulus currents were used and current spread was assumed to be greater, average SGN densities of profiles A, B, and C were computed.
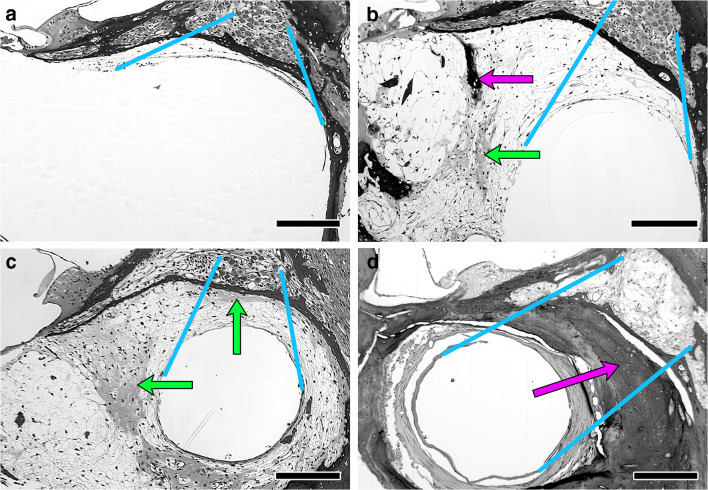
To test whether the observed variation in intrascalar tissue was affected by treatment, the tissue in scala tympani of profile A was scored on a 3-level scale (Low, Medium, or High). These scores were also used in statistical analyses of the correlations of the intrascalar tissue level with other histological measures and with the measures of CI function. The assignment of scores was based on evaluation of a region of interest defined as the area between the wall of Rosenthal’s canal and the open space within which the implant would have been located, where the presence of intrascalar tissue was expected to have the greatest effect on current flow between the implant and the spiral ganglion (Fig. 2). When the intrascalar tissue was limited to a thin layer along the scala tympani wall, the implant’s position could not be determined. In these cases, the region of interest was considered to be the area of intrascalar tissue immediately adjacent to Rosenthal’s canal (Fig. 2a). When the proportion of the scala tympani occupied by intrascalar tissue was larger, the region of interest was defined by projection of Rosenthal’s canal onto the open space that could have been occupied by the implant (Fig. 2b–d). In this study, ears had either less than 25 μm or more than 60 μm of intrascalar tissue in the region of interest. No bone or cartilage was observed in the region of interest when the intrascalar tissue in that region was less than 25 μm thick. Therefore, “Low” was defined as having less than 25 μm of tissue in the region of interest (e.g., Fig. 2a; N = 21). This included 2 ears with no visible intrascalar tissue anywhere in scala tympani. “Medium” was defined as having more intrascalar tissue in the region of interest than in the Low group but no bone or cartilage in that area (e.g., Fig. 2b; N = 12). “High” was defined as having more intrascalar tissue in the region of interest than in the Low group and having any amount of bone or cartilage in the region of interest (Fig. 2c, d; N = 19). Ears scored as High ranged from those with only a thin lens of cartilage in a fibrous mesh (e.g., Fig. 2c) to those with bone nearly filling the area around the implant (e.g., Fig. 2d). Of the 19 ears with the High tissue level classification, 3 had only cartilage (no bone) in the region of interest, 9 had a mixture of tissue types, and 7 had all bone.
Fig. 2.
Variability of intrascalar tissue found in implanted guinea pigs. a Minimal tissue. b More extensive tissue with patches of bone (dark stain and laminated) and cartilage (lighter and unlaminated). c Similar to b, but with cartilage between the implant and the spiral ganglion. d Implant completely surrounded by bone that fills most of the scala. Blue lines illustrate the criteria used to delineate the regions of interest for scoring the intrascalar tissue (the tissue potentially between the SGN cell bodies in Rosenthal’s canal and the implant), connecting the edges of the area occupied by the SGN to the edges of the open space in scala tympani. Arrows indicate patches of cartilage (green) and bone (magenta) in the intrascalar tissue. Bar = 200 μm. Panel a is an example of a cochlea classified as Low intrascalar tissue, b is an example of Medium intrascalar tissue, and c and d are examples of High intrascalar tissue. See text for further details
Statistical Analyses
All the following analyses were performed in R version 3.5.1 (R Core Team 2018). To test for associations between intrascalar tissue level and treatment (two categorical variables), contingency tables were constructed with the numbers of ears having each possible combination of intrascalar tissue level and treatment group. Then, Pearson’s χ2 test was performed using function chisq.test to determine whether treatment had a significant effect on the intrascalar tissue level. This analysis was performed first with all three treatment groups to test for an overall effect of treatment, then with each pair of treatments to determine which pairs were significantly different. To avoid type I errors when judging statistical significance of the post hoc pairwise tests, we used the table-wide Bonferroni criterion (p = 0.05 divided by the number of tests).
Effects of intrascalar tissue level and treatment on SGN density were evaluated by ANOVA, using the function aov, which computes sequential (type I) sums of squares to determine the variation attributable to a factor. Separate analyses were performed on the density in profile A and the average of the densities in profiles A–C because profile A (which was closest to the stimulating electrode) was used in analyses of psychophysical measures and the average of densities in profiles A–C (the 3 most basal profiles) was used in analyses of electrophysiological measures (as explained above). These analyses were performed first with all three treatment groups to test for an overall effect of treatment, then with each pair of treatments to determine which pairs were significantly different, using the table-wide Bonferroni criterion to judge significance in the post hoc pairwise tests.
ANOVA was performed to test for significant effects of the three proposed factors, one continuous (SGN density) and two categorical (level of intrascalar tissue development and treatment groups). We also tested for significant effects of all possible interactions of those factors on each of the functional measures. Distance from the implant to neural tissue could not be determined from the histological specimens of most animals, including all animals with intrascalar tissue classified as Low; therefore, it was not included as a factor. For psychophysical measures, SGN density in profile A, only, was used; for electrophysiological measures, the average SGN density in profiles A–C was used. When the initial analysis demonstrated that none of the interaction effects was significant, a second test was performed using a reduced statistical model that omitted the interaction terms, providing better estimates of the effects of the factors. The continuous factor, SGN density, was the first term evaluated in each model, so the results of the other tests in the ANOVA represent the effects of the factors on the variation that was not explained by correlation with SGN density. To provide a visual reference for interpreting these statistical results, a linear regression of each functional measure on SGN density was computed using the lm function, which yields the same proportion of variation explained and the same p value for the significance of the continuous factor as the ANOVA. That regression line was graphed on a scatterplot of the data, with symbols coded to indicate the treatment group and intrascalar tissue level, thereby illustrating the deviations from the expected values for members of the various subgroups.
RESULTS
Occurrence of Intrascalar Tissue
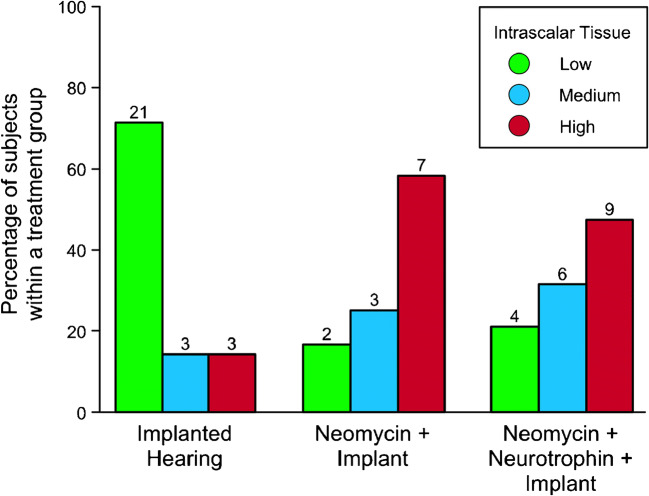
The proportion of ears with Medium or High levels of intrascalar tissue was markedly lower in the IH group than in the NI and NNI groups, which had received neomycin prior to implantation (Fig. 3). A contingency table test performed on the 3 groups demonstrated that there were significant differences among groups in the proportions of ears in the three intrascalar tissue levels (χ2(4) = 14.8, p = 0.005). Post-hoc pairwise tests demonstrated that the IH group, with 71 % of ears at the Low level, was significantly different from the NI and NNI groups, with < 22 % at that level (χ2(2) > 9, p < 0.008). The two neomycin treated groups were not significantly different from each other (χ2(2) = 0.35, p = 0.84); however, the NNI group, which also received a neurotrophin treatment, had a smaller proportion of ears with the High level of intrascalar tissue (47 % vs. 58 %) and slightly larger proportions of ears with Medium and Low levels than the NI group. Thus, neomycin-treated ears tended to have more extensive development of intrascalar tissue than those that received only an implant. Adding a neurotrophin treatment as well as the neomycin did not induce an additional increase in the likelihood of reaching at least the Medium level of intrascalar tissue and may have slightly reduced the likelihood of reaching the High level. Within the NNI group, timing of neurotrophin treatment also did not have a meaningful effect on the level of fibrosis (e.g., the proportion of animals with intrascalar tissue scored as High was 40 % in the delayed group vs. 42 % in the simultaneous group).
Fig. 3.
Occurrence of intrascalar tissue differed between treatment groups. Bars indicate the percentage of subjects in each treatment group with the indicated level of intrascalar tissue in the treated ear. Numbers above the bars indicate the number of subjects in each treatment group with each level of intrascalar tissue
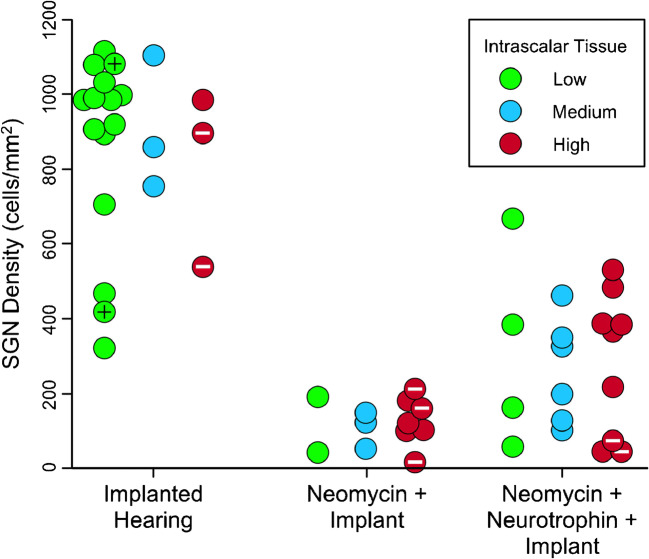
SGN density in profile A was highest in the IH treatment group, whether all intrascalar tissue levels were combined or each level was analyzed separately (Fig. 4). The NI group had the lowest average SGN densities (whether intrascalar tissue levels were separated or combined), and the NNI group had SGN densities that were intermediate between the IH and NI groups. Quantitative analysis confirmed that there was a strong effect of treatment on SGN density in profile A (F(2,43) = 64.99, p < 0.001), and also that there was not a significant effect of intrascalar tissue level on SGN density in profile A, either independently (F(2,43) = 0.033, p = 0.967) or via interaction with treatment (F(4,43) = 0.127, p = 0.972). Post hoc pairwise tests indicated that even the smallest of the differences between groups (120.5 cells/mm2 for NI vs. 282.6 for NNI) was very significantly different (F(1,29) = 8.5, p = 0.007) with a p value well below the table-wide Bonferroni criterion for significance (p = 0.017).
Fig. 4.
Within treatment groups, intrascalar tissue development was independent of SGN density. Ears within each treatment group are sorted and color-coded to indicate both SGN density and intrascalar tissue category. Special cases of intrascalar tissue are indicated by marks inside the symbols: black + = no visible intrascalar tissue, white – = bone surrounding the implant filled the remaining space in the scala tympani
Results for the effects of intrascalar tissue level and treatment group on the average SGN density in profiles A through C were comparable to those obtained for profile A alone. There was a strong effect of treatment on the average SGN density across the 3 profiles (F(2,43) = 121.4, p < 0.001), and there was not a significant effect of intrascalar tissue level on the average SGN density, either independently (F(2,43) = 0.483, p = 0.620) or via interaction with treatment (F(4,43) = 0.234, p = 0.918). In addition, NI and NNI groups differed least in average SGN density across profiles A–C (105.6 vs. 309.8 cells/mm2), but were still very significantly different (F(1,29) = 10.35, p = 0.003) with a p value below the table-wide Bonferroni criterion for significance.
These results demonstrate that reduction of SGN density was associated with neomycin treatment and that neurotrophin treatment moderated that reduction. In addition, differences in the level of intrascalar tissue were not associated with differences in the response of SGN density to these treatments. Neomycin and neurotrophin may affect both SGN density and intrascalar tissue level, but the response of SGN density to those treatments was not correlated with the observed differences in intrascalar tissue level.
Relationships of Intrascalar Tissue to Psychophysical Measures
MPI slope was negatively correlated with SGN density (t(50) = 5.77, p < 0.001, Fig. 5a), indicating the negative slopes were steeper when SGN density was higher. The correlation accounted for 40 % of the variation in MPI slope. Individuals lacking intrascalar tissue had MPI slopes in the same range as other animals with similar SGN density. Likewise, individuals with bone-filled scala tympani also had MPI slopes in the same range as other animals with similar SGN density. Animals in the IH group that had Low level of intrascalar tissue and high SGN density spanned a much larger range of variation for MPI slope than any other subgroup in this study, and a large proportion of animals in this subgroup had steeper MPI slopes than were seen in any other subgroup; however, ANOVA indicated that treatment group did not account for a significant proportion of the variation left unexplained by SGN density (F(2,46) = 0.07, p = 0.932) and neither did the intrascalar tissue level (F(2,46) = 1.37, p = 0.265).
Fig. 5.
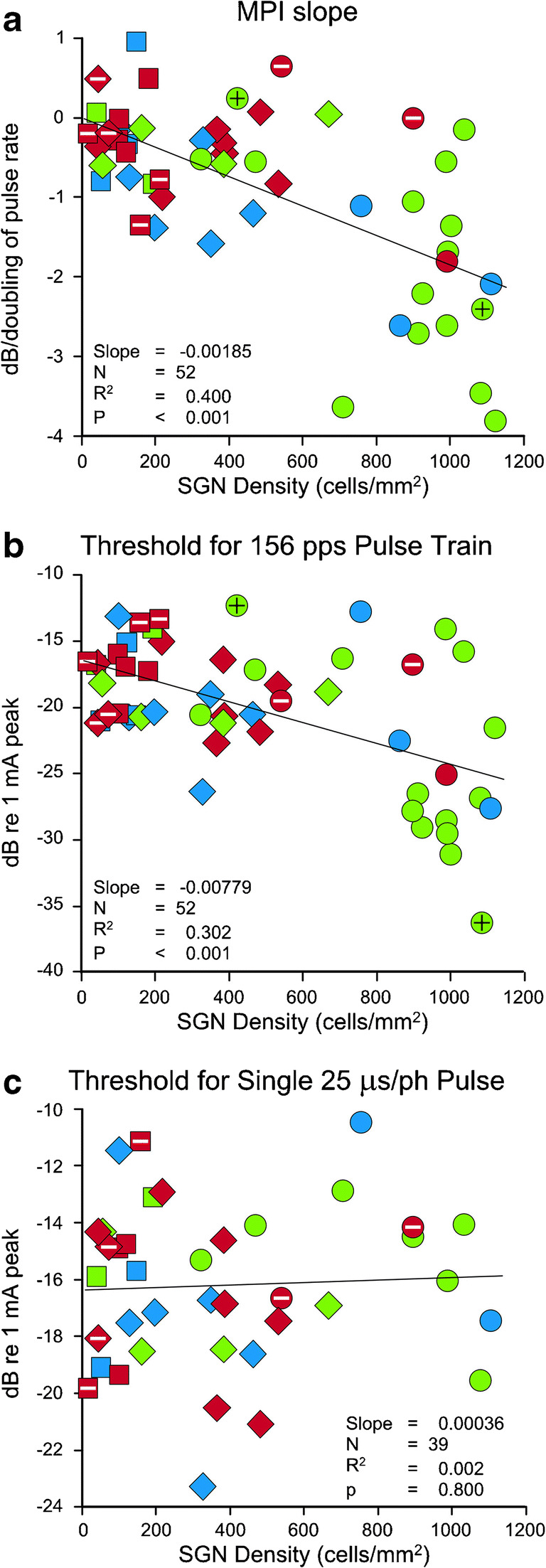
Relationships between psychophysical measures and SGN density in profile A. Statistics are for regression of the functional measure on SGN density using all individuals. Treatment group is indicated by symbol shape (circle = IH, square = NI, diamond = NNI) and intrascalar tissue level is indicated by color (red = High, blue = Medium, green = Low). Special cases of intrascalar tissue are indicated by marks inside symbols: black + = no visible tissue, white – = bone surrounding the implant filled the remaining space in the scala tympani
Thresholds for 156-pps pulse trains also were negatively correlated with SGN density (t(50) = 4.65, p < 0.001, Fig. 5b), indicating the stimulus level needed to elicit a response decreased as SGN density increased. The correlation accounted for 30 % of the variation in these thresholds. As with MPI slopes, individuals with a bone-filled scala tympani had a broad range of thresholds similar to all NI and NNI animals, combined. One of the two animals with no tissue in scala tympani had the highest threshold for 156-pps pulse trains in this study, the other had the lowest. Again, animals in the IH group that had Low level intrascalar tissue and high SGN density spanned a much larger range of variation than any other subgroup in this study. A large proportion of animals in this subgroup also had lower thresholds than were seen in any other subgroup. ANOVA indicated that the treatment group did not account for a significant proportion of the variation left unexplained by SGN density (F(2,46) = 0.88, p = 0.421) and neither did the intrascalar tissue level (F(2,46) = 0.37, p = 0.692).
Thresholds for single 25-μs pulses were not significantly correlated with SGN density (t(37) = 0.26, p < 0.800, Fig. 5c); thus, the stimulus level needed to elicit a response did not change as SGN density increased. Again, individuals with bone-filled scala tympani had a broad range of thresholds similar to all NI and NNI animals, combined. There also were no significant effects of treatment group (F(2,33) = 3.05, p = 0.061) or intrascalar tissue levels (F(2,33) = 0.07, p = 0.931) on differences in thresholds for a single, short-duration pulse.
Relationships of Intrascalar Tissue to Electrophysiological Measures
ECAP AGF slopes were significantly correlated with SGN density (t(26) = 5.39, p < 0.001, Fig. 6a), indicating slopes increased as SGN density increased. The correlation accounted for 53 % of the variation, but variability of this measure may be underrepresented because the sample size for this data set was only 28 and many subgroups had 2 or fewer individuals. The small subgroup samples meant that some interaction effects could not be tested; but those that could be tested were not significant (p > 0.24). The available data also did not demonstrate significant effects of treatment group (F(2,22) = 0.11, p = 0.893) or intrascalar tissue levels (F(2,22) = 0.13, p = 0.877) on ECAP AGF slopes.
Fig. 6.
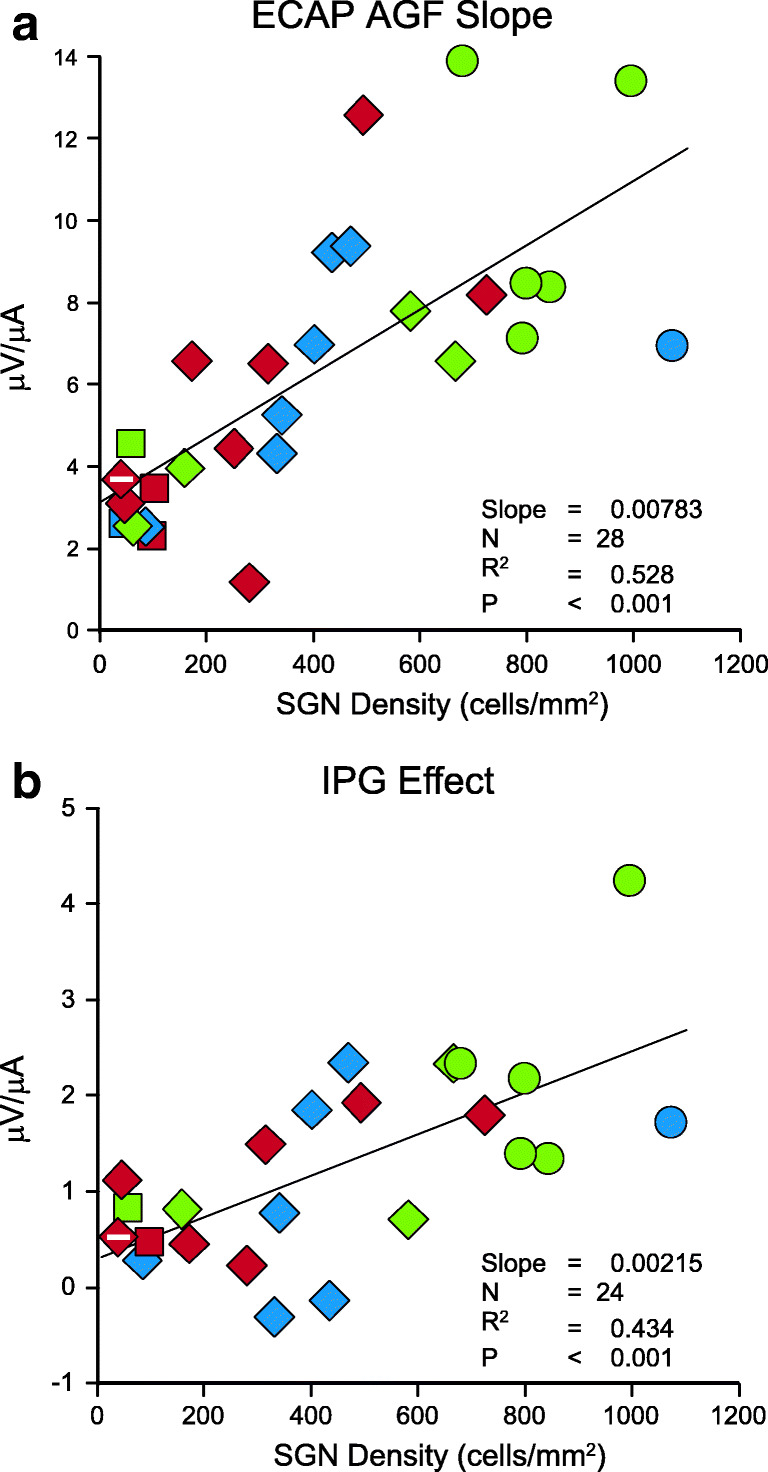
Relationships between electrophysiological measures and average SGN density in profiles A–C. Statistics are for regression of the functional measure on SGN density using all individuals. Treatment group is indicated by symbol shape (circle = IH, square = NI, diamond = NNI) and intrascalar tissue level is indicated by color (red = High, blue = Medium, green = Low). Special cases of intrascalar tissue are indicated by marks inside symbols: black + = no visible tissue, white – = bone surrounding the implant filled the remaining space in the scala tympani
The IPG Effect on ECAP AGF slopes was significantly correlated with SGN density (t(22) = 4.11, p < 0.001, Fig. 6b). Thus, the IPG Effect increased as SGN density increased, meaning the increase in ECAP AGF slope due to the larger IPG was greater when SGN density increased. The correlation accounted for 43 % of the variation in the IPG Effect. As with ECAP AGF slopes, the sample size for this data set was small and many subgroups were represented by few individuals. The small subgroup samples meant that some interaction effects could not be tested, but those that could be tested were not significant (p > 0.33). The available data also did not demonstrate significant effects of treatment group (F(2,18) = 0.08, p = 0.924) or intrascalar tissue levels (F(2,18) = 0.75, p = 0.485) on IPG effect.
DISCUSSION
The aim of this study was to investigate the relationship between measures of CI function and variation in the tissue that formed in the scala tympani between the implant and the nerves it stimulates, after accounting for the variation in function that can be attributed to variation in SGN density. We examined variation in 5 measures of CI function in guinea pigs with variation in SGN densities produced by implanting CIs in hearing ears (IH), ears deafened with neomycin (NI), or neomycin-deafened ears treated with neurotrophin (NNI). The IH group had a lower average level of intrascalar tissue than the NI and NNI groups, which were not different from each other. In addition, all three groups differed in SGN density, but the NI and NNI groups were more similar to each other than either was to the IH group. However, in each treatment group, all three tissue levels were observed and spanned similar broad ranges of SGN density. Because of this overlap, the level of intrascalar tissue was not significantly correlated with SGN density. This independent variation of intrascalar tissue and SGN density permitted testing for independent effects of these factors on CI function.
Effect of Intrascalar Tissue on CI Function
The principal finding of this study was that incorporating the variation of the level of intrascalar tissue with the variation of SGN density in the statistical analyses did not explain more variation in electrical hearing than was explained by SGN density alone. SGN density explained more than half of the variation in only 1 of the 5 functional measures (ECAP AGF slope, 52.8 %). For 2 measures, SGN density explained less than a third of the variation (threshold for a pulse train at 156 pps, 30.2 % and threshold for a single 25 μs/ph pulse, 0.2 %). Variation in intrascalar tissue did not increase the proportion of variance explained in any of the functional measures, including two measures shown to predict inter-ear differences in speech recognition in human subjects: MPI (Zhou and Pfingst 2014) and IPG Effect (Schvartz-Leyzac and Pfingst 2018). Still, we did not present complex sounds comparable to speech and therefore cannot rule out the possibility that intrascalar tissue may have affected sound perception in ways that could not be detected in this study.
Failure to find a significant effect of intrascalar tissue in this study could be due to scoring the tissue in a small number of broad categories. Such scoring tends to produce within-group variances of the dependent variable that are larger than would be obtained with smaller categories, making it difficult to demonstrate that an effect is significant even when it is large. However, in our analyses, intrascalar tissue accounted for < 5 % of the total variation, which is unlikely to be significant even with much larger sample sizes. A more consequential limitation of our data was that the small numbers of implanted-hearing animals with Medium or High levels of intrascalar tissue prevented evaluations of the effect of intrascalar tissue on electrical hearing within this group. However, both groups of neomycin-deafened animals (with and without neurotrophin treatment) had more substantial numbers of animals with Medium and High levels of intrascalar tissue and in these groups there was no evidence of differences in electrical hearing due to differences in levels of intrascalar tissue. Either the effect of fibrosis on perception is more subtle than can be detected by these tests or it is much smaller than the effects of other, uncontrolled factors.
Our results have implications for the effect of intrascalar tissue on signal transmission from the electrode to the neuron. Two of the measures explicitly quantify psychophysical detection thresholds and the third, MPI slope, represents the effect of pulse rate on thresholds, while ECAP AGF slopes and IPG Effects represent responses to variation in intensity of stimulus. Thus, all 5 measurements could be affected by increased impedance resulting in weaker signals reaching the neurons. Our finding that there was no independent effect of intrascalar tissue on these measurements may seem to contradict data showing that the fibrous protein and bone in the intrascalar tissue have much higher impedances than the perilymph that normally fills the scala tympani (Geddes and Baker 1967; Gabriel et al. 1996). One explanation for the apparent contradiction might be that the intrascalar tissue is porous and is immersed in, and permeated by, residual perilymph. Another possibility is that implant position may be a confounding factor (Shepherd et al. 1993; Schvartz-Leyzac et al. 2020). All functional data used in this study were collected after electrical detection thresholds were considered stable, so it is unlikely that the data reflected changes in electrode position. However, our histological data suggest that across-subject variation in position during the stable period could be substantial (distance from modiolar wall could be 10–200 μm, cf. Figure 2). Because implant position could not be determined in a majority of animals, it is possible that difference in electrode position could be a factor in function after stabilization. Thus, the combination of implant position and intrascalar tissue might affect processing complex signals (words and sentences) even if a gross change in impedance is not apparent. To investigate this further may require assessment intrascalar tissue development and implant position at multiple time points.
Multiple Interacting Factors Determine the Amount of Intrascalar Fibrosis and Bone
Another important result of this study is that Medium and High levels of intrascalar tissue were more frequent in animals deafened with neomycin (NI and NNI) than in non-deafened (IH) animals. Because IH animals received the same surgery and the same implant but usually had little or no intrascalar tissue, differences in the level of intrascalar tissue cannot be attributed to the implant or to activation of the immune system by the aspects of the surgical procedure experienced by all animals. Instead, the differences in level of intrascalar tissue can only reflect processes in the inner ear that differed between repetitions of the procedure. We hypothesize that variation in local trauma associated with implant insertion, which was reduced but not eliminated by using a single surgeon, contributes to variation in intrascalar tissue in both groups and that the response is amplified by the reaction to neomycin-induced cell death.
Intrascalar soft tissue and bone are reported to be common findings in temporal bones of human CI recipients (Eshraghi et al. 2003; Fayad and Linthicum 2006; Seyyedi and Nadol 2014) and are common complications in revision/reimplantation surgeries (Côté et al. 2007). Soft insertion has been proposed to reduce the frequency of extreme fibrosis in human recipients (Fayad et al. 2009; Kamakura and Nadol 2016). Studies in mice suggest fibrosis is produced by normal wound-healing processes, which may lead to an amplified foreign body response (Bas et al. 2015). Thus, different levels of intrascalar tissue may reflect different levels of trauma. The implanted human temporal bones with dense fibrous tissue and bone often have evidence that implants passed through the spiral ligament or basilar membrane, or fractured the osseous spiral lamina (Eshraghi et al. 2003; Fayad and Linthicum 2006). These severe injuries are rare in our guinea pigs and did not occur in the 52 animals in this study; however, the CI was inserted through a cochleostomy in all animals. Our results suggest that the trauma and debris associated with the cochleostomy usually were not sufficient by themselves to produce that High level of intrascalar tissue. In addition to insertion trauma, the main factor affecting immune system activation in our study was the extensive hair cell death induced by the neomycin treatment. Toxin-induced hair cell death has been shown to cause upregulation of the immune system in mice (Kaur et al. 2015a, 2015b). In our study, implanted ears that also received neomycin (NI and NNI groups) typically had a higher level of intrascalar tissue consistent with a more strongly activated immune system. Even so, many NI and NNI ears had less severe intrascalar tissue than human temporal bones with translocated implants, suggesting that neomycin-induced cell death does not induce a local immune response as strong as that induced by the rupture of membranes or breakage of bone.
Neomycin treatment was the sole known variable associated with an increase in the level of intrascalar tissue in this study. Although there was a range of outcomes in each treatment group, severe fibrosis is more common after neomycin treatment, and that frequency was not significantly altered by neurotrophin treatment. These results raise the possibility that the magnitude and mechanism of hearing loss may be factors leading to higher levels of intrascalar tissue. Additional studies are needed to determine whether those higher levels are due to the aminoglycoside or the activated state of the immune system induced by an extensive ototoxic lesion. A better understanding of the time course of immune system activity after ototoxic drug exposure could have important implications for the treatment of patients exposed to such compounds, ranging from the utility of immune system suppression to the anticipation of complications after implantation.
Further Studies
Our analyses did not find a significant effect of intrascalar tissue on electrical hearing beyond that which was attributable to SGN density. Consequently, the variation in CI function that was not attributable to SGN density remained unexplained. Only SGN density accounted for a significant proportion of variation in any measure analyzed in this study, and it usually accounted for less than 50 % of that variation. It is possible that effects of intrascalar tissue were obscured by factors unrelated to that tissue. Some of these factors may be changes in nerve health and functional state that are only partially correlated with SGN density. For example, high counts of peripheral processes are associated with high survival of SGN somata and HCs in cochleae exposed to relatively little trauma from CI insertion or other treatments; however, the correlation is not perfect (Pfingst et al. 2011). Thus, independent variation in peripheral process survival may contribute to variation in CI function. Reduction of soma size and distortion of soma shape are associated with neuronal degeneration after HC loss (Ylikoski et al. 1974; Spoendlin 1984) and may affect the cell’s response to CI stimulation (Ramekers et al. 2014). In addition, the condition of Schwann cells and myelin sheaths may affect nerve function even if the neurons, themselves, are healthy (Prado-Guitierrez et al. 2006). Also, there is variation in sensitivity and firing dynamics among neurons within a tonotopic region in normal individuals (Borg et al. 1988; Crozier and Davis 2014; Shrestha et al. 2018); the surviving neurons represent a sample of that variation, which may or may not be biased. In addition, the variation of that sample may have been transformed by the effects of the treatment on the survivors. A quantitative investigation of biased mortality and effects of treatments or age on sensitivity to stimuli might improve our understanding of variation in CI function.
Even if additional studies were to support the absence of an effect of intrascalar tissue on electrical hearing that would not mean the tissue is wholly benign. The development of this tissue has been linked to loss of residual hearing in CI recipients (O'Leary et al. 2013). One possible mechanism for the loss is that the tissue forms a barrier preventing bulk fluid displacement in response to stapedial loading, eliminating the impedance matching function of the middle ear (Quesnel et al. 2016). The tissue may also block or dampen basilar membrane displacement, impeding progress of sound waves through the ear (Choi and Oghalai 2005; Kiefer et al. 2006). These mechanical changes could also limit the effectiveness of therapies that might be developed in the future, such as hair cell regeneration treatments to repopulate the membrane with cells that are sensitive to its movements (Kanzaki et al. 2002; Izumikawa et al. 2005). Also, wholly implantable devices that depend on oscillation of piezoelectric materials by cochlear fluids (Inaoka et al. 2011; Knisely et al. 2015; Zhao et al. 2019) could be immobilized by tissue filling scala tympani. Better understanding of intrascalar tissue could benefit future therapies and protect residual hearing.
Acknowledgements
The authors thank Lisa Beyer and Laila Al-Jerdi for technical assistance. This work was supported by NIH NIDCD grants R01 DC010786 and R01 DC015809, and contracts from MED-EL.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bas E, Dinh CT, Garnham C, Polak M, Van de Water TR. Conservation of hearing and protection of hair cells in cochlear implant patients' with residual hearing. Anat Rec. 2012;295:1909–1927. doi: 10.1002/ar.22574. [DOI] [PubMed] [Google Scholar]
- Bas E, Gupta C, Van De Water TR. A novel organ of Corti explant model for the study of cochlear implantation trauma. Anat Rec. 2012;295:1944–1956. doi: 10.1002/ar.22585. [DOI] [PubMed] [Google Scholar]
- Bas E, Goncalves S, Adams M, Dinh CT, Bas JM, Van De Water TR, Eshraghi AA. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front Cell Neurosci. 2015;9:16. doi: 10.3389/fncel.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey PJ, Pyman BC, Clark GM, Dowell RC, Gordon M, Brown AM, Hollow RD. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol. 1992;101:342–348. doi: 10.1177/000348949210100410. [DOI] [PubMed] [Google Scholar]
- Borg E, Engstrom B, Linde G, Marklund K. 8th nerve-fiber firing features in normal-hearing rabbits. Hear Res. 1988;36:191–201. doi: 10.1016/0378-5955(88)90061-5. [DOI] [PubMed] [Google Scholar]
- Budenz CL, Cosetti MK, Coelho DH, Birenbaum B, Babb J, Waltzman SB, Roehm PC. The effects of cochlear implantation on speech perception in older adults. J Am Geriatr Soc. 2011;59:446–453. doi: 10.1111/j.1532-5415.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- Burghard A, Lenarz T, Kral A, Paasche G. Insertion site and sealing technique affect residual hearing and tissue formation after cochlear implantation. Hear Res. 2014;312:21–27. doi: 10.1016/j.heares.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Chen HC, Xing YZ, Xia L, Chen ZN, Yin SK, Wang J. AAV-mediated NT-3 overexpression protects cochleae against noise-induced synaptopathy. Gene Ther. 2018;25:251–259. doi: 10.1038/s41434-018-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Oghalai JS. Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hear Res. 2005;205:193–200. doi: 10.1016/j.heares.2005.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GM, Tong YC, Patrick JF, Seligman PM, Crosby PA, Kuzma JA, Money DK. A multi-channel hearing prosthesis for profound-to-total hearing-loss. J Med Eng Technol. 1984;8:3–8. doi: 10.3109/03091908409032065. [DOI] [PubMed] [Google Scholar]
- Côté M, Ferron P, Bergeron F, Bussières R. Cochlear reimplantation: causes of failure, outcomes, and audiologic performance. Laryngoscope. 2007;117:1225–1235. doi: 10.1097/MLG.0b013e31805c9a06. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Davis RL. Unmasking of spiral ganglion neuron firing dynamics by membrane potential and neurotrophin-3. J Neurosci. 2014;34:9688–9702. doi: 10.1523/jneurosci.4552-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi AA, Yang NW, Balkany TJ. Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope. 2003;113:415–419. doi: 10.1097/00005537-200303000-00005. [DOI] [PubMed] [Google Scholar]
- Eshraghi AA, Gupta C, Van de Water TR, Bohorquez JE, Garnham C, Bas E, Talamo VM. Molecular mechanisms involved in cochlear implantation trauma and the protection of hearing and auditory sensory cells by inhibition of c-jun-n-terminal kinase signaling. Laryngoscope. 2013;123:S1–S14. doi: 10.1002/lary.23902. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Makarem AO, Linthicum FH. Histopathologic assessment of fibrosis and new bone formation in implanted human temporal bones using 3d reconstruction. Otolaryngol Head Neck Surg. 2009;141:247–252. doi: 10.1016/j.otohns.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson A, de Medina P, Paillasse MR, Silvente-Poirot S, Poirot M, Ulfendahl M. Dendrogenin a and b two new steroidal alkaloids increasing neural responsiveness in the deafened Guinea pig. Front Aging Neurosci. 2015;7:7. doi: 10.3389/fnagi.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues .2. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41:2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- Garadat SN, Zwolan TA, Pfingst BE. Across-site patterns of modulation detection: relation to speech recognition. J Acoust Soc Am. 2012;131:4030–4041. doi: 10.1121/1.3701879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes LA, Baker LE. Specific resistance of biological material-a compendum of data for biomedical engineer and physiologist. Med Biol Eng. 1967;5:271–293. doi: 10.1007/bf02474537. [DOI] [PubMed] [Google Scholar]
- Green KMJ, Bhatt YM, Mawman DJ, O'Driscoll MP, Saeed SR, Ramsden RT, Green MW. Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants International. 2007;8:1–11. doi: 10.1179/cim.2007.8.1.1. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Hickman TT, Suzuki J, Ji LC, Kohrman DC, Corfas G, Liberman MC. Protection from noise-induced cochlear synaptopathy by virally mediated overexpression of NT3. Sci Rep. 2019;9:12. doi: 10.1038/s41598-019-51724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydebrand G, Hale S, Potts L, Gotter B, Skinner M. Cognitive predictors of improvements in adults' spoken word recognition six months after cochlear implant activation. Audiol Neuro-Otol. 2007;12:254–264. doi: 10.1159/000101473. [DOI] [PubMed] [Google Scholar]
- Hochmair ES, Hochmair-Desoyer IJ. An implanted auditory 8-channel stimulator for the deaf. Med Biol Eng Comput. 1981;19:141–148. doi: 10.1007/BF02442707. [DOI] [PubMed] [Google Scholar]
- Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Heydebrand G, Skinner MW. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34:342–360. doi: 10.1097/AUD.0b013e3182741aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeder C, Landegger LD, Engleder E, Gabor F, Plasenzotti R, Plenk H, Kaider A, Hirtler L, Gstoettner W, Arnoldner C. Effects of intraoperatively applied glucocorticoid hydrogels on residual hearing and foreign body reaction in a guinea pig model of cochlear implantation. Acta Otolaryngol. 2015;135:313–319. doi: 10.3109/00016489.2014.986758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaoka T, Shintaku H, Nakagawa T, Kawano S, Ogita H, Sakamoto T, Hamanishi S, Wada H, Ito J. Piezoelectric materials mimic the function of the cochlear sensory epithelium. Proc Natl Acad Sci. 2011;108:18390–18395. doi: 10.1073/pnas.1110036108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai R, Herrmann BS, Nadol JB, Quesnel AM. The pattern and degree of capsular fibrous sheaths surrounding cochlear electrode arrays. Hear Res. 2017;348:44–53. doi: 10.1016/j.heares.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jahn KN, Arenberg JG. Evaluating psychophysical polarity sensitivity as an indirect estimate of neural status in cochlear implant listeners. J Assoc Res Otolaryngol. 2019;20:415–430. doi: 10.1007/s10162-019-00718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura T, Nadol JB. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res. 2016;339:132–141. doi: 10.1016/j.heares.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki S, Kawamoto K, Oh SH, Stover T, Suzuki M, Ishimoto S, Yagi M, Miller JM, Lomax MI, Raphael Y. From gene identification to gene therapy. Audiol Neuro-Otol. 2002;7:161–164. doi: 10.1159/000058303. [DOI] [PubMed] [Google Scholar]
- Kaur T, Hirose K, Rubel EW, Warchol ME. Macrophage recruitment and epithelial repair following hair cell injury in the mouse utricle. Front Cell Neurosci. 2015;9:9. doi: 10.3389/fncel.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, Warchol ME. Fractalkine signaling regulates macrophage recruitment into the cochlea and promotes the survival of spiral ganglion neurons after selective hair cell lesion. J Neurosci. 2015;35:15050–15061. doi: 10.1523/jneurosci.2325-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Yagi M, Stover T, Kanzaki S, Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF. Mol Ther. 2003;7:484–492. doi: 10.1016/S1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Kel GE, Tan J, Eastwood HT, Wongprasartsuk S, O'Leary SJ. Early cochlear response and ICAM-1 expression to cochlear implantation. Otol Neurotol. 2013;34:1595–1602. doi: 10.1097/MAO.0b013e31828f4929. [DOI] [PubMed] [Google Scholar]
- Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants? Laryngoscope. 2005;115:672–677. doi: 10.1097/01.mlg.0000161335.62139.80. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Bohnke F, Adunka O, Arnold W. Representation of acoustic signals in the human cochlea in presence of a cochlear implant electrode. Hear Res. 2006;221:36–43. doi: 10.1016/j.heares.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Knisely K, Zhao CM, Grosh K (2015) A MEMS AlN transducer array with flexible interconnections for use as a cochlear implant. In: Karavitaki KD and Corey DP.(eds.) mechanics of hearing: protein to perception. Amer Inst Physics, Melville. 10.1063/1.4939432
- Li PMMC, Somdas MA, Eddington DK, Nadol JB. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116:731–738. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Okada T, Shimazaki K, Sheykholeslami K, Nomoto T, Muramatsu S, Mizukami H, Kume A, Xiao S, Ichimura K, Ozawa K. Protection against aminoglycoside-induced ototoxicity by regulated AAV vector-mediated GDNF gene transfer into the cochlea. Mol Ther. 2008;16:474–480. doi: 10.1038/sj.mt.6300379. [DOI] [PubMed] [Google Scholar]
- Moberly AC, Bates C, Harris MS, Pisoni DB. The enigma of poor performance by adults with cochlear implants. Otol Neurotol. 2016;37:1522–1528. doi: 10.1097/MAO.0000000000001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, Marovic P, O'Leary JS, Richardson R, Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Parkins CW. The bionic ear - principles and current status of cochlear prostheses. Neurosurgery. 1985;16:853–865. doi: 10.1227/00006123-198506000-00025. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Hembrador S, Kang SY, Middlebrooks JC, Raphael Y, Su GL. Detection of pulse trains in the electrically stimulated cochlea: effects of cochlear health. J Acoust Soc Am. 2011;130:3954–3968. doi: 10.1121/1.3651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Hughes AP, Colesa DJ, Watts MM, Strahl SB, Raphael Y. Insertion trauma and recovery of function after cochlear implantation: evidence from objective functional measures. Hear Res. 2015;330:98–105. doi: 10.1016/j.heares.2015.07/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Swiderski DL, Hughes AP, Strahl SB, Sinan M, Raphael Y. Neurotrophin gene therapy in deafened ears with cochlear implants: long-term effects on nerve survival and functional measures. J Assoc Res Otolaryngol. 2017;18:731–750. doi: 10.1007/s10162-017-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Guitierrez P, Fewster LM, Heasman JM, Mckay CM, Shepherd RK. Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res. 2006;215:47–55. doi: 10.1016/j.heares.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB. Delayed loss of hearing after hearing preservation cochlear implantation: human temporal bone pathology and implications for etiology. Hear Res. 2016;333:225–234. doi: 10.1016/j.heares.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SF, Grolman W. Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol. 2014;15:187–202. doi: 10.1007/s10162-013-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis M, Boisvert I, Looi V, da Cruz M. Speech recognition outcomes after cochlear reimplantation surgery. Trends Hear. 2017;21:9. doi: 10.1177/2331216517706398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, Fretwell NJ, Fallon JB, Wallace GG, Shepherd RK, Clark GM, O'Leary SJ. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009;30:2614–2624. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AF, Harris JP, Keithley EM. Immune-mediated hearing loss: basic mechanisms and options for therapy. Acta Oto-Laryngol. 2002;122 supplement 548:38–43. doi: 10.1080/00016480260094965. [DOI] [PubMed] [Google Scholar]
- Ryu KA, Lyu AR, Park H, Choi JW, Hur GM, Park YH. Intracochlear bleeding enhances cochlear fibrosis and ossification: an animal study. PLoS One. 2015;10:13. doi: 10.1371/journal.pone.0136617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, Pfingst BE. Across-site patterns of electrically evoked compound action potential amplitude-growth functions in multichannel cochlear implant recipients and the effects of the interphase gap. Hear Res. 2016;341:50–65. doi: 10.1016/j.heares.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, Pfingst BE. Assessing the relationship between the electrically evoked compound action potential and speech recognition abilities in bilateral cochlear implant recipients. Ear Hear. 2018;39:344–358. doi: 10.1097/AUD.0000000000000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, Holden TA, Zwolan TA, Arts HA, Firszt JB, Buswinka CJ, Pfingst BE (2020) Effects of electrode location on estimates of neural health in humans with cochlear implants. J Assoc Res Otolaryngol (early view). 10.1007/s10162-020-00749-0 [DOI] [PMC free article] [PubMed]
- Seyyedi M, Nadol JB., Jr Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol. 2014;35:1545–1551. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyyedi M, Viana LM, Nadol JB. Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol Neurotol. 2014;35:1446–1450. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Clark GM, Black RC, Patrick JF. The histopathological effects of chronic electrical-stimulation of the cat cochlea. J Laryngol Otol. 1983;97:333–341. doi: 10.1017/s0022215100094202. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Hatsushika S, Clark GM. Electrical-stimulation of the auditory-nerve - the effect of electrode position on neural excitation. Hear Res. 1993;66:108–120. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- Shrestha BR, Chia C, Wu L, Kujawa SG, Liberman MC, Goodrich LV. Sensory neuron diversity in the inner ear is shaped by activity. Cell. 2018;174:1229–1246. doi: 10.1016/j.cell.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly DJ, Campbell L, Uschakov A, Saief ST, Lam M, O'Leary SJ. Applying neurotrophins to the round window rescues auditory function and reduces inner hair cell synaptopathy after noise-induced hearing loss. Otol Neurotol. 2016;37:1223–1230. doi: 10.1097/mao.0000000000001191. [DOI] [PubMed] [Google Scholar]
- Souter M, Eastwood H, Marovic P, Kel G, Wongprasartsuk S, Ryan AF, O'Leary SJ. Systemic immunity influences hearing preservation in cochlear implantation. Otol Neurotol. 2012;33:532–538. doi: 10.1097/MAO.0b013e31824bac44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol. 1984;93:76–82. doi: 10.1177/00034894840930S415. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yagi M, Brown JN, Miller AL, Miller JM, Raphael Y. Effect of transgenic GDNF expression on gentamicin-induced cochlear and vestibular toxicity. Gene Ther. 2000;7:1046–1054. doi: 10.1038/sj.gt.3301180. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep. 2016;6:11. doi: 10.1038/srep24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Green SH. Functional role of Neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31:7938–7949. doi: 10.1523/jneurosci.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk M, Hessler R, Mugridge K, Jolly C, Fehr M, Lenarz T, Scheper V. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS One. 2016;11:19. doi: 10.1371/journal.pone.0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahara K, Nishimura K, Ogita H, Ito J, Nakagawa T, Furuta I, Kita T, Omori K, Yamamoto N. Hearing preservation at low frequencies by insulin-like growth factor 1 in a Guinea pig model of cochlear implantation. Hear Res. 2018;368:92–108. doi: 10.1016/j.heares.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Wersäll J, Björkroth B. Degeneration of neural elements in the cochlea of the guinea-pig after damage to the organ of Corti by ototoxic antibiotics. Acta Otolaryngol. 1974;78(suppl 326):23–41. doi: 10.3109/00016487409129730. [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Stark G, Reiss L. Changes in gene expression and hearing thresholds after cochlear implantation. Otol Neurotol. 2015;36:1157–1165. doi: 10.1097/mao.0000000000000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Knisely KE, Colesa DJ, Pfingst BE, Raphael Y, Grosh K. Voltage readout from a piezoelectric intracochlear acoustic transducer implanted in a living guinea pig. Sci Rep. 2019;9:3711. doi: 10.1038/s41598-019-39303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Pfingst BE. Relationship between multipulse integration and speech recognition with cochlear implants. J Acoust Soc Am. 2014;136:1257–1268. doi: 10.1121/1.4890640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am. 1997;102:3673–3685. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]