Abstract
Bipolar disorder is often comorbid with anxiety, which is itself associated with poorer clinical outcomes, including suicide. A better etiologic understanding of this comorbidity could inform diagnosis and treatment. The present study aims to test whether comorbid anxiety in bipolar disorder reflects shared genetic risk factors. We also sought to assess the contribution of genetic risk for anxiety to suicide attempts in bipolar disorder. Polygenic risk scores (PRS) were calculated from published genome-wide association studies of samples of controls and cases with anxiety (n = 83,566) or bipolar disorder (n = 51,710), then scored in independent target samples (total n = 3369) of individuals with bipolar disorder who reported or denied lifetime anxiety disorders or suicidal attempts in research interviews. Participants were recruited from clinical and nonclinical settings and genotyped for common genetic variants. The results show that polygenic risk for anxiety was associated with comorbid anxiety disorders and suicide attempts in bipolar disorder, while polygenic risk for bipolar disorder was not associated with any of these variables. Our findings point out that comorbid anxiety disorders in bipolar disorder reflect a dual burden of bipolar and anxiety-related genes; the latter may also contribute to suicide attempts. Clinical care that recognizes and addresses this dual burden may help improve outcomes in people living with comorbid bipolar and anxiety disorders.
Subject terms: Clinical genetics, Bipolar disorder
Introduction
Anxiety and bipolar disorder (BP) are highly comorbid conditions1,2, but the basis of this comorbidity is uncertain. There is some overlap in diagnostic criteria, but this is not substantial.
It is possible that both conditions share environmental risk factors but these do not appear to be much more common in people with comorbid anxiety and BP than in those with BP alone1. Familial co-aggregation of anxiety and bipolar disorder3,4 suggests shared genetic risk factors, but this has not been tested using molecular genetic methods.
Comorbid anxiety has important clinical implications for people living with BP. Several studies have reported unfavorable outcomes in BP with comorbid anxiety, including more frequent mood episodes5,6, more severe depressive episodes7, higher rates of substance abuse6, less favorable treatment response6,8,9, and increased suicide attempts10–12. The United States National Comorbidity Survey found that the diagnosis of anxiety disorder is particularly frequent among individuals with suicide attempts13. A deeper understanding of the comorbidity between anxiety and BP could shed light on these clinical problems and could in future point toward new treatment approaches.
BP is a highly heritable disease (~80%), and many genome wide association studies (GWAS) have been published, together finding close to 40 replicated genetic loci associated with the disorder14,15. Molecular genetic studies of anxiety are still relatively uncommon16, with many studies having small samples and as a result inconsistently replicated results. Two recent large-scale GWAS of anxiety and anxiety disorders have made considerable progress in identifying robust genetic risk factors17,18.
Both studies found that anxiety is a highly polygenic trait, like most other psychiatric conditions, with thousands of associated alleles that each contribute a small share to the total risk. Polygenic risk scores (PRS) are a powerful tool that capitalize on this cumulative genetic risk. One way in which these scores can be used is to identify shared genetic liability—if people with one disorder have a high polygenetic risk score for a second disorder it may indicate a shared genetic liability. In the field of psychiatric disorders, the PRS was initially used by Purcell et al. in a study of schizophrenia19. The PRS approach has since been applied to BP20–22, Schizoaffective Disorder23, Schizophrenia21, and Major Depression Disorder24, among other traits. PRS have also demonstrated a shared genetic risk factors among many of these disorders25,26.
The primary aim of the present study is to examine the genetic relationship between genetic risk for anxiety disorders and anxiety comorbidity in bipolar disorder. We aim to answer the question of whether anxiety comorbidity in bipolar disorder reflects distinct genetic risk factors, such that this comorbidity occurs if an individual has high genetic liability for both bipolar disorder and anxiety disorder. Alternatively, this comorbidity may reflect an alternative clinical manifestation (pleiotropy) of the known genetic risk for bipolar disorder. Our secondary research question is whether genetic risk for anxiety contributes to the increase in suicide attempts among people with comorbid bipolar and anxiety disorders. The results shed light on the mechanisms that underlie variable clinical presentations of BP and might help inform clinical management of patients with comorbid BP and anxiety disorders.
Subjects and methods
Sample description
The present study used existing data from five independent samples: First, a GWAS of lifetime anxiety disorder undertaken in the UK Biobank18, referred to herein as the “discovery sample”. We also include three additional samples; the Genetic Association Information Network (GAIN) bipolar, the Translational Genomics Research Institute (TGEN), and the Swedish Bipolar Disorder Cohort (SWEBIC), which we combined and referred to herein as the “target samples.” Ascertainment, diagnosis, and genotyping of these samples have been previously described18,27,28. Further details of the target samples are presented in Table 1. Written informed consent was obtained from all participants. Each study was approved by a local Ethics Committee.
Table 1.
Sample description.
| Sample | Target | |||
|---|---|---|---|---|
| Study name | GAIN | TGEN | SWEBIC | Combined |
| Sample Size | 528 | 1110 | 1731 | 3369 |
| Female (%) | 298 (56) | 733 (66) | 1067 (61) | 2098 (62) |
| Age at BP onset (SD) | 18.5 (9) | 18.5 (9) | na | 18.5 (9)e |
| Comorbidy | ||||
| Any anxiety | 192 | 459 | 518c | 1169 (51) |
| Panic disorder (%) | 139 (72) | 304 (66) | 425 (82) | 868 (74) |
| Agoraphobia (%) | 69 (36) | 169 (37) | na | 238 (20) |
| Specific phobia (%) | 55 (29) | 167 (36) | na | 222 (19) |
| Social anxiety (%) | 56 (29) | 169 (37) | na | 225 (19) |
| Other/unspecified anxiety disorder | na | na | 93 (18) | 105 (8) |
| Suicide attempt (%) | 257a (49) | 518b (48) | 615d (36) | 1390 (42 |
SA suicide attempt, na not recorded/available.
aRecorded in 527 subjects.
bAssessed in 1068 subjects.
cAssessed in 636 subjects.
dAssessed in 1719 subjects.
eAge at BP onset was available only for 1595 subjects (GAIN and TGEN samples).
The discovery sample comprised the largest available GWAS on anxiety disorders, including individuals of western European-ancestry who took part in the UK Biobank online mental health follow-up questionnaire. This sample represented people reporting a lifetime diagnosis by a professional of panic disorder (PD), agoraphobia, social phobia (SP), social anxiety disorder (SAD), or generalized anxiety disorder (GAD)18.
Target sample: GAIN/TGEN/SWEBIC
The GAIN, TGEN, and SWEBIC samples included 3369 individuals of European ancestry assessed by a comprehensive psychiatric interview. All individuals included in the present analysis had received a final DSM-III-R/IV diagnosis of Bipolar I (BPI) or Schizoaffective disorder—bipolar type (SA-BP).
The GAIN and TGEN participants (N = 1638) were assessed by a comprehensive psychiatric interview29 supplemented by family informant and medical record data. Phenotypic data was harmonized and compiled within the Bipolar Disorder Phenome Database30. Four individuals were excluded owing to missing genotype or phenotype data.
The SWEBIC participants (N = 1731) were assessed according to the DSM-IV criteria. Genotype data have been previously reported31.
Phenotypes
Anxiety comorbidity
Anxiety comorbidity was defined as a lifetime diagnoses of PD, Agoraphobia, SP, SAD, and/or GAD occurring before or after a lifetime diagnosis of BP as assessed during comprehensive psychiatric interview described above.
Suicidal attempts
Suicide attempt was defined as a lifetime self-report of self-harm with lethal intent, reported during a structured interview according to previous studies32.
Polygenic risk score (PRS)
PRS for anxiety were calculated using summary statistics from the UK Biobank discovery sample18. We followed the guide for genomic profile risk scoring (Box 1 of reference25) to calculate the PRS. In the discovery sample, p value informed pruning of correlated SNPs was done from summary statistics by use of LD-based clumping (r2 threshold < 0.25 across a 500 kilobase window) as implemented in PLINK33, using the 1000 Genomes European-ancestry sample (excluding Finns) as a reference panel. The pruned SNPs were used to compute the anxiety PRS in the target samples based on 65,415 shared SNPs. Alleles were weighted by the effect sizes from the discovery sample (log[OR]), including all SNPs that were significant at p < 0.5.
We used the same approach to calculate a BP PRS in the target samples, based on summary statistics available from a recently-published BP GWAS34. P value threshold from the discovery sample was set to 0.2, since this captured the most phenotypic variance.
PRS were standardized using means and SDs from the respective distributions.
BP-PRS showed a small but significant correlation with anxiety-PRS (Pearson r = 0.07, p < 0.0001).
Statistical methods
Association between anxiety PRS and phenotypic information was tested by logistic regression, as implemented in SAS vs. 9.4. Population stratification was corrected with ancestry principal components analysis (PC) based on the variance-standardized relationship matrix in Plink33, using the first 5 PCs as covariates. Sex was used as a covariate for anxiety comorbidity, since we observed an association between Anxiety and Sex in the target (p < 0.001) sample, consistent with previous findings8,9. In addition, the source study (GAIN,TGEN, SWEBIC) was included as a covariate to control for any batch effects.
PRS association p values were Bonferroni-corrected for four different tests: Anxiety PRS and BP PRS versus comorbid anxiety or suicide attempts (p < 0.0125). Only one p value threshold was used for calculating PRS in each of the discovery samples (p < 0.5 for anxiety PRS, p < 0.2 for bipolar PRS, as noted above).
Power calculations
The power of PRS was carried out in AVENGEME35,36. The proportion of phenotypic variance explained by common SNPs was estimated from the target sample. The power of anxiety PRS and BP PRS to predict anxiety on BP sample was 90 and 74%, considering the h2SNP for anxiety (12%) obtained from the discovery sample18. The power of anxiety PRS and BP PRS to detect suicide attempt was 91 and 76%, respectively, given an h2SNP of 10% for suicide attempt32.
Results
Sample characteristics
A total of 3369 patients with a diagnosis of BPI or SA-BP, from 3 different studies, comprised the target sample (Table 1). The studies were similar in terms of European ancestry and sex ratio. A lifetime diagnosis of any anxiety disorder (“Any Anxiety”) was present in 51.6% of the target sample (Table 1). 41.92% of the target sample reported (N = 1390) one or more suicide attempts (Table 1).
Anxiety comorbidity
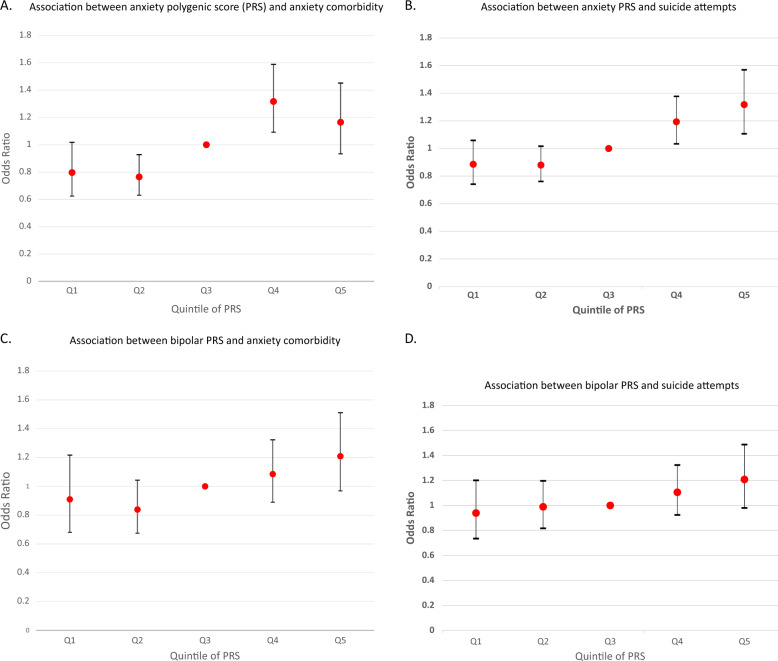
Anxiety-PRS was significantly and positively associated with “Any Anxiety” in the target sample (Fig. 1a). Each unit increase in anxiety-PRS led to a 15.3% increase in the odds of a comorbid anxiety disorder (OR = 1.153, 95% CI: 1.048–1.269, p = 0.0034, Nagelkerke’s R2 = 0.08).
Fig. 1. Association of anxiety or bipolar disorder polygenic scores with anxiety comorbidity and suicide attempts.
a The association between anxiety polygenic score (PRS) and anxiety comorbidity in patients with bipolar disorder. Anxiety comorbidity was evaluated as a categorical phenotype (“Any Anxiety”). b The association between anxiety PRS and suicide attempts in patients with bipolar disorder. c The association between bipolar PRS and anxiety comorbidity in patients with bipolar disorer. d The association between bipolar PRS and suicide attempts in patients with bipolar disorder. Logistic regression results are reported as odds ratios (OR). 95% confidence intervals (CI) are also shown. The PRS data are distributed in quintile.
In contrast, we detected no significant main effect of BP PRS on anxiety comorbidity in the target sample (Fig. 1c) (OR = 1.118, 95% CI: 1.000–1.248, p = ns, Nagelkerke’s R2 = 0.05).
Suicidal behavior
Anxiety PRS was significantly and positively associated with suicide attempts (SA) in the target sample (Fig. 1b) (OR = 1.106, 95% CI: 1.030–1.189, p = 0.0055, Nagelkerke’s R2 = 0.06). In contrast, we detected no significant association between BP PRS and SA in the target sample (OR = 1.078, 95% CI: 0.998–1.164, p = ns, Nagelkerke’s R2 = 0.02) (Fig. 1d).
Discussion
To our knowledge, this is the first study to address the impact of genetic risk for anxiety on the clinical course of BP and to provide evidence for a molecular genetic distinction between bipolar disorder with and without comorbid anxiety. The results suggest that bipolar disorder with comorbid anxiety reflects a dual burden of bipolar and anxiety-related genes. Clinical approaches that address this dual genetic burden may help improve outcomes in people living with comorbid bipolar and anxiety disorders.
There were two main findings. First, anxiety disorder comorbidity in bipolar disorder was associated with anxiety PRS in the samples we studied. Second, anxiety PRS was also associated with suicide attempts in BP.
In previous population-based studies, about 60% of bipolar probands met criteria for comorbid anxiety disorders1,2,37. In the present study, we found that anxiety risk alleles play a significant role in this comorbidity. Our findings thus contribute to heavily-debated questions concerning the nosologic relationship between mood and anxiety disorders. Our data support the view that anxiety comorbidity in BP is due, at least in part, to the same common genetic risk variants as anxiety in general, while bipolar risk alleles do not. This finding is consistent with the known low genetic correlations between bipolar disorder and anxiety-related traits38, which suggests that the disorders share few genetic risk factors.
A second important result arising from the polygenic scoring analyses is the association between anxiety PRS and suicidal behavior. In our target sample we observed a positive association between suicide attempts and genetic risk for anxiety in individuals with a diagnosis of bipolar disorder. These results are consistent with the increased rates of suicidal behavior in people with anxiety disorders reported by population-based studies11 and call attention to the importance of monitoring suicide risk in people with BP and comorbid anxiety.
Our results show that anxiety PRS accounts for only a small proportion of overall anxiety comorbidity in BP, so other factors may be involved. One factor could be assortative mating where people with BP are more likely to partner with people with anxiety disorders, leading to increased comorbidity in the offspring. However, Nordsletten et al. have reported a maximal rate of assortative mating between anxiety and Bipolar I of 18%39. There is also a small but significant genetic correlation between anxiety and BP, which suggests that BP risk alleles may also contribute to comorbid anxiety18. Correlated nongenetic risks may also contribute to comorbidiy. Nongenetic risk factors that might contribute to both BD and anxiety disorders include social isolation, unstable relationships, socioeconomic disadvantage, and traumatic life events40. Some anxiety comorbidity may also arise as a complication of BD or its treatment. For example, according to the staging model41, anxiety may manifests as a residual symptom following an acute mood episode.
This study should be viewed in the light of several limitations. This is a cross-sectional study, that relied upon retrospective reports. Although the best-estimate diagnosis procedure considers convergent data from family informants and medical records, data on GAD was not available, and the results may underestimate any association between anxiety PGS and the full range of comorbid anxiety disorders. The target sample was underpowered to detect association between BP-PRS with both comorbid anxiety and suicide attempt, so the failure to detect a significant result in this study does not rule out a contribution of BP risk alleles to those trait. Moreover, the overall direction of the relationship between comorbid anxiety or suicide attempt is similar for both ANX-PRS and BP-PRS (Fig. 1c, d), suggesting that a larger target sample may uncover significant associations with BP-PRS. While we used the largest published BP GWAS available, most controls were not formally screened for anxiety. This would not create a false-positive result, but would further reduce the power of a BP-based PRS to detect anxiety in another sample. The results should be considered preliminary until replicated in an independent sample. The lack of a replication sample here reflects the scarcity of available samples worldwide that have been fully characterized for both BP and anxiety. Anxiety-PRS indexes only a small proportion of the variance in anxiety disorder risk. This is an inherent weakness with the PRS method but is expected to improve with increased size of the discovery samples. A further limitation of this study is the reliance of the anxiety sumstats on self-report of a past diagnosis made by an unknown professional or retrospective recall of lifetime symptoms, both of which may contain error. However, we note that Purves et al.18 report high genetic correlation between these summary statistics and several other anxiety phenotypes, reassuring us that this case selection approach has utility.
In conclusion, anxiety and suicidal behavior in bipolar disorder are influenced by genetic risk factors involved in anxiety disorders. Patients with comorbid bipolar and anxiety disorders thus carry a dual genetic burden, suggesting the need for clinical approaches that address both disorders. More research is needed to understand the interplay between genetic and non-genetic influences on the clinical presentation and course of BP. Better powered discovery samples for both BP and anxiety will be needed to further elucidate this relationship.
Acknowledgements
We are greatly thankful to all study participants without whom this research would not have been accomplished. This research was funded by the NIMH Intramural Research Program (ZIAMH002843). The SWEBIC collection was funded by the Broad Institute from a grant from Stanley Medical Research Institute. Weighting files were received from Purves K and Thalia E. This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD. ClinicalTrials.gov Identifier NCT00001174.
Conflict of interest
M.L. reports grants from the Swedish Medical Research Council, grants from the Broad Institute, during the conduct of the study; personal fees from Lundbeck pharmaceuticals, outside the submitted work. J.N. reports a grant from Janssen, outside the submitted work. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appear at the end of the paper
Contributor Information
Fabiana L. Lopes, Email: fabiana.lopes@nih.gov
Bipolar Genome Study Consortium (BiGS):
Ney Alliey-Rodriguez, Judith A. Badner, Wade Berrettini, William Byerley, William Coryell, David W. Craig, Howard J. Edenberg, Tatiana Foroud, Elliot S. Gershon, Tiffany A. Greenwood, Yiran Guo, Brendan J. Keating, Daniel L. Koller, William B. Lawson, Chunyu Liu, Pamela B. Mahon, Melvin G. McInnis, Sarah S. Murray, John L. Nurnberger, Jr., Evaristus A. Nwulia, Corrie B. Panganiban, John Rice, Nicholas J. Schork, Erin N. Smith, Peng Zhang, Sebastian Zöllner, Fernando S. Goes, John R. Kelsoe, Caroline M. Nievergelt, James B. Potash, Tatyana Shekhtman, Paul D. Schilling, and Peter P. Zandi
References
- 1.Olfson M, et al. Reexamining associations between mania, depression, anxiety and substance use disorders: results from a prospective national cohort. Mol. Psychiatry. 2017;22:235–241. doi: 10.1038/mp.2016.64. [DOI] [PubMed] [Google Scholar]
- 2.Pavlova B, Perlis RH, Alda M, Uher R. Lifetime prevalence of anxiety disorders in people with bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:710–717. doi: 10.1016/S2215-0366(15)00112-1. [DOI] [PubMed] [Google Scholar]
- 3.MacKinnon DF, McMahon FJ, Simpson SG, McInnis MG, DePaulo JR. Panic disorder with familial bipolar disorder. Biol. Psychiatry. 1997;42:90–95. doi: 10.1016/S0006-3223(96)00299-5. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak J, Biederman J, Monuteaux MC, Richards J, Faraone SV. Parsing the comorbidity between bipolar disorder and anxiety disorders: a familial risk analysis. J. Child Adolesc. Psychopharmacol. 2002;12:101–111. doi: 10.1089/104454602760219144. [DOI] [PubMed] [Google Scholar]
- 5.Frank E, et al. Clinical significance of lifetime panic spectrum symptoms in the treatment of patients with bipolar I disorder. Arch. Gen. Psychiatry. 2002;59:905–911. doi: 10.1001/archpsyc.59.10.905. [DOI] [PubMed] [Google Scholar]
- 6.Young LT, Cooke RG, Robb JC, Levitt AJ, Joffe RT. Anxious and non-anxious bipolar disorder. J. Affect. Disord. 1993;29:49–52. doi: 10.1016/0165-0327(93)90118-4. [DOI] [PubMed] [Google Scholar]
- 7.Toniolo RA, Caetano SC, da Silva PV, Lafer B. Clinical significance of lifetime panic disorder in the course of bipolar disorder type I. Compr. Psychiatry. 2009;50:9–12. doi: 10.1016/j.comppsych.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Feske U, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am. J. Psychiatry. 2000;157:956–962. doi: 10.1176/appi.ajp.157.6.956. [DOI] [PubMed] [Google Scholar]
- 9.Henry C, et al. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J. Clin. Psychiatry. 2003;64:331–335. doi: 10.4088/JCP.v64n0316. [DOI] [PubMed] [Google Scholar]
- 10.Simon NM, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am. J. Psychiatry. 2004;161:2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- 11.Sareen J, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch. Gen. Psychiatry. 2005;62:1249–1257. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- 12.Simon NM, et al. The association of comorbid anxiety disorders with suicide attempts and suicidal ideation in outpatients with bipolar disorder. J. Psychiatr. Res. 2007;41:255–264. doi: 10.1016/j.jpsychires.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Pagura J, Cox BJ, Sareen J, Enns MW. Factors associated with multiple versus single episode suicide attempts in the 1990–1992 and 2001–2003 United States national comorbidity surveys. J. Nerv. Ment. Dis. 2008;196:806–813. doi: 10.1097/NMD.0b013e31818b6a77. [DOI] [PubMed] [Google Scholar]
- 14.Hou, L. et al. Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum. Mol. Genet. 10.1093/hmg/ddw181 (2016). [DOI] [PMC free article] [PubMed]
- 15.Goes FS. Genetics of bipolar disorder. Psychiatr. Clin. 2016;39:139–155. doi: 10.1016/j.psc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage JE, Sawyers C, Roberson-Nay R, Hettema JM. The genetics of anxiety-related negative valence system traits. Am. J. Med Genet. Part B Neuropsychiatr. Genet. Publ. Int Soc. Psychiatr. Genet. 2017;174:156–177. doi: 10.1002/ajmg.b.32459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otowa T, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol. Psychiatry. 2016;21:1485. doi: 10.1038/mp.2016.11. [DOI] [PubMed] [Google Scholar]
- 18.Purves, K. L. et al. A major role for common genetic variation in anxiety disorders. Mol Psychiatry10.1038/s41380-019-0559-1 (2019). [DOI] [PMC free article] [PubMed]
- 19.Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins AL, et al. Identifying bipolar disorder susceptibility loci in a densely affected pedigree. Mol. Psychiatry. 2013;18:1245–1246. doi: 10.1038/mp.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruderfer DM, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol. Psychiatry. 2014;19:1017–1024. doi: 10.1038/mp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amare AT, et al. Association of polygenic score for schizophrenia and HLA antigen and inflammation genes with response to lithium in bipolar affective disorder: a Genome-Wide Association Study. JAMA Psychiatry. 2018;75:65–74. doi: 10.1001/jamapsychiatry.2017.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamshere ML, et al. Polygenic dissection of the bipolar phenotype. Br. J. Psychiatry J. Ment. Sci. 2011;198:284–288. doi: 10.1192/bjp.bp.110.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colodro-Conde, L. et al. A direct test of the diathesis-stress model for depression. Mol Psychiatry. 10.1038/mp.2017.130 (2017). [DOI] [PMC free article] [PubMed]
- 25.Wray NR, et al. Research review: polygenic methods and their application to psychiatric traits. J. Child Psychol. Psychiatry. 2014;55:1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- 26.Visscher PM, et al. 10 Years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EN, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol. Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, E. N. et al. Genome-wide association of bipolar disorder suggests an enrichment of replicable associations in regions near genes. PLoS Genet. 7. 10.1371/journal.pgen.1002134 (2011). [DOI] [PMC free article] [PubMed]
- 29.Nurnberger JL, Jr, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiave. Arch. Gen. Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 30.Potash JB, et al. The bipolar disorder phenome database: a resource for genetic studies. Am. J. Psychiatry. 2007;164:1229–1237. doi: 10.1176/appi.ajp.2007.06122045. [DOI] [PubMed] [Google Scholar]
- 31.Bergen SE, et al. Genome-wide association study in a Swedish population yields support for greater CNV and MHC involvement in schizophrenia compared with bipolar disorder. Mol. Psychiatry. 2012;17:880–886. doi: 10.1038/mp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullins N., Bigdeli T. B., Børglum A. D. GWAS of Suicide Attempt in Psychiatric Disorders and Association with Major Depression Polygenic Risk Scores. Am J Psychiatry. 10.1176/appi.ajp.2019.18080957 (2019). [DOI] [PMC free article] [PubMed]
- 33.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahl EA, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 2019;51:793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palla L, Dudbridge F. A fast method that uses polygenic scores to estimate the variance explained by genome-wide marker panels and the proportion of variants affecting a trait. Am. J. Hum. Genet. 2015;97:250–259. doi: 10.1016/j.ajhg.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merikangas KR, Kalaydjian A. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Curr. Opin. Psychiatry. 2007;20:353–358. doi: 10.1097/YCO.0b013e3281c61dc5. [DOI] [PubMed] [Google Scholar]
- 38.Ohi K. et al. Shared genetic etiology between anxiety disorders and psychiatric and related intermidiate phenotypes. Psychol. Med.10.1017/S003329171900059X (2019). [DOI] [PubMed]
- 39.Nordsletten AE, et al. Patterns of nonrandom mating within and across 11 major psychiatric disorders. JAMA Psychiatry. 2016;73:354–361. doi: 10.1001/jamapsychiatry.2015.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reinares M, et al. Family functioning in bipolar disorder: characteristics, congruity between patients and relatives, and clinical correlates. Psychiatry Res. 2016;245:66–73. doi: 10.1016/j.psychres.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Cosci F, Fava GA. Staging of mental disorders: systematic review. Psychother. Psychosom. 2013;82:20–34. doi: 10.1159/000342243. [DOI] [PubMed] [Google Scholar]