Abstract
Reporting and analysis of Adverse Events Following Immunization (AEFIs) are the cornerstones of vaccine safety surveillance prompting causality assessment and signal detection. This paper describes the impact of the Italian Pharmacovigilance System of vaccines over a 10-year period (2008–2017). The reporting rate (RR) per all distributed dose was calculated. Serious AEFIs and causality assessments for fatal cases were described. The main results from signal detection were reported. During the study period, 46,430 AEFIs were reported with an overall RR of 17.2 per 100,000 distributed doses. Italy showed the highest number of reports among European countries. Only 4.4% of the reports came from citizens. Of the total, 12.7% were classified as serious with a RR over the study period of 2.20 per 100,000 distributed doses. They were mainly related to hyperpyrexia and usually had a positive outcome. Fatal outcomes were reported in 0.3% of the cases and were primarily associated with the influenza vaccine in elderly patients. None of these outcomes had a consistent causal association with the vaccination. Febrile convulsions by the measles, mumps, rubella and varicella vaccines and intussusception by the rotavirus vaccine were among the highlighted signals. The reporting rate and the analysis of serious events from 10 years support the good risk/benefit profiles of vaccines.
Subject terms: Drug regulation, Public health
Introduction
Vaccination is one of the most important achievements in public health. It prevented at least 10 million deaths between 2010 and 20151. Despite substantial evidence for the benefits of immunization, they may not be immediately perceived by the general population. Moreover, misconceptions about vaccine safety still influence public confidence and adherence to immunization programmes. In fact, safety concerns are the main reason for vaccination refusal in Western countries2. Nevertheless, the risks of adverse events following immunization (AEFIs) are very low compared with those associated with the target diseases3–6.
Low public confidence in the safety of immunization programmes may impede vaccination coverage and compromise herd immunity protection. This effect has serious consequences for population7,8. A dramatic recent example was the decline in measles vaccination coverage in Italy. It caused a large outbreak since January 2017 which accounted for 29% of all measles cases reported by EU countries in 2017–20189. At the same time, poliomyelitis vaccine coverage fell below the 95% threshold. Consequently, in June 2017, the Italian Ministry of Health increased the number of compulsory vaccinations from 4 to 10 (diphtheria, tetanus, hepatitis B, poliomyelitis, pertussis, Haemophilus influenzae type b, measles, mumps, rubella, and varicella) for children up to 16 years of age (Law no 119/2017). This policy had a positive impact on all vaccination coverage10.
The new legislation increased public awareness about the importance of vaccines and vaccination. However, parental adherence to vaccination programmes is complex and requires further evaluation11. The EMA Guideline on Good Pharmacovigilance Practices (GVP) states that a significant factor affecting public vaccine confidence is the knowledge that systems are in place to ensure complete and rapid assessment and to take precautionary measures if needed12.
The two main goals of an effective vaccinovigilance system are to: (1) monitor and evaluate vaccine safety through early and timely detection and management of any AEFIs to ensure favourable risk/benefit profiles and the safe use of vaccines, and (2) ensure transparent and up-to-date communication of vaccine risk/benefit profiles and provide information and answers to the public to allay their vaccine safety concerns. Vaccinovigilance, then, can effectively contend with the “social amplification of risk” phenomenon and foster a vaccination culture by increasing public confidence in it13.
The foundation of any safety surveillance system is timely reporting. These systems can trigger safety signals or awareness of new or known adverse events requiring further investigation. The purpose of the changes made to the European Pharmacovigilance (PhV) Legislation of 2012 was to increase the transparency and efficiency of relevant activities, to enhance patient/consumer participation in the PhV system, and to promote incident reporting using various tools, such as the internet14,15. Vaccinovigilance in the Italian PhV system was presented in the form of a survey of 26 European countries16. It was also briefly described in 201517. To date, however, very little has been published on the Italian vaccinovigilance system. Studies describing national vaccinovigilance data have been published for Australia18, China19, Oman20, Switzerland21, and the United States22. Other vaccinovigilance data have been reported for specific regions such as Veneto, Italy23, Valencia, Spain24, and Rondonia, Brazil25. Most of the published data focused either on paediatric populations26 or on specific vaccines27.
The main aim of this study was to describe and analyse the Italian PhV system on vaccines in the last 10 years. Specific objectives were: (i) to describe and analyse the Italian AEFI reporting rate (within the EU context), between 2008 and 2017, (ii) to present a detailed overview of all reported AEFIs and (iii) to provide a brief overview of main signals detected during the study period.
Methods
Data source: Italian pharmacovigilance system and database
The Italian PhV System is based on a network connecting the Italian Medicines Agency (Agenzia Italiana del Farmaco [AIFA]) to 21 regional authorities, including the Regional PhV Centers (RPC), the local health authorities/hospitals/research institutes, the local PhV representatives (LRP), and pharmaceutical companies. The AIFA manages post-marketing drug and vaccine surveillance. RCPs collaborate with the AIFA to monitor adverse drug reactions and detect safety signals. They cooperate with the prevention measures implemented in vaccinovigilance programmes. Adverse reaction reports were collected and analyzed through the National PhV Database (RNF), which was created in 2001. Most of the RNF data consist of spontaneous reports of suspected adverse reactions made by health care professionals (HCPs), patients, and active surveillance projects. Spontaneous reports are transmitted to LRPs in hard copy form or through a web-based system (Vigifarmaco). AEFIs, laboratory tests, and therapeutic indications are coded according to the Medical Dictionary for Regulatory Activities (MedDRA®)28.
All reports are transferred to the European database EudraVigilance managed by the European Medicines Agency (EMA), and to the World Health Organization (WHO) global database (Vigibase).
Data extraction and analysis
AEFI reports from 2008–2017 were extracted from the RNF database. The annual reporting rate (RR) per distributed doses was calculated both overall and per reports with at least one serious AEFI (denominator available since 2009). Moreover, the number of reports from other countries in the same period was obtained from the global database Vigibase. The reporting rate per 1,000,000 inhabitants was calculated for Italy and other EU countries (denominators calculated from Eurostat database)29.
All AEFIs collected by the RNF in the study period were categorized according to primary source, age of vaccine administration, outcome, and seriousness according to the definitions in DIR 2001/83/EC Art 130 and the Important Medical Event (IME) List31.
AEFI reports are grouped according to five age classes: infants (< 23 months), children (2–11 years), adolescents (12–17 years), adults (18–64 years), and elderly (> 65 years).
A report is defined as serious when at least a serious adverse reaction is reported.
Serious adverse reaction is defined as “any untoward medical occurrence that at any dose results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a congenital anomaly/birth defect”30. An AEFI may be deemed serious according to medical judgement if a clinically relevant event is present. EMA developed a list of IME Terms to facilitate the classification of clinically relevant events31.
All reports with at least a serious event are evaluated for the likelihood of a causal relationship between the vaccine and the reported AEFI. Causality is assessed by the RCPs and the AIFA and, since 2015, is evaluated in accordance with the new tool developed by WHO, classifying reports as consistent, inconsistent, indeterminate and unclassifiable32,33.
All collected AEFIs are regularly evaluated for signal detection activity. Signal detection is carried out according to a specific standard operating procedure, including disproportion analysis with the Reporting Odds Ratios and compliance with European provisions and guidelines like such as Good Pharmacovigilance Practices34.
Results
AEFI reporting rates
A total of 46,430 reports were submitted between 2008 and 2017. They referred to 63,457 suspected vaccines and described 96,926 AEFIs. The mean number of AEFIs per report was 2.1.
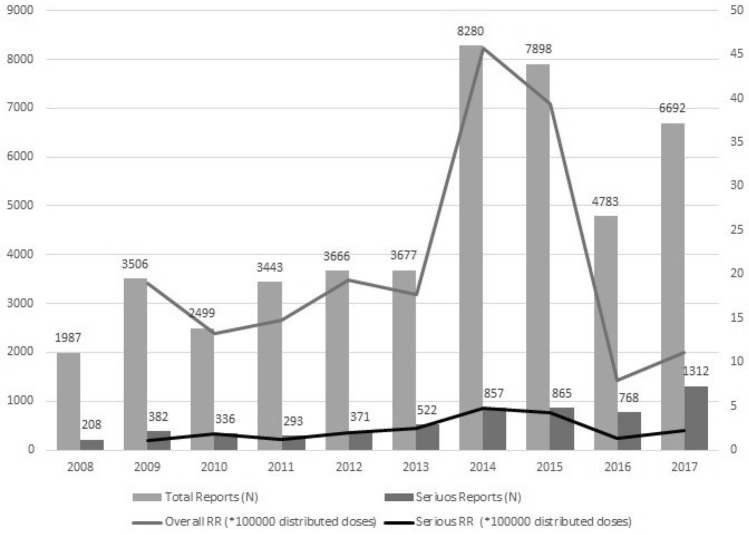
Figure 1 shows the annual RR per distributed dose both overall and per report with at least a serious event (denominator available since 2009). The overall trend shows an increase with time and three peaks in 2009, 2014–15, and 2017 while RR per serious reports show a lower variability over time ranging from 1.3 (2003) to 2.2 (2017) with two peaks in 2014 (4.7) and 2015 (4.3).
Figure 1.
Distribution (N) of reports and Reporting Rate per distributed dose of AEFI by year, from 2008 to 2017.
The increase in 2014 and 2015 is related to the doubled proportion of cases of hyperpyrexia in comparison to previous years. Considering the whole study period, overall RR and RR per reports with at least one serious AEFI were respectively 17.1 and 2.2 per 100,000 distributed doses. More than 770,000 vaccine related reports were sent to the WHO Vigibase during this decade. Most of them originated in the US (57.2%) and Europe (29.0%). Italy had the second-highest absolute number of reports worldwide after the US. Among the European countries, Italy accounted for the highest number of reports (19.4%), followed by Germany (17.4%), the Netherlands (10.2%), France (8.9%), the UK (8.4%), and Spain (6.9%).
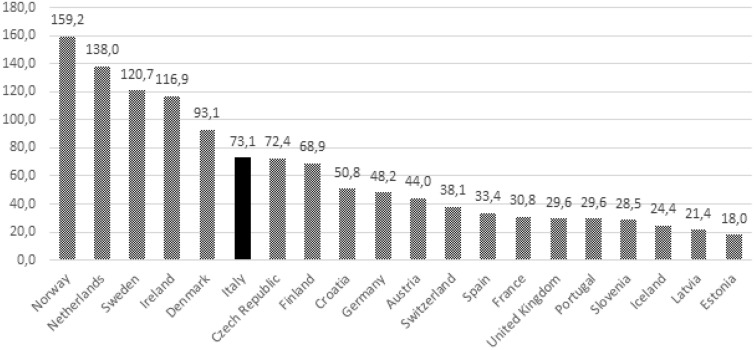
Figure 2 shows the AEFI RR per 1,000,000 inhabitants by EU country. The 20 countries with the highest reporting rates from 2008 to 2017 were considered.
Figure 2.
Reporting rate of AEFI per 1,000,000 inhabitants by EU Country among the study period (2008–2017).
General report characteristics
Almost all reports (95.6%) had the most important information needed for a causality assessment evaluation, including time of onset of the reaction, date of vaccine administration, reporter role, age group, gender, and seriousness based on the seriousness criteria. Moreover, all reports with the most serious adverse events had not only the AEFI description, but also further narratives of the whole case written by the reporter, with the attachment of detailed documents such as hospital clinical charts.
Table 1 shows the distribution of reports according to the source. A large number of reports came from doctors: among these, most worked in vaccination centres (50%), 15% worked in hospitals, 5,9% were general practitioners and 5,3% were paediatricians. Other health operators include nurses and health care assistants at the vaccine centres. The number of reports from citizens was significantly higher in 2017 than the previous years (889 reports in 2017, 43% of total reports from citizens). More than half of these referred to events which occurred in earlier years.
Table 1.
Distribution of reports by source.
| Source | N. of reports | % |
|---|---|---|
| Doctors | 32,233 | 69.4 |
| Pharmacists | 2,148 | 4.6 |
| Other health operators | 9,928 | 21.4 |
| Citizens | 2,054 | 4.4 |
| Not available | 67 | 0.1 |
| Total | 46,430 | 100.0 |
Table 2 shows the distribution of reports according to age groups. The highest part of reports are related to infants. The proportions of serious AEFIs varied among the different age classes, with the highest value in the elderly.
Table 2.
Distribution of reports by age group with percentages of serious reports.
| Age group | N. of reports | % | % serious |
|---|---|---|---|
| Infants (< 23 months) | 25,863 | 55.7 | 13.5 |
| Children (2–11 years) | 9,115 | 19.6 | 9.6 |
| Adolescents (12–17 years) | 3,051 | 6.6 | 11.6 |
| Adults (18–64 years) | 5,747 | 12.4 | 9.8 |
| Elderly (> 65 years) | 2,466 | 5.3 | 24.0 |
| Not reported | 188 | 0.4 | 19.7 |
| Total | 46,430 | 100.0 | 12.7 |
Description of reports with at least a serious AEFI
Of the 46,430 reports collected for the decade, 5,914 (12.7%) were classified as serious. Among these, 2,794 (47.2%) were cases requiring hospitalization (including ER admission) or where hospitalization was prolonged. Another 2,617 (44.3%) referred to other relevant events. Causality assessment evaluation of reported adverse events has been performed for reports with at least a serious event. Since 2015, when the new tool for causality assessment developed by the WHO was used, only 7.7% of reports lacked adequate information and were categorized as unclassifiable. The majority of serious reports were considered consistent with the vaccination (64.0%), 11.1% were indeterminate and 17.2% were inconsistent. The proportion of both unclassifiable and inconsistent reports increased from 2015 to 2017, mainly due to reports from patients. The most frequently reported serious reactions in inconsistent reports were hyperpyrexia and febrile convulsions (due to unrelated time of onset) and autism spectrum disorders. Outcome data were available for 90.9% (42,184/46,430) of the reports. Of these, 84% recovered and 11.2% improved. Fatal outcomes were reported for 0.3% of the total AEFIs (N = 129). None of these outcomes in the causality assessment showed a consistent causal association with vaccination. More than 80% of fatal reports involved influenza vaccines administered to the elderly adults.
Table 3 shows the distribution of AEFIs by most commonly reported vaccine type and the proportion of serious reported events.
Table 3.
Distribution of AEFIs with > 500 reports per vaccine type, and AEFI seriousness.
| Vaccine | Total AEFIs (N) | Serious AEFIs (%) |
|---|---|---|
| DTaP-HB-IPV-HIB | 10,429 | 13.7 |
| PNEUMO-con13 | 7,950 | 12.7 |
| MMR | 7,909 | 13.4 |
| MMRV | 5,375 | 14.3 |
| HPV | 5,243 | 5.9 |
| VAR | 4,589 | 9.3 |
| MENB | 3,967 | 13.0 |
| FLU | 2,587 | 21.8 |
| DTaP-IPV | 2,443 | 8.3 |
| MENC-con | 1,968 | 18.0 |
| PNEUMO-con | 1,409 | 14.9 |
| FLU_H1N1 | 1,356 | 8.0 |
| MEN_4-con | 1,122 | 16.8 |
| DT | 844 | 7.9 |
| DTaP | 820 | 13.8 |
| PNEUMO | 763 | 9.8 |
| DTP | 713 | 13.6 |
| HA | 677 | 14.9 |
| TT | 563 | 9.4 |
| FLU_ad | 524 | 31.5 |
| ROTAVIRUS | 524 | 16.8 |
DTaP-HB-IPV-HIB: diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio, and Haemophilus influenzae type b; PNEUMO-con13: pneumococcal conjugate vaccine (13-valent); MMR: measles, mumps & rubella; MMRV: measles, mumps, rubella & varicella; HPV: human papillomavirus vaccine; VAR: varicella vaccine; MENB: serogroup B meningococcal vaccine; FLU: flu vaccine; DTaP-IPV: diphtheria, inactivated polio; MENC-con: meningococcal C conjugate vaccine; PNEUMO-con: pneumococcal conjugate vaccine; FLU_H1N1: flu H1N1 vaccine; MEN_4-con: meningococcal conjugate vaccine, quadrivalent; DT: diphtheria, tetanus; DTaP: diphtheria and tetanus toxoids and acellular pertussis vaccine; PNEUMO: pneumococcal vaccine; DTP: diphtheria and tetanus toxoids and whole-cell pertussis vaccine; HA: hepatitis A virus vaccine; TT: tetanus vaccine; FLU_ad: influenza vaccine, adjuvanted.
Often more than one vaccine is administered to the same person during the same vaccination session. The most commonly reported vaccine combinations were: hexavalent + pneumococcal conjugate 13 (PCV-13) (6,082/46,430, 13.1%), MMR + V (3,929/46,430, 8.5%) and hexavalent + pneumococcal conjugate (PCV7) (1,030/46,430, 2.2%). The vaccines with the highest proportion of serious events were INF (21.8%) and INF-ad (31.5%). Table 4 shows the most frequently reported AEFIs according to MedDRA Preferred Terms.
Table 4.
List of the most frequently reported AEFIs (> 500).
| MedDRA PT | MedDRA SOC | Reports (N) |
|---|---|---|
| Pyrexia | General disorders and administration site conditions | 16,763 |
| Hyperpyrexia | General disorders and administration site conditions | 5,165 |
| Vaccination site pain | General disorders and administration site conditions | 2,898 |
| Headache | Nervous system disorders | 2,741 |
| Post-vaccinal Irritability | General disorders and administration site conditions | 2,687 |
| Morbilliform rash | Skin and subcutaneous tissue disorders | 2,494 |
| Pain | General disorders and administration site conditions | 2,161 |
| Injection site erythema | General disorders and administration site conditions | 2,083 |
| Vaccination site swelling | General disorders and administration site conditions | 2,039 |
| Somnolence | Nervous system disorders | 1,835 |
| Erythema | Skin and subcutaneous tissue disorders | 1,809 |
| Rash | Skin and subcutaneous tissue disorders | 1,679 |
| Crying | General disorders and administration site conditions | 1,515 |
| Urticaria | Skin and subcutaneous tissue disorders | 1,499 |
| Vomiting | Gastrointestinal disorders | 1,470 |
| Vaccination site reaction | General disorders and administration site conditions | 1,376 |
| Irritability | Psychiatric disorders | 1,295 |
| Cough | Respiratory, thoracic and mediastinal disorders | 1,228 |
| Nasopharyngitis | Infections and infestations | 1,187 |
| Local reaction | General disorders and administration site conditions | 1,096 |
| Diarrhea | Gastrointestinal disorders | 1,094 |
| Myalgia | Musculoskeletal and connective tissue disorders | 1,079 |
| Injection site pain | General disorders and administration site conditions | 1,062 |
| Asthenia | General disorders and administration site conditions | 1,048 |
| Restlessness | Psychiatric disorders | 1,011 |
| Arthralgia | Musculoskeletal and connective tissue disorders | 989 |
| Nausea | Gastrointestinal disorders | 949 |
| Flushing | Vascular disorders | 921 |
| Agitation | Psychiatric disorders | 906 |
| Pruritus | Skin and subcutaneous tissue disorders | 853 |
| Decreased appetite | Metabolism and nutrition disorders | 824 |
| Pain in extremity | Musculoskeletal and connective tissue disorders | 754 |
| Lymphadenopathy | Blood and lymphatic system disorders | 700 |
| Swelling | General disorders and administration site conditions | 605 |
| Edema | General disorders and administration site conditions | 570 |
| Malaise | General disorders and administration site conditions | 559 |
| Abdominal pain | Gastrointestinal disorders | 556 |
| Hypotonia | Nervous system disorders | 548 |
| Vaccination site edema | General disorders and administration site conditions | 504 |
| Febrile convulsion | Nervous system disorders | 502 |
There were 8,831/96,731 reported IMEs (9.1%). The most frequently reported IMEs were hyperpyrexia, seizures, and loss of consciousness. Various types of seizures/epilepsy have been reported along with fever (pyrexia or hyperpyrexia) and described as febrile convulsions. There were 502 cases reported as febrile convulsions. Another 227 cases presented with these symptoms but were described using a combination of terms related to both seizures and fever. Sixty-five reports described autism spectrum disorders. These were deemed unrelated to the vaccination in the causality assessment.
Most significant safety signals
During the study period, all potential signals were routinely and closely investigated and analysed. In most cases, the potential signals were not confirmed during the validation procedure. However, when needed, monitoring was integrated with active surveillance by conducting epidemiological studies. The most relevant safety signals in the study period included the risk of febrile seizures related to measles, mumps, rubella, varicella (MMRV) vaccine, neurological events and convulsions related to the co-administration of 13-valent pneumococcal polysaccharide conjugate (PCV13) and hexavalent vaccines, Guillain–Barré Syndrome (GBS) after influenza vaccination and intussusception after rotavirus vaccine. Finally, no reports of postural orthostatic tachycardia syndrome (POTS) or complex regional pain syndrome (CRPS) were identified following HPV vaccination.
Discussion
Due to the known limitations of the pre-marketing clinical trials, the spontaneous reporting systems are the cornerstone of post-marketing drug safety surveillance35. Spontaneous reporting is essential in signal detection and the identification of new risks associated with drugs, but can also be very useful in the evaluation of the safety profile of both drugs and vaccines36. There are no globally accepted indicators which could demonstrate the functionality of an AEFI surveillance system. However, underreporting and incomplete reports are the most important limitations of spontaneous reporting37,38, so a high number of reports and a high good quality of the reported information are clearly related to the strength of the system. Moreover, a strong collaboration between the drug regulatory agency and the national immunization programme and the availability of reliable information on the actual number of immunizations have both been reported as essential components of an efficient surveillance system of AEFIs39.
The absolute number of reports in Italy is the highest one in Europe and the worldwide second after that the US. Despite this huge amount of information, up to our knowledge this is the first attempt to provide an overview of Italian vaccinovigilance safety data.
The numbers show an increasing trend since 2008, in line with what has been observed in many other countries40,41. Of all European nations, Italy had highest number of reports among European countries with at least 10 million inhabitants.
The increase in reports is the result of the combined global efforts to improve vaccinovigilance systems.
In Italy, the collaboration between the AIFA and the RPCs promoted reporting activity substantially using various strategies such as enhancements endorsed at the policy level, interventions to stimulate AEFI reporting in vaccination districts, and the development of health care education and training programmes. Since 2009, the AIFA committed to disseminating vaccinovigilance safety data to ensure transparent management of emerging issues and to favour the diffusion of accurate information to the public. Italy submitted the most AEFIs of all European countries to the WHO during the survey period, indicating that the Italian vaccinovigilance system has expanded over time. The number of reports and the reporting rates related to population or to administered doses are very useful as performance indicators of the system to monitor the trends over time. However, they do not provide information on the quality of the system, particularly on the ability of the system to detect vaccine safety problems40.
According to our results, AEFI reports were of high quality, allowing for the analysis of a large amount of detailed information. A study by the WHO reported that Italy had the highest rate of well-documented reports (65%) among nations submitting > 1,000 reports per year42. The observed data quality, together with the ability of the system to detect serious AEFIs, supports the reliability of the results.
The peaks in the observed Italian AEFI reporting rates per 100,000 distributed doses correlate with those of active surveillance programmes complementing passive monitoring systems43–45. They help to raise awareness towards specific potential safety issues and increase the attention paid to new vaccines when market experience is still limited (e.g. HPV or MenB introduction).The 2009 peak in AEFI reporting may be explained by the introduction of the HPV vaccine in 2008 and monitoring of the H1N1 cases during the flu pandemic.
The AEFI reporting peak observed in 2017 may be associated with the increase in the number of compulsory vaccinations from 4 to 10 mandated by Law No. 119/2017 enforced in July 201746. Moreover, in 2017 the National Vaccinating Plan47 expanded the scope of free vaccinations. The changes contributed to increasing the AEFI RR in 2017.
Reporting rates per administered doses were not calculated due to unavailability of the data along the study period in Italy. Efforts focus on developing and implementing computerized immunization registries in as many regions as possible. The comparison between reporting rates and background rates of the diseases is very important in AEFI detection. Background rates of possible adverse events are a crucial part of the assessment of possible vaccine safety concerns and help to separate causally related events from those only temporally associated with but not caused by vaccination48,49.
A survey conducted in 2012 showed that only six of the 15 fully computerized regions could automatically calculate vaccination coverage50. This number increased to 9 of the 18 fully computerized on 21 total regions in 201651. The regional variability can be explained by the decentralization of the Italian health care system51. However, Law No. 119 of July 31, 2017 ordered the implementation of a national immunization registry at the Ministry of Health. To improve the relationship between the Drug Agency and the Public Health Authority, in 2014, a Vaccinovigilance Working Group (VWG) was created by the AIFA. It included the National Institute of Health, the Minister of Health, and both PhV and public health members. The main aim of this group was to contribute to signal detection activities.
More than half of the AEFIs originated either from doctors or other health care professionals. Patients have the lowest reporting frequency of all groups. Patient reporting may play an important role in pharmacovigilance. Patients not only contribute to reducing the underreporting of adverse reactions, but the quality of their reports is similar to the quality of healthcare operators. In comparison to doctors and pharmacists the description of the reported adverse reactions is often more detailed with a different perspective52–54. Patient empowerment projects improve the efficacy of a passive surveillance system and facilitate the creation of a trusting relationship that dispels false beliefs and allays fears regarding vaccination.
In 2013, a project to stimulate AEFI reporting from both patient and health care operators significantly increased the reporting rate per 100,000 administered vaccine doses (from a mean of 1,7 per 100,000 administered doses in 2009–2012 to approximately 14.0 per 100,000 in 2013 after implementing the intervention)55. The lower variability of RR per reports with at least a serious event compared with RR per administered doses suggests a limited underreporting for serious AEFIs. Approximately 13% of the reports included at least a serious AEFI. This percentage is higher in comparison to other published data56–58 and could suggest a higher risk of serious events associated to vaccinations. However, all serious AEFIs must be closely investigated to establish any causal relationship with vaccine administration.
Nearly all cases reported as serious either required hospitalization/prolonged hospitalization or experienced important medical events. Together, they accounted for > 90% of all reported AEFIs. However, hospitalized cases included also those treated in the ER and discharged without admission, because the reported event was resolved.
Among the important medical events, one of the most frequently reported serious AEFIs was high fever which accounted for almost 2/3 of the total reported IME. It is a relatively common adverse event, but not usually dangerous. The second most commonly reported event was seizure. Nevertheless, most of these cases were associated with hyperpyrexia. According to the CDC, febrile seizures are fairly common, with a lifetime prevalence ≤ 5%. Nevertheless, febrile seizures rarely occur after vaccination59. The proportion of reported AEFIs that were rated serious was significantly higher among the elderly adults than among those in other age groups. This age group includes a higher percentage of frail people including patients suffering from chronic diseases or other medical conditions. Therefore, this age group may substantially increase the total rate of reported serious events including those not attributable to the vaccines.
Approximately 2 out of 3 of the fatal cases were adults or elderly patients, who had received influenza vaccine. Most of these reports were posted in 2014 and were related with the prudent withdrawal of two batches of conjugated flu vaccine (trivalent influenza vaccine with MF59C.1 adjuvant) after three post-vaccination deaths were reported60. After the evaluation by EMA the two flu vaccine batches were reintroduced, since none of the reported deaths had any consistent causal relationship with the flu vaccine.
The reporting rate of serious report was quite stable over time indicating the capacity of the system to detect safety issues. The highest incidences of AEFI reporting occurred for the most frequently administered vaccines, namely, hexavalent, pneumococcus, MMR, and MMRV. The high number of AEFIs reported for HPV can be explained by active surveillance. For this vaccine, even minor reactions and incidents were reported to the RNF. Compared with the other vaccines, HPV accounted for only a small proportion of the reported serious events. Flu vaccines were associated with the highest proportion of serious reported events (≤ 1/3 of all reports for MF59 adjuvanted flu vaccine). The most frequently reported AEFIs included fever, pain, asthenia, injection site reactions, rash, allergic reactions, headache, drowsiness, and febrile seizures.
The main goal of spontaneous reporting systems is to identify signals, and during the study period, all potential signals were closely investigated and analysed. The international literature was reviewed to evaluate each signal. Moreover, formal studies were conducted to investigate the most significant signals. The most important signals in the study period included the following:
Higher risk of febrile seizures with the measles, mumps, rubella and varicella (MMRV) vaccine compared with separate vaccination with measles, mumps and rubella (MMR) and varicella. Product information of the involved vaccines was updated, and a communication was sent to all the health professionals61
Risks of neurological events and convulsions after the co-administration of 13-valent pneumococcal polysaccharide conjugate (PCV13) and hexavalent vaccines. A comparative safety evaluation of 7-valent and 13-valent pneumococcal vaccines in routine paediatric vaccinations was conducted in four Italian regions from 2009 to 201162. A slightly increased risk of neurological events or convulsions was found but, due to the study limitations, no regulatory decision was taken.
Risk of Guillain–Barré Syndrome (GBS) after influenza vaccination. A case–control study was conducted in 2010 in seven regions of Italy to explore this correlation (GBS). Influenza vaccination was associated with GBS, with a relative risk of 2.1 (95% CI 1.1, 3.9). The results of the study did not modify the risk–benefit profile of seasonal influenza vaccination. The attributable risk in adults ranged from two to five GBS cases per 1,000,000 vaccinations63,64
Risk of intussusception associated with the administration of rotavirus vaccines. A study was conducted to investigate the representative intussusception incidence background in the different age groups and its temporal trend in Italy65. The overall intussusception incidence rate was 21 per 100,000 children aged ≤ 15 years and higher among boys than girls.
Strength and limitations
To our knowledge, no studies have been published on global Italian vaccinovigilance data. Previous literature presented data either at the regional level or for the paediatric population. No comprehensive view of Italian vaccinovigilance activity or its evolution over time has yet been reported. The strength of this study is to fill in this information gap and make data available for international comparison. A limitation of the present study is that the AEFI reporting rates cannot be related to the actual number of administered doses. Another limitation concerns the timing of the report updates. In the present study, the AEFIs were analysed according to the date of receipt of reports by the RNF and not by the date of vaccine administration. This could have an impact on the reporting rate due to the delayed reporting.
Conclusion
This report is an overview of the Italian vaccinovigilance data. Over the past decade, the system has allowed for the detection of a large amount of reliable data, including information regarding serious AEFIs. About ten multicentre active surveillance projects were conducted to collect and monitor a larger amount of safety-related data. Timely analyses of all signals were performed across the nation. The reporting rate and the analysis of serious reports from 10 years of AEFIs support the risk/benefit profiles of vaccines. The low participation rate of citizens in vaccine safety management emerged as an important issue. The quality of the data is strictly related to the availability of precise denominators (most importantly the number of administered doses or at least the number of distributed doses). Further efforts need to be implemented to have national vaccine registries and to link this information with surveillance data. Transparency, information, and population empowerment on immunization safety must be promoted through effective communication.
Author contributions
U.M. and L.G. developed the research idea. F.M. and G.S. wrote the first draft of the manuscript, which was reviewed and integrated by each author according to his or her personal expertise. U.M., F.M., S.G., and L.G. compiled and analysed the data and the main results. All other authors helped interpret the data and provided significant intellectual contributions to the manuscript. All authors approved the final version of the manuscript.
Funding
The study was not funded.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO: Ten years in public health, 2007–2017. Report by Dr Margaret Chan, Director-General, World Health Organization. https://www.who.int/publications/10-year-review/en/ (2017).
- 2.McIntosh ED, Janda J, Ehrich JH, Pettoello-Mantovani M, Somekh E. Vaccine hesitancy and refusal. J. Pediatr. 2016;175:248–249. doi: 10.1016/j.jpeds.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Giambi C, et al. Parental vaccine hesitancy in Italy—results from a national survey. Vaccine. 2018;36:779–787. doi: 10.1016/j.vaccine.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 4.My C, Danchin M, Willaby HW, Pemberton S, Leask J. Parental attitudes, beliefs, behaviours and concerns towards childhood vaccinations in Australia: a national online survey. Aust. Fam. Phys. 2017;46:145–151. [PubMed] [Google Scholar]
- 5.Principi N, Esposito S. Adverse events following immunization: real causality and myths. Expert Opin. Drug Saf. 2016;15:825–835. doi: 10.1517/14740338.2016.1167869. [DOI] [PubMed] [Google Scholar]
- 6.Di Pasquale A, et al. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016;34:6672–6680. doi: 10.1016/j.vaccine.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Rashid H, Khandaker G, Booy R. Vaccination and herd immunity: what more do we know? Curr. Opin. Infect Dis. 2012;25:243–249. doi: 10.1097/QCO.0b013e328352f727. [DOI] [PubMed] [Google Scholar]
- 8.Pollard SL, et al. Estimating the herd immunity effect of rotavirus vaccine. Vaccine. 2015;33:3795–3800. doi: 10.1016/j.vaccine.2015.06.064. [DOI] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control (ECDC). Monthly measles and rubella monitoring report, January 2019. Stockholm: ECDC. https://ecdc.europa.eu/sites/portal/files/documents/measles-rubella-monthly-surveillance-report-january-2019.pdf (2019).
- 10.D’Ancona F, et al. Introduction of new and reinforcement of existing compulsory vaccinations in Italy: first evaluation of the impact on vaccination coverage in 2017. Euro Surveill. Preprint at: https://www.igienistionline.it/docs/2018/19eurosurveillance.pdf (2018). [DOI] [PMC free article] [PubMed]
- 11.Karafillakis E, Larson HJ; ADVANCE consortium. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine.35, 4840–4850 (2017). [DOI] [PubMed]
- 12.European Medicines Agency (EMA). Guideline on good pharmacovigilance practices (GVP) Product- or Population-Specific Considerations I: Vaccines for prophylaxis against infectious diseases.https://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/12/WC500157839.pdf (2013).
- 13.Kasperson RE, et al. The social amplification of risk: a conceptual framework. Risk Anal. 1988;8:177–187. [Google Scholar]
- 14.EU Regulation No 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, and Regulation (EC) No 1394/2007 on advanced therapy medicinal products. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol1/reg_2010_1235/reg_2010_1235_en.pdf (2010).
- 15.EU Directive No 2010/84/ of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol1/dir_2010_84/dir_2010_84_en.pdf (2010)
- 16.Zanoni G, et al. Vaccine adverse event monitoring systems across the European Union countries: time for unifying efforts. Vaccine. 2009;27:3376–3384. doi: 10.1016/j.vaccine.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 17.Santuccio C, Trotta F, Felicetti P. Ongoing pharmacovigilance on vaccines. Pharmacol. Res. 2015;92:2–5. doi: 10.1016/j.phrs.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Clothier HJ, et al. Consumer reporting of adverse events following immunization. Hum. Vaccin. Immunother. 2014;10:3726–3730. doi: 10.4161/hv.34369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, et al. Surveillance for adverse events following immunization from 2008 to 2011 in Zhejiang Province, China. Clin. Vaccine Immunol. 2013;20:211–217. doi: 10.1128/CVI.00541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Awaidy S, Bawikar S, Prakash KR, Al Rawahi B, Mohammed AJ. Surveillance of adverse events following immunization: 10 years' experience in Oman. East Mediterr. Health J. 2010;16:474–480. [PubMed] [Google Scholar]
- 21.Schumacher Z, Bourquin C, Heininger U. Surveillance for adverse events following immunization (AEFI) in Switzerland 1991–2001. Vaccine. 2010;28:4059–4064. doi: 10.1016/j.vaccine.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhou W, et al. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. MMWR Surveil. Summ. 2003;52:1–24. [PubMed] [Google Scholar]
- 23.Micheletti F, Moretti U, Tridente G, Zanoni G. Consultancy and surveillance of post-immunization adverse events in the Veneto region of Italy for 1992–2008. Hum. Vaccine. 2011;7:1–6. doi: 10.4161/hv.7.0.14613. [DOI] [PubMed] [Google Scholar]
- 24.Alguacil-Ramos AM, et al. Surveillance for adverse events following immunization (AEFI) for 7 years using a computerized vaccination system. Public Health. 2016;135:66–74. doi: 10.1016/j.puhe.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Cunha MPL, Dórea JG, Marques RC, Leão RS. Vaccine adverse events reported during the first ten years (1998–2008) after introduction in the State of Rondonia, Brazil. Biomed. Res. Int. 2013;1:853083. doi: 10.1155/2013/853083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrajolo C, et al. Pediatric drug safety surveillance in Italian pharmacovigilance network: an overview of adverse drug reactions in the years 2001–2012. Expert Opin. Drug Saf. 2014;13(suppl 1):S9–S20. doi: 10.1517/14740338.2014.939581. [DOI] [PubMed] [Google Scholar]
- 27.Härmark L, van Hunsel F, Hak E, van Grootheest K. Monitoring the safety of influenza A (H1N1) vaccine using web-based intensive monitoring. Vaccine. 2011;29:1941–1947. doi: 10.1016/j.vaccine.2010.12.123. [DOI] [PubMed] [Google Scholar]
- 28.MedDRA MSSO. Introductory Guide MedDRA Version 23.0.https://www.meddra.org/sites/default/files/guidance/file/intguide_23_0_english.pdf (2020)
- 29.Eurostat: Population: demography, population projections, census, asylum and migration. https://ec.europa.eu/eurostat/web/population/overview (2020).
- 30.EU Directive 2010/84/EU of the European Parliament and of the council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use on the Community code relating to medicinal products for human use. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol (2010).
- 31.EMA. Inclusion/exclusion criteria for the “Important Medical Events” list.https://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/08/WC500212100.pdf (2018).
- 32.WHO. Causality Assessment of an Adverse Event Following Immunization (AEFI), User manual for the revised WHO classification (second edition). https://www.who.int/vaccine_safety/publications/gvs_aefi/en/ (2018).
- 33.Tozzi AE, et al. Assessment of causality of individual adverse events following immunization (AEFI): a WHO tool for global use. Vaccine. 2013;31:5041–5046. doi: 10.1016/j.vaccine.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 34.EMA. Guidelines on good pharmacovigilance practices (GVP).https://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2017/10/WC500236404.pdf. (2017).
- 35.Härmark L, van Grootheest AC. Pharmacovigilance: methods, recent developments and future perspectives. Eur. J. Clin. Pharmacol. 2008;64:743–752. doi: 10.1007/s00228-008-0475-9. [DOI] [PubMed] [Google Scholar]
- 36.Bonaldo G, Vaccheri A, D'Annibali O, Motola D. Safety profile of human papilloma virus vaccines: an analysis of the US Vaccine Adverse Event Reporting System from 2007 to 2017. Br. J. Clin. Pharmacol. 2019;85:634–643. doi: 10.1111/bcp.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Niu R, et al. The quality of spontaneous adverse drug reaction reports from the pharmacovigilance centre in western China. Expert Opin. Drug Saf. 2019;18:51–58. doi: 10.1080/14740338.2019.1559812. [DOI] [PubMed] [Google Scholar]
- 39.Lankinen KS, et al. Vaccinovigilance in Europe—need for timeliness, standardization and resources. Bull. World Health Organ. 2004;82:828–835. [PMC free article] [PubMed] [Google Scholar]
- 40.Lei J, Balakrishnan MR, Gidudu JF, Zuber PLF. Use of a new global indicator for vaccine safety surveillance and trends in adverse events following immunization reporting 2000–2015. Vaccine. 2018;36:1577–1582. doi: 10.1016/j.vaccine.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dey A, Wang H, Quinn H, Pillsbury A, Glover C, Hickie M, Wood N, Beard F, Macartney K. Surveillance of adverse events following immunisation in Australia: annual report, 2018. Commun. Dis. Intell. 2020;44:1–28. doi: 10.33321/cdi.2020.44.12. [DOI] [PubMed] [Google Scholar]
- 42.Bergvall T, Nore GN, Lindquist M. VigiGrade: a tool to identify well-documented Individual case reports and highlight systematic data quality issues. Drug Saf. 2014;37:65–77. doi: 10.1007/s40264-013-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasparini R, et al. Safety and tolerability of bivalent HPV vaccine: an Italian post-licensure study. Hum. Vaccin. 2011;7:136–146. doi: 10.4161/hv.7.0.14576. [DOI] [PubMed] [Google Scholar]
- 44.Spila-Alegiani S, Da Cas R, Giambi C, Raschetti R, Salmaso S. Il registro del vaccino anti HPV. [The register of anti HPV vaccine] Recent Prog. Med. 2013;104:262–266. doi: 10.1701/1295.14327. [DOI] [PubMed] [Google Scholar]
- 45.Cocchio S, et al. A postmarket safety comparison of 2 vaccination strategies for measles, mumps, rubella and varicella in Italy. Hum. Vaccin. Immunother. 2016;12:651–654. doi: 10.1080/21645515.2015.1101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AIFA. Rapporto sulla sorveglianza postmarketing dei vaccini in Italia 2017. [Report of vaccine postmarketing surveillance in Italy 2017]. https://www.agenziafarmaco.gov.it/content/rapporto-vaccini-2017-sorveglianza-postmarketing-italia (2018).
- 47.Piano Nazionale Prevenzione Vaccinale 2017–19 [National prevention plan 2017–19]. https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (2017).
- 48.Deeks SL, et al. Estimating background rates of Guillain–Barré Syndrome in Ontario in order to respond to safety concerns during pandemic H1N1/09 immunization campaign. BMC Public Health. 2011;11:329. doi: 10.1186/1471-2458-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, et al. The expected number of background disease events during mass immunization in China. PLoS ONE. 2013;8:e71818. doi: 10.1371/journal.pone.0071818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alfonsi V, et al. Regional coordinators for infectious diseases and vaccinations. Immunisation registers in Italy: a patchwork of computerisation. Euro Surveill.https://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20156 (2012). [DOI] [PubMed]
- 51.D'Ancona F, Gianfredi V, Riccardo F, Iannazzo S. Immunisation Registries at regional level in Italy and the roadmap for a future Italian National Registry. Ann. Ig. 2018;30:77–85. doi: 10.7416/ai.2018.2199. [DOI] [PubMed] [Google Scholar]
- 52.Hazell L, et al. On behalf of the yellow card study collaboration. how do patients contribute to signal detection? a retrospective analysis of spontaneous reporting of adverse drug reactions in the UK’s yellow card scheme. Drug Saf. 36, 199–206 (2013). [DOI] [PubMed]
- 53.Inácio P, Cavaco A, Airaksinen M. The value of patient reporting to the pharmacovigilance system: a systematic review. Br J. Clin. Pharmacol. 2017;83:227–246. doi: 10.1111/bcp.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Härmark L, et al. A patient-reported safety information: a renaissance of pharmacovigilance? Drug Saf. 2016;39:883–890. doi: 10.1007/s40264-016-0441-x. [DOI] [PubMed] [Google Scholar]
- 55.Alicino C, et al. Routine surveillance of adverse events following immunization as an important tool to monitor vaccine safety. The two-years’ experience of the Liguria Region, Italy. Hum. Vaccin. Immunother. 2015;11:91–94. doi: 10.4161/hv.34360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmadipour N, et al. Vaccine safety surveillance in Canada: Reports to CAEFISS, 2013–2016. Can. Commun. Dis. Rep. 2018;44:206–214. doi: 10.14745/ccdr.v44i09a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomsen PO, Saldaña VA, Cerda LJ, Abarca VK. Vaccine safety: description of the adverse events reported to the surveillance system in Chile, 2014 to 2016. Rev. Chilena Infectol. 2019;36:461–468. doi: 10.4067/S0716-10182019000400461. [DOI] [PubMed] [Google Scholar]
- 58.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2015;33:4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CDC Vaccine Safety. Common concerns: childhood vaccines and febrile seizures.https://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html (2016).
- 60.AIFA. AIFA dispone il divieto di utilizzo per due lotti del vaccino antinfluenzale FLUAD 27/11/2014 [AIFA disposal on a ban on the use of two batches of FLUAD flu vaccine 11/27/2014]. https://www.agenziafarmaco.gov.it/content/aifa-dispone-il-divieto-di-utilizzo-due-lotti-del-vaccino-antinfluenzale-fluad-0 (2014).
- 61.AIFA. Comunicato AIFA per gli operatori sanitari: Aggiornamento sul rischio di febbre e convulsioni febbrili dopo somministrazione della prima dose di vaccino tetravalente morbillo, parotite, rosolia e varicella [AIFA release for health professionals: Update on the risk of fever and febrile convulsions after administration of the first dose of tetravalent measles, mumps, rubella and varicella vaccine]. https://www.aifa.gov.it/sites/default/files/comunicato_AIFA_MPRV.pdf (2013).
- 62.Trotta F, Rizzo C, Santuccio C, Bella A. the Pharmacovigilance Study Group on Pneumococcal Comparative safety evaluation of 7-valent and 13-valent pneumococcal vaccines in routine paediatric vaccinations in four Italian regions, 2009 to 2011. Euro Surveill. 2015;20:21–28. doi: 10.2807/1560-7917.es2015.20.7.21041. [DOI] [PubMed] [Google Scholar]
- 63.Benedetti MD, et al. A multicentric prospective incidence study of Guillain–Barré syndrome in Italy. The ITANG study. Neuroepidemiology. 2015;45:90–99. doi: 10.1159/000438752. [DOI] [PubMed] [Google Scholar]
- 64.Galeotti F, et al. Risk of Guillain–Barré syndrome after 2010–2011 influenza vaccination. Eur. J. Epidemiol. 2013;28:433–444. doi: 10.1007/s10654-013-9797-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trotta F, Da Cas R, Bella A, Santuccio C, Salmaso S. Intussusception hospitalizations incidence in the pediatric population in Italy: a nationwide cross-sectional study. Ital. J. Pediatr. 2016;42:89. doi: 10.1186/s13052-016-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]