Abstract
A novel coronavirus was emerged in December 2019 from Wuhan city, China and has now become a global threat to human health. Currently, the coronavirus disease 2019 (COVID-19) has spread to more than 34 countries with 2,445 deaths and 78,811 confirmed cases. Currently, there is no vaccine available against COVID-19. The traditional vaccines development requires more time and high cost and due to this, the disease outbreaks becomes more challenging. Now a days, plants have become more attractive platform for edible vaccine production than the other system. The development of an edible vaccine in a selected plant system has many significant advantages such as; easy and efficient oral delivery, low cost with higher scale production, avoidance of any trained medical personnel for delivery, lack of any pathogenic infection, multicomponent expression in a single plant, and so forth. In this manuscript, the concept, development, and importance of an edible vaccine have been discussed. By using this plant-based platform, an edible vaccines can be produced in many crops like banana, cucumber, carrot, lettuce, and tomato against various diseases. Due to increasing cases globally with COVID-19, there is an urgent requirement to develop an ideal vaccine and antiviral therapy against this virus to control the disease worldwide.
Keywords: Edible vaccines, COVID-19, Wuhan, Pneumonia, Coronavirus
Introduction
The pneumonia of unexplained etiology was reported from Wuhan city, Hubei province, China in mid-December, 2019 by the Wuhan Municipal Health Commission and on 8 January 2020 [1]. The first case of the onset of illness with pneumonia symptoms was reported on 8 December 2019 by Municipal Health Commission and on 31 December 2019. The cases were linked with seafood and animal market indicated that the virus transmission from animal to human. The increasing number of cases with no exposure to seafood market supporting the evidence of human to human transmission. The causative agent was initially suspected to be associated with coronaviruses (CoVs), based on the developed symptoms like fever, cough, and shortness of breath and designated as Wuhan CoV-1. But later, the viral RNA was isolated and sequencing was performed. Based on sequencing and phylogenetic analysis, the virus showed the higher sequence identity with severe acute respiratory syndrome coronavirus (SARS-CoV) of bat origin and designated as coronavirus disease 2019 (COVID-19) [1,2]. As on 23, February 2020 the virus has been spread into more than 34 countries including China with 2,445 deaths and 78,811 laboratory confirmed cases [1,2]. Based on medical and healthcare workers, the medical supplies are getting low due to the high number of cases in Wuhan city. To manage the cases, China has announced to build two new hospitals with 1,000 beds within 6 days which is functional now. The outbreak caused by COVID-19 in Wuhan is an epidemic threat to global health [1,2].
Currently, there are many systems available such as bacteria, yeast, and mammalian cell lines to express the recombinant subunit vaccines and therapeutic proteins but all the above systems have some disadvantages like high cost, safety, and target integrity. While, by using plant-based system is very useful and avoids all the issue as compared to other system and provides low cost and better safety with high scalability without harm with any pathogenic infections. This is the most recent and alternative technology for vaccine development. The expected protein folding and post-translational modifications of the proteins can be produced in the correct form in plant-based systems with the desired biological functions [3]. Plant-based edible vaccines have been recently introduced for vaccine production. The main goals of plant-based edible vaccines are the transformation and production of antigens into plants and the oral consumption of such vaccine to induce antigen specific immune responses. Currently, the use of plant-based expression system platform have been extensively utilized for the expression and purification of vaccines, recombinant proteins, enzymes, and many bio-pharmaceuticals in a variety of plant species, including potato, corn, tomato, carrot, lettuce, and spinach and have reached at advanced stage of pre-clinical and clinical evaluation. The oral administration of edible vaccines is a preferable route of vaccination for being a simple and safe route of administration; the low production cost allows for local production and minimal plant material processing; natural bio-encapsulation and hence, stability in the gastrointestinal (GI) tract; and protective immunogenicity at the GI mucosa. The current status of plant-based vaccine and their clinical evaluation are summarized in Table 1 [4,5]. The plant-based vaccine development idea was started 30 years ago and since then many vaccine proteins have been produced in plants for human and animal diseases [6,7,8,9,10]. Since more than 20 years, plant science has grown tremendously and shown great importance towards molecular pharming and have significant achievements in large scale with low cost production of recombinant proteins, enzymes, and pharmaceuticals compounds. Currently, various pharmaceutical compounds, antigen, antibodies, hormones, enzymes, and growth regulators have been developed into multiple plants system [10,11,12,13]. The main objective of this review is to provide the latest information about the use of plant-based platform for the development of an edible vaccine.
Table 1. Current status of plant-based vaccines and therapeutic proteins [5].
| Product plant | Plant host | Indication | Delivery | Product stage |
|---|---|---|---|---|
| Norwalk virus CP | Potato | Diarrhea | Oral | Phase 1 |
| HBsAg | Lettuce, potato | Hepatitis B | Oral | Phase 1 |
| Rabies virus GP/NP | Spinach | Rabies | Oral | Phase 1 |
| Newcastle disease virus HN | Tobacco cell suspension | Newcastle disease | Subcutaneous | USDA approved |
| Personalized anti-idiotype scFVs | Nicotiana benthamiana | Non-Hodgkin's lymphoma | Subcutaneous | Phase 1 |
| Personalized anti-idiotype dcFVs | Nicotiana benthamiana | Non-Hodgkin's lymphoma | Subcutaneous | Phase 1 (ongoing) |
| H5N1 influenza HA, VLP | Nicotiana benthamiana | H5N1 “avian” influenza | Intramuscular | Phase 1 (ongoing); phase 2 (Health Canada approved) |
| H5N1 influenza HA/1 | Nicotiana benthamiana | H5N1 | Intramuscular | Phase 1 (ongoing) |
| H1N1 influenza HA C1 | Nicotiana benthamiana | H1N1 “swine” influenza | Intramuscular | Phase 1 (ongoing) |
| Anti-Streptococcus, surface antigen I/III | Tobacco | Dental caries | Topical | Phase 2; EU approved |
| Anti-αCCR 5 | Nicotiana benthamiana | HIV | Topical | Pre-clinical |
| Anti-HIV gp120 | Maize, Nicotiana benthamiana | HIV | Topical | Pre-clinical |
| Anti-HBsAg scFV | Tobacco | Hepatitis B | Not applicable | On market |
| Glucocerebrosidase | Carrot cell suspension | Gaucher disease | Intravenous | Phase 3 |
| Insulin | Safflower | Diabetes | Subcutaneous | Phase 1/2 |
| Gastric lipase | Maize | Cystic fibrosis, pancreatitis | Oral | Phase 2 |
CP, capsid protein; HBsAg, hepatitis B surface antigen; GP, glycoprotein; NP, nucleoprotein; HN, hemagglutinin-neuraminidase; USDA, United States Department of Agriculture; scFVs, single-chain Fv fragments; HA, hemagglutinin; VLP, virus-like particles; EU, European Union; HIV, human immunodeficiency virus.
The Concept of an Edible Vaccine Development
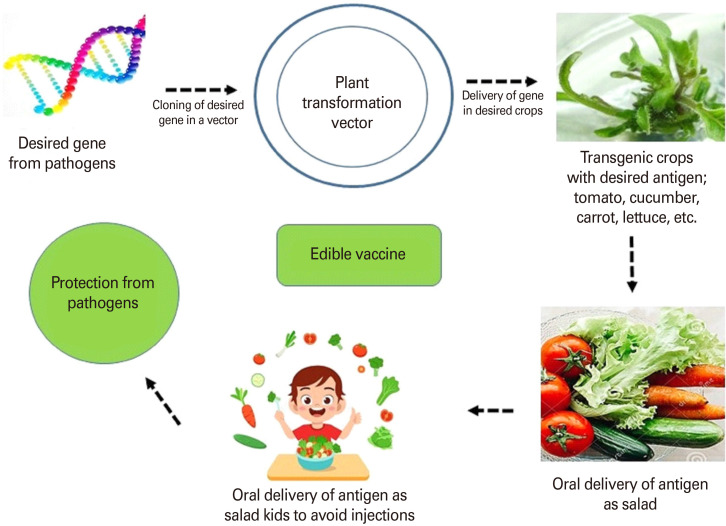
For plant-based vaccine development, the desired plant with efficient and quick regeneration and transformation properties should be selected. The desired gene can be delivered into plant cells to express desired proteins/pharmaceuticals compounds with low cost and high scale [5,6,9]. This technology has attracted the researchers because of elimination of a specific animal requirement to conduct the experiment. The edible vaccines can be designed in such a way that, the expressed and produced proteins should not be pathogenic. The production of conventional vaccines is very expensive, and they require purification and refrigeration. Apart from that, the plant-based vaccines are the most suitable for children as an oral delivery. Currently, World Health Organization has suggested new technologies for the vaccine development. The specific proteins can be expressed into desired plants with very less cost and can be grown to the required locations so that, an edible vaccine can be available to the needy population globally, especially in the developing countries. Due to high cost, storage, refrigeration, transportation, and requirements of trained medical personnel, an injectable vaccine cannot be easily taken in developing countries. Additionally, various pathogenic organism, bacterial and viral diseases can be easily transmitted by re-use of needles. Monoclonal antibodies are being used in the treatment of arthritis and cancer. The monoclonal antibodies can be easily produced in desired transgenic plants with very less cost and time duration. It has been reported that the developed transgenic rice was stable at room temperature for 18 months. Because these vaccines are needle-free [14], they have the added advantage of eliminating the associated waste and potential for dissemination of blood borne-infections. Antigens are released in the form of vaccine through bio-encapsulation which protects them from gastric enzymes. The released proteins were absorbed by M cells in the intestinal wall and passed on to the macrophages and antigen-presenting cells and local lymphocyte populations generating serum immunoglobulin (Ig)G, IgE, and local IgA responses and memory cells which neutralize the attack by a real pathogen. The development and stability of edible vaccine as an antigen has been investigated in many recent reports. The current status of plant-based vaccines has been reviewed and presented [5]. The concept of edible vaccine development has been presented in Fig. 1.
Fig. 1. Concept of an edible vaccine development.
Advantages of Edible Vaccine
Currently, the plant-based vaccine has many valuable advantages as compared to traditional vaccines which include. (1) Edible vaccine can be used orally as fruits and vegetables. (2) Capsules can be made from dried leaf tissue powder. (3) No adjuvants require to enhance immune responses. (4) Orally-introduced antigens elicit mucosal immunity. (5) It is easy to bulk produce onsite, transport and stored with less cost and without refrigeration. (6) No injection and need for trained medical person. (7) Easy to express, separate and purify the protein. (8) They can be stored as seeds and oils and dried tissue without any refrigeration. (9) They do not have any risk of contamination and disease spread. (10) There is the possibility of enhanced compliance, especially in children [5]. Edible vaccines have received considerable attention from researchers in both academia and industry. The first plant-derived rabies vaccine was produced in tomatoes and offered the advantages of high biomass yields and the increased containment by growth in greenhouses. Lettuce and bananas have also been utilized for the production of plant-based vaccines [8,9,10].
Vaccines in Desired Plant-Based System
The edible can be developed in many desired plants. Recently, there are different crops like tomato, carrot, corn, cucumber, lettuce, and spinach plants that have been utilized as a green factory to express, and purify the desired protein, enzymes, antigens, and biopharmaceuticals compounds [3,4,5,6,7,8,9,10,11,12]. The most important advantage of plant expression systems is the low cost. Tomato can be used as for vaccine production and the used as salad which facilitates the easy oral delivery of a particular vaccine. As an example, tomato has excellent biomass and easily transformed and generate the whole plant within short period and this provides an ideal choice for vaccine production. Therefore, tomato is no doubt a green vaccine factory.
An Edible Vaccine for Coronavirus Disease 2019
Currently, there is no approved vaccine or treatment with proven efficacy against newly emerged CoV COVID-19. While previous work on SARS-CoV vaccines have shown that the Spike glycoprotein (S) is the main inducer of neutralizing antibodies. The CoVs infection causes severe respiratory disease with clinical symptoms; specifically, patients develop acute respiratory symptoms. The Spike protein can be used to develop a vaccine against the COVID-19. The Spike (S) protein gene or subunit of Spike like S1 subunit can be cloned into a plant expression vector and the desired plant like tomato, cucumber, or lettuce can be transformed. The developed transgenic plants can be used as salad and easily delivered orally and immunized the human against the newly emerged virus. Several teams are working on a vaccine to protect against the new CoV. Recently, the therapeutic options for COVID-19 has been discussed and suggested in a published paper [15]. However, the development of a protective vaccine is of great importance to prevent and control the spread of the virus as well as any future outbreaks.
Conclusion
The newly emerged CoV has now become a global threat to human health. As on February 23, 2020, this virus has been spread to more than 34 countries including China with 2,445 deaths and 78,811 confirmed cases [1,2]. The fast spread of this virus has attracted the researchers towards the development of an urgent vaccine to control the virus infection and disease spread. The full-genome of COVID-19 has provided the valuable information which can be used to design and develop an effective vaccine [16]. The plant-based edible vaccine has now attracted many researchers and pharmaceutical companies to develop fast and cheaper vaccines against many pathogens. This technology has got more publicity in the last 10 years and have proven the efficiency to express the desired protein either as antigens or pharmaceuticals therapeutic compounds in the desired plant cells. This novel technology provides the high and fast expression, purification, and better stability of desired proteins in to plant cells as well as their removal of refrigeration requirement and trained medical personnel for delivery. The edible vaccine also removes the risk factors and safety and regulatory issues related to living organisms. The edible vaccine can be grown at the site of requirement and orally delivered in the form of salad. Therefore, this technology is emerging as a novel and alternative methods for large scale edible vaccine production, manufacturing, and processing as well as commercialization and easily availability to the needy persons globally. This technology will provide an effective vaccination against many diseases in human and animal globally. Based on current information, it is concluded that there is an urgent need to develop a vaccine against this fast spreading 2019 novel CoV by using various vaccine development technology. The edible vaccine technology will provide a fast and cheaper vaccine not only this new virus but also against many other pathogens.
Footnotes
No potential conflict of interest relevant to this article was reported.
Author would like to gratefully acknowledge the research facility provided by Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia.
References
- 1.World Health Organization. Disease outbreak news (DONs) [Internet] Geneva: World Health Organization; 2020. [cited 2020 Feb 23]. Available from: https://www.who.int/csr/don/en/ [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2019 novel coronavirus (2019-nCoV) situation summary [Internet] Atlanta, GA: Centers for Disease Control and Prevention; 2020. [cited 2020 Feb 23]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/locations-confirmed-cases.html. [Google Scholar]
- 3.Yusibov V, Rabindran S. Recent progress in the development of plant derived vaccines. Expert Rev Vaccines. 2008;7:1173–1183. doi: 10.1586/14760584.7.8.1173. [DOI] [PubMed] [Google Scholar]
- 4.Yusibov V, Streatfield SJ, Kushnir N. Clinical development of plant-produced recombinant pharmaceuticals: vaccines, antibodies and beyond. Hum Vaccin. 2011;7:313–321. doi: 10.4161/hv.7.3.14207. [DOI] [PubMed] [Google Scholar]
- 5.Sohrab SS, Suhail M, Kamal MA, Husen A, Azhar EI. Recent development and future prospects of plant-based vaccines. Curr Drug Metab. 2017;18:831–841. doi: 10.2174/1389200218666170711121810. [DOI] [PubMed] [Google Scholar]
- 6.Rybicki EP. Plant-based vaccines against viruses. Virol J. 2014;11:205. doi: 10.1186/s12985-014-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucka M, Kowalczyk T, Szemraj J, Sakowicz T. Plants as an alternative source of therapeutic proteins. Postepy Hig Med Dosw (Online) 2015;69:362–373. doi: 10.5604/17322693.1145824. [DOI] [PubMed] [Google Scholar]
- 8.Aryamvally A, Gunasekaran V, Narenthiran KR, Pasupathi R. New strategies toward edible vaccines: an overview. J Diet Suppl. 2017;14:101–116. doi: 10.3109/19390211.2016.1168904. [DOI] [PubMed] [Google Scholar]
- 9.Rybicki EP. Plant-made vaccines and reagents for the One Health initiative. Hum Vaccin Immunother. 2017;13:2912–2917. doi: 10.1080/21645515.2017.1356497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Eerde A, Gottschamel J, Bock R, et al. Production of tetravalent dengue virus envelope protein domain III based antigens in lettuce chloroplasts and immunologic analysis for future oral vaccine development. Plant Biotechnol J. 2019;17:1408–1417. doi: 10.1111/pbi.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buyel JF. Process development strategies in plant molecular farming. Curr Pharm Biotechnol. 2015;16:966–982. doi: 10.2174/138920101611150902115413. [DOI] [PubMed] [Google Scholar]
- 12.Liew PS, Hair-Bejo M. Farming of plant-based veterinary vaccines and their applications for disease prevention in animals. Adv Virol. 2015;2015:936940. doi: 10.1155/2015/936940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laere E, Ling AP, Wong YP, Koh RY, Mohd Lila MA, Hussein S. Plant-based vaccines: production and challenges. J Bot. 2016;2016:4928637 [Google Scholar]
- 14.Giudice EL, Campbell JD. Needle-free vaccine delivery. Adv Drug Deliv Rev. 2006;58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Kruse RL. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China: version 2. F1000Res. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rambaut A. Preliminary phylogenetic analysis of 11 nCoV-2019 genomes, 2020-01-19 [Internet] [place unknown]: ARTIC Network; 2020. [cited 2020 Feb 23]. Available from: http://virological.org/t/preliminary-phylogenetic-analysis-of-11-ncov2019-genomes-2020-01-19/329. [Google Scholar]