Abstract
Background:
Plasma exchange (PE) is often considered as an effective treatment for neuromyelitis optica spectrum disorder (NMOSD) and several inflammatory demyelinating disorders of the central nervous system. This study aimed to evaluate the visual outcomes of Chinese patients with severe acute isolated optic neuritis (ON) who received PE therapy after high-dose intravenous methylprednisolone (IVMP) treatment.
Methods:
Thirty-seven acute isolated ON patients experiencing their first attack of severe visual impairment without neurological disability were recruited. All subjects received five cycles of double-filtration plasmapheresis. Visual acuity (VA) was documented at onset, 1 day before PE treatment, at each cycle of PE therapy and at the 1-month follow-up visit.
Results:
This study included 26 female (70.3%) and 11 male (29.7%) subjects, and 18 subjects (48.6%) had bilateral involvement. The time window between onset and PE treatment was 27.3 ± 12.7 days (range: 6–53 days). Mean VA (logMAR) of the studied eyes at onset, 1-day before PE treatment/after IVMP and after the fifth PE treatment were 3.41 ± 1.50, 2.61 ± 1.64 and 1.66 ± 1.52, respectively (p < 0.001). Nineteen eyes (51.4%) showed no light perception at the onset, and 17 eyes (45.9%) improved to Snellen VA >20/800 after IVMP and PE treatments, among which five eyes (13.5%) recovered to Snellen VA 20/20 (p < 0.001). Predictors of good visual outcome included body mass index [odds ratio (OR) = 0.734, p = 0.044], serum AQP4 antibody-positive status (OR = 0.004, p = 0.001), bilaterality (OR = 0.042, p = 0.008) and time window from onset to PE therapy per 1 day (OR = 0.79, p = 0.002).
Conclusion:
This study revealed that PE treatment effectively improves the visual outcomes of patients experiencing their first attack of severe acute isolated ON after high-dose IVMP treatment. Better visual outcomes can be achieved with early PE treatment.
Keywords: acute severe optic neuritis, plasma exchange, vision improvement, visual acuity
Introduction
Inflammatory optic neuritis (ON) is one of the main manifestations of inflammatory demyelinating disorders in the central nervous system and is sometimes the only initial symptom of demyelination.1 Some ON patients develop severe and permanent visual impairment with visual acuity (VA) worse than 0.1.2 Visual impairment negatively affects the vision-related quality of life (QoL) in neuromyelitis optica spectrum disorder (NMOSD) patients.3,4 Severe ON is often associated with NMOSD in Chinese populations and primarily affects the spinal cord and the optic nerve.5 Brainstem symptoms have been reported in NMOSD patients, with a higher frequency in non-Caucasian populations.6 Immediate unilateral blindness occurs in one-third of patients with NMOSD-related ON at the first attack.7 Autoantibodies against the aquaporin-4 (AQP4) water channel on the surface of astrocytes have been suggested to be involved in the pathogenesis of NMOSD.8 NMOSD can be treated in the acute phase by 5-day intravenous methylprednisolone (IVMP), with or without subsequent plasma exchange (PE) treatments, followed by oral steroids as well as immunosuppressive and immunomodulatory drugs for prevention of further relapse.9
PE therapy involves the filtration and replacement of patients’ plasma with artificial plasma.10 According to the American Society for Apheresis, PE is considered as an effective treatment for NMO/NMOSD or multiple sclerosis (MS),11 acting through the elimination of the inflammatory mediators,12,13 especially in patients with poor response to initial IVMP. PE has also been applied against NMO/NMOSD relapses and steroid-resistant or severe ON, the latter of which is most likely related to NMO/NMOSD or MS.14–18 However, most previous investigations pooled patients with monophasic and relapsing disease course and with isolated and complex manifestation.19 Some evidences suggested the significant association of past history of ipsilateral ON with poor visual outcome.19 Factors including fewer prior attacks, shorter disease duration and lower pre-existing disability are associated with favourable outcome of PE in other inflammatory demyelinating diseases.16 Better visual outcomes can be expected for early treatment by PE upon the first attack of isolated ON. However, the efficacy of adding PE to steroid therapy for the treatment of severe acute isolated ON at the first attack has not been quantified. This study aimed to evaluate the visual outcomes of Chinese patients with severe acute isolated ON who received PE therapy after high-dose IVMP treatment, and further identify the predictive factors for good visual outcomes.
Methods
Study subjects
In this prospective case series study, 37 patients with severe acute isolated ON after high-dose IVMP treatment were recruited in the Department of Neuro-ophthalmology at the Chinese People’s Liberation Army General Hospital from January 2015 to December 2018. The study protocol was approved by the Ethics Committee for Human Research at the Chinese People’s Liberation Army General Hospital (approval number S2017-093-01) and was in accordance with the tenets of the Helsinki Declaration and the ICH-GCP guidelines. Written informed consent was obtained from all study subjects.
ON was diagnosed in accordance with the criteria described by the Optic Neuritis Treatment Trial.20,21 The inclusion criteria for the subjects in this study were as follows: (1) first attack of acute vision loss in one or both eyes with or without ocular pain; (2) no presentation of neurological disability other than visual impairment at the onset; (3) severe visual loss defined as best corrected VA (BCVA; Snellen) worse than 20/200 at the onset; (4) consistent visual field defect; (5) presence of relative afferent pupillary defect or visual evoked potential abnormalities; (6) presence of optic disc oedema; (7) detection of demyelinating lesions at the optic nerve with enhancement on T1-weighted orbit magnetic resonance imaging (MRI); (8) administration of IVMP treatment at 1000 mg/day for 3–5 days after onset and no other treatment; (9) no obvious visual improvement of more than three lines of VA after IVMP treatment; (10) disease duration from onset to PE treatment less than 60 days; (11) tolerance for and completion of the course of five-cycle PE treatment without serious adverse events. Only patients who met all of the inclusion criteria were included. Patients were excluded if their eyes were simultaneously affected by other types of optic neuropathy, corneal opacity, dense cataract, vitreoretinal diseases or glaucoma. Patients with distinct demyelinating lesions on the brain MRI were also excluded.22,23
Ophthalmic examinations
All patients received complete ophthalmic examinations. BCVA was measured at every visit, including the onset, after IVMP treatment (1 day before PE therapy), the day after each PE therapy and at the 1-month follow-up visit. For subjects who were unable to read letters at 1-m distance, the VA was assessed and categorised into four levels, in descending order: ability to count fingers (CF), perception of hand motion (HM), light perception (LP) or no light perception (NLP). The Snellen scale was converted into the logMAR (log of the minimum angle of resolution) scale for the statistical analysis.24 At 1 day before PE therapy, the visual field (VF) was documented by static automated white-on-white threshold perimetry using the Humphrey automated perimetry (Humphrey Field Analyzer II). In addition, orbital MRI was also performed at 1 day before PE therapy to evaluate the lesions on optic nerves, restricted to T1-weighted imaging (T1WI), T2-weighted imaging, post-contrast T1WI and fluid-attenuated-inversion recovery sequences (3.0 T MRI, DISCOVERY MR 750, GE Healthcare, United States).
AQP4 and myelin oligodendrocyte glycoprotein autoantibody serotyping
The serum samples from all study subjects were tested for the presence of AQP4 autoantibodies using a cell-based assay (Euroimmun, Lübeck, Germany) before the first PE treatment. Myelin oligodendrocyte glycoprotein (MOG) autoantibody detection by cell-based assay (Euroimmun, Lübeck, Germany) was also performed as an additional test during the follow-up period.
PE treatment
Double-filtration plasmapheresis (Plasauto EZ, Asahi Kasei Medical, Tokyo, Japan) was performed in all recruited study subjects. One to 1.5 volumes of circulating plasma were dialysed in each cycle for 2–4 h on every other day. Each patient received five cycles of PE treatment over 10 days.
Clinical outcome assessment
The visual outcome was categorised into 10 levels: Snellen VA of 20/20, scotoma but VA better than 20/30, 20/30 ⩾ VA > 20/60, 20/60 ⩾ VA > 20/200, 20/200 ⩾ VA > 20/800, 20/800 ⩾ VA > 20/2000, CF, HM, LP and NLP. Patients with severe visual impairment were defined as having BCVA (Snellen) worse than 20/200 at onset.25 Good visual outcome was defined as final Snellen VA > 20/40.10,19
Statistical analysis
To avoid the bias of inter-relationship between two eyes in the same patient, only one eye was randomly selected using a random number table if the patients were bilaterally involved in univariate analysis. Continuous data are presented as the mean ± standard deviation (SD). Mann–Whitney U test was used to compare the difference in clinical characteristics between good and poor final visual outcomes, whereas χ2 or Fisher’s exact test was used to compare the categorical variables of clinical characteristics with the final visual outcomes. The differences in the VA value (logMAR) and the numbers of patients in each VA category at the eight time points (at onset, 1 day before PE treatment, at each cycle of PE therapy and at the 1-month follow-up visit) were evaluated using the Friedman test along with the post-hoc Wilcoxon signed-rank test. Two different models, logistic regression and multivariate generalised estimating equation (GEE) analyses, were executed to identify the critical factors associated with the final visual outcome (VA > 20/40 versus VA ⩽ 20/40), including age at onset, body mass index (BMI), sex, bilateral or unilateral affected eye, serum AQP4 antibody status, logMAR VA at onset and time window from onset to PE treatment. The backward stepwise method of multivariate logistic regression was performed based on one selected eye from each patient, and the multivariate GEE analysis was performed on all affected eyes, including those of bilaterally involved patients, to account for the bias of relatedness. p < 0.05 was considered as statistically significant. Statistical significance in the Wilcoxon signed-rank test was adjusted by Bonferroni corrections. All statistical analyses were performed using commercially available software (IBM SPSS Statistics 23; SPSS Inc., Chicago, IL, United States).
Results
Patients’ demographics
A total of 37 patients with severe acute isolated ON, including 26 (70.3%) female and 11 (29.7%) male subjects, were included in this study (Table 1). The mean recruitment age was 38.7 ± 14.8 years, and 18 (48.6%) of the total subjects showed bilateral involvement. Twenty (54.1%) patients were seropositive for AQP4 antibody. Twenty-four patients were tested for serum MOG antibody, but only one showed positive in the test (Tables 1 and 2). Lesions visible in orbital MRI indicated the involvement of retrobulbar, canalicular and intracranial segments of the optic nerve in 29 (78.4%), 15 (40.5%) and 10 (27.0%) patients, respectively, whereas optic chiasma was involved in four (10.8%) patients seronegative for AQP4 antibody, and they retained poor visual outcomes after PE therapy (Table 2). Longitudinally extensive optic nerve lesion, which is defined as a lesion involving more than half of the segments of the whole optic nerve, was found in 33 (89.2%) patients. The time window between the onset and PE therapy was 27.2 ± 12.7 days (range: 6–53 days). A significant difference in the onset-to-PE therapy time window was identified between the patients seropositive and seronegative for AQP4 antibody (p = 0.001; Table 2). The mean VA (logMAR) of the studied eyes at onset (3.4 ± 1.5) showed no statistically significant difference from that at 1 day before PE therapy (representing the VA after IVMP treatment, 2.6 ± 1.6, adjusted p > 0.999; Table 1 and Figure 1).
Table 1.
Major clinical characteristics of the recruited severe acute isolated optic neuritis patients with different visual acuity outcomes.
| Total | Final Snellen VA ⩽20/40 | Final Snellen VA >20/40 | p | p c | OR (95% CI)c | |
|---|---|---|---|---|---|---|
| Study subjects [n, eyes] | 37 (37) | 27 (27) | 10 (10) | – | – | – |
| Onset age (mean ± SD, years) | 38.7 ± 14.8 | 42.2 ± 14.6 | 29.1 ± 11.2 | 0.017 a | 0.120 | 0.90 (0.79–1.03) |
| BMI (mean ± SD, kg/m2) | 24.2 ± 4.2 | 25.2 ± 4.2 | 21.7 ± 3.0 | 0.014 a | 0.166 | 0.75 (0.49–1.13) |
| Gender (female, n, %) | 26 (70.3%) | 19 (70.4%) | 7 (70.0%) | >0.999b | –d | – |
| Affected eye (bilateral, n, %) | 18 (48.6%) | 16 (59.3%) | 2 (20.0%) | 0.062b | 0.096 | 0.06 (0.00–1.64) |
| Serum AQP4-antibody (positive, n, %) | 20 (54.1%) | 15 (55.6%) | 5 (50.0%) | >0.999b | 0.051 | 0.01 (0.00–1.01) |
| Serum MOG-antibody status (n, %) | ||||||
| Positive | 1 (2.7%) | 0 (0.0%) | 1 (10.0%) | |||
| Negative | 23 (62.2%) | 15 (55.6%) | 8 (80.0%) | 0.053b | – | – |
| Unknown | 13 (35.1%) | 12 (44.4%) | 1 (10.0%) | |||
| Orbital MRI lesion (n, %) | ||||||
| Retrobulbar | 29 (78.4%) | 20 (74.1%) | 9 (90.0%) | 0.404b | – | – |
| Canalicular | 15 (40.5%) | 12 (44.4%) | 3 (30.0%) | 0.481b | – | – |
| Intracranial | 10 (27.0%) | 8 (29.6%) | 2 (20.0%) | 0.694b | – | – |
| Chiasmal involvement | 4 (10.8%) | 4 (14.8%) | 0 (0.0%) | 0.557b | – | – |
| Longitudinally extensive optic nerve lesion | 33 (89.2%) | 24 (88.9%) | 9 (90.0%) | >0.999b | – | – |
| VA at onset (logMAR, mean ± SD) | 3.4 ± 1.5 | 3.8 ± 1.4 | 2.5 ± 1.4 | 0.021 a | –d | – |
| Onset to PE window (mean ± SD, days) | 27.2 ± 12.7 | 29.1 ± 13.0 | 22.4 ± 11.1 | 0.139a | 0.038 | 0.80 (0.64–0.99) |
Mann–Whitney U test.
Fisher’s exact test.
Logistic regression test.
Removed at the last step by backward stepwise method.
Bold: p < 0.05.
CI, confidence interval; AQP4, aquaporin-4; BMI, body mass index; logMAR, log of the minimum angle of resolution; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; OR, odds ratio; PE, plasma exchange; SD, standard deviation; VA, visual acuity.
Table 2.
Major clinical characteristics of the recruited severe acute isolated optic neuritis patients with different serum AQP4-antibody status.
| Total | Serum AQP4-antibody
positive |
Serum AQP4-antibody
negative |
p c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Final Snellen VA ⩽ 20/40 | Final Snellen VA > 20/40 | p a | All | Final Snellen VA ⩽ 20/40 | Final Snellen VA > 20/40 | p b | |||
| Study subjects [eyes (n)] | 37 (37) | 20 (20) | 15 | 5 | – | 17 (17) | 12 | 5 | – | – |
| Onset age (mean ± SD, years) | 38.7 ± 14.8 | 42.0 ± 16.2 | 46.2 ± 15.3 | 29.3 ± 12.2 | 0.042 d | 34.8 ± 12.4 | 37.2 ± 12.4 | 28.9 ± 11.6 | 0.328d | 0.149d |
| BMI (mean ± SD, kg/m2) | 24.2 ± 4.2 | 23.5 ± 4.4 | 24.5 ± 4.5 | 20.4 ± 2.7 | 0.053d | 25.1 ± 3.7 | 26.0 ± 3.7 | 22.9 ± 3.1 | 0.130d | 0.167d |
| Gender (female, n) | 26 | 17 | 12 | 5 | 0.539e | 9 | 7 | 2 | 0.620e | 0.069e |
| Affected eye (bilateral, n) | 18 | 7 | 7 | 0 | 0.114e | 11 | 9 | 2 | 0.280e | 0.103e |
| MOG antibody status (n) | ||||||||||
| Positive | 1 | 0 | 0 | 0 | 1 | 0 | 1 | |||
| Negative | 23 | 12 | 8 | 4 | 0.603e | 11 | 7 | 4 | – | 0.472e |
| Unknown | 13 | 8 | 7 | 1 | 5 | 5 | 0 | |||
| Orbital MRI lesion (n) | ||||||||||
| Retrobulbar | 29 | 14 | 10 | 4 | >0.999e | 15 | 10 | 5 | >0.999e | 0.246e |
| Canalicular | 15 | 5 | 4 | 1 | >0.999e | 10 | 8 | 2 | 0.593e | 0.050e |
| Intracranial | 10 | 4 | 3 | 1 | >0.999e | 6 | 5 | 1 | 0.600e | 0.460e |
| Chiasmal involvement | 4 | 0 | 0 | 0 | – | 4 | 4 | 0 | 0.261e | 0.036 e |
| Longitudinally extensive optic nerve lesion | 33 | 16 | 12 | 4 | >0.999e | 17 | 12 | 5 | – | 0.109e |
| VA at onset (logMAR, mean ± SD) | 3.4 ± 1.5 | 3.1 ± 1.6 | 2.8 ± 1.7 | 4.1 ± 0.9 | 0.168d | 3.8 ± 1.3 | 3.5 ± 1.4 | 4.3 ± 0.9 | 0.328d | 0.297d |
| VA at final visit (logMAR, mean ± SD) | 1.7 ± 1.5 | 1.7 ± 1.5 | 1.6 ± 1.5 | 2.0 ± 1.7 | 0.612d | 1.6 ± 1.6 | 1.2 ± 1.3 | 2.4 ± 2.2 | 0.442d | 0.357d |
| Good final visual acuity outcome (VA > 20/40, n) | 10 | 5 | 0 | 5 | – | 5 | 0 | 5 | – | >999e |
| Onset to PE window (mean ± SD, days) | 27.2 ± 12.7 | 21.6 ± 12.6 | 23.5 ± 13.8 | 16.0 ± 6.1 | 0.349d | 33.9 ± 9.5 | 36.1 ± 8.0 | 28.8 ± 11.7 | 0.130d | 0.001 d |
p-value for comparison between final visual acuity (VA) outcomes in serum AQP4-antibody positive group.
p-value for comparison between final VA outcomes comparison in serum AQP4-antibody negative group.
p-value for comparison between serum AQP4-antibody positive and negative groups.
Mann–Whitney U test.
Fisher’s exact test.
Bold: p < 0.05.
AQP4, aquaporin-4; BMI, body mass index; logMAR, log of the minimum angle of resolution; MOG, myelin oligodendrocyte glycoprotein; MRI, magnetic resonance imaging; PE, plasma exchange; SD, standard deviation; VA, visual acuity.
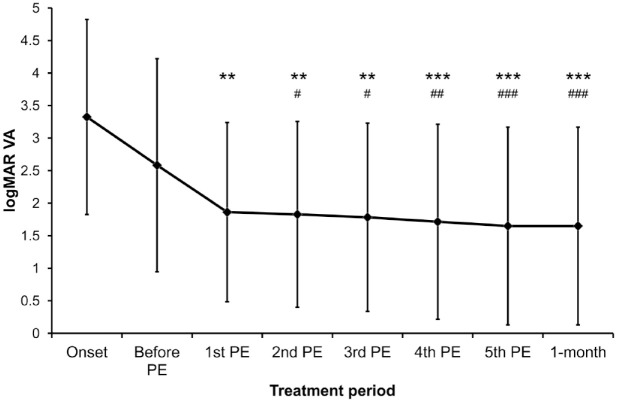
Figure 1.
Average visual acuity of the studied eyes along the plasma exchange treatment period.
The mean VA (logMAR) values of the studied eyes at the onset, 1 day before PE therapy (after IVMP treatment), after each PE cycle and at the 1-month follow-up visit were 3.4 ± 1.5, 2.6 ± 1.6, 1.9 ± 1.4, 1.8 ± 1.4, 1.8 ± 1.5, 1.7 ± 1.5, 1.7 ± 1.5 and 1.7 ± 1.5 respectively. The VA gradually improved along the treatment period (Friedman p < 0.001). The data are presented as the mean ± standard deviation.
**Adjusted p < 0.01 and ***adjusted p < 0.001, compared with the VA at onset; #adjusted p < 0.05, ##adjusted p < 0.01 and ###adjusted p < 0.001, compared with the VA before the PE therapy.
IVMP, intravenous methylprednisolone; PE, plasma exchange; VA, visual acuity.
Vision improvement after PE therapy
The mean logMAR VA measurements of the studied eyes gradually improved after each of the five PE treatments (1.9 ± 1.4, 1.8 ± 1.4, 1.8 ± 1.5, 1.7 ± 1.5 and 1.7 ± 1.5, respectively, Friedman p < 0.001; Figure 1). Compared with the VA at the onset, the logMAR VA of the majority of the studied eyes improved after the first cycle of PE therapy (adjusted p = 0.008; Supplemental Material Figure 1 online). The VA after the fifth PE therapy was better than that before the beginning of PE therapy (adjusted p < 0.001; Figure 1). Only three eyes showed reduced VA and seven eyes retained the same VA after the PE therapy (Supplemental Figure 2). At 1 month after the PE therapy, the mean logMAR VA of the studied eyes was 1.7 ± 1.5 (Figure 1), and the VA change between the fifth PE therapy and the 1-month follow-up was 0.0 ± 0.0 (adjusted p > 0.999), indicating that the vision of all study subjects was stably maintained after the five-cycle PE therapy. The average logMAR VA improvement for the improved eyes was −2.4 ± 1.2 at the 1-month follow-up compared with that at the onset.
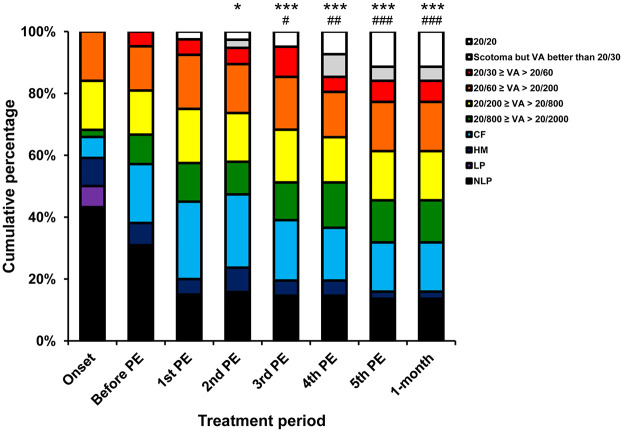
At the onset, 19 (51.4%) of the studied eyes were categorised as NLP, three (8.1%) eyes as LP, four (10.8%) eyes as HM, three (8.1%) eyes as CF and eight (21.6%) eyes as 20/200 ⩾ VA > 20/2000 (Supplemental Table 1). The proportions of eyes in each visual outcome category at 1 day before PE therapy (after IVMP treatment) were not significantly different from those at the onset (adjusted p > 0.999). A significant trend of increase in the visual outcome improvement was observed from the first to the fifth PE therapy as compared with the visual outcome before the PE therapy (Friedman p < 0.001; Figure 2). The proportions of eyes in each visual outcome category shifted significantly towards an improved distribution after the second PE therapy as compared with those at the onset (adjusted p = 0.014), and after the third PE therapy as compared with those at 1 day before PE therapy (after IVMP treatment; adjusted p = 0.012). After the fifth PE therapy, six (16.2%) of the studied eyes were categorised as NLP, no (0.0%) eyes as LP, one (2.7%) eye as HM, seven eyes (18.9%) as CF, 13 eyes (35.1%) as 20/200 ⩾ VA > 20/2000 and 10 (27.0%) eyes as VA > 20/60 (adjusted p < 0.001; Supplemental Table 1). Critically, five (13.5%) eyes recovered to normal vision (Snellen VA = 20/20). There was no difference in the proportions of eyes in each visual outcome category between the day after the fifth PE therapy and at the 1-month follow-up (adjusted p > 0.999).
Figure 2.
Visual outcome categorisation of the studied eyes along the plasma exchange treatment period.
The proportions of eyes in each visual outcome category at 1 day before PE treatment (after IVMP treatment) were not significantly different from those at the onset. A significant trend of increase in visual outcome improvement was observed from the first to the fifth PE therapy as compared with the visual outcome before the PE therapy (Friedman p < 0.001). The proportions of eyes in each visual outcome category shifted significantly towards an improved distribution after the second PE therapy as compared with those at the onset, and after the third PE therapy as compared with those at 1 day before PE therapy (after IVMP treatment). The proportions in each category of visual outcome at the 1-month follow-up visit were the same as those after the fifth PE therapy. The data are presented as cumulative percentages.
*Adjusted p < 0.05 and ***adjusted p < 0.001, compared with the VA at onset; #adjusted p < 0.05, ##adjusted p < 0.01 and ###adjusted p < 0.001, compared with the VA before the PE therapy.
CF, ability to count fingers; HM, ability to perceive hand motion; IVMP, intravenous methylprednisolone; LP, light perception; NLP, no light perception; PE, plasma exchange; VA, visual acuity.
Visual outcome improvement-associated factors
A total of 10 (27.0%) selected eyes showed good visual outcome with Snellen VA > 20/40 after the five-cycle PE therapy, and 27 (73.0%) eyes showed Snellen VA ⩽ 20/40 (Table 1). No significant difference was found between the good and poor visual outcomes with regard to the sex proportions (p > 0.999), the serum AQP4 antibody status (p > 0.999), bilaterality (p = 0.062) or the distribution of orbital MRI lesions (retrobulbar: p = 0.404; canalicular: p = 0.481; intracranial: p = 0.694; chiasmal involvement: p = 0.557; longitudinally extensive optic nerve lesion: p > 0.999). The patients with good visual outcomes were significantly younger at disease onset (29.1 ± 11.2 years) than those with poor visual outcomes (42.2 ± 14.6 years; p = 0.017). The patients with good visual outcomes also had a lower mean BMI (21.7 ± 3.0 kg/m2) than those with poor visual outcomes (25.2 ± 4.2 kg/m2; p = 0.014). In addition, the patients with poor visual outcomes showed more severely defective vision at onset (logMAR VA: 3.8 ± 1.4) than those with good visual outcomes (logMAR VA: 2.5 ± 1.4; p = 0.021). However, logistic regression analysis revealed no association of the visual outcome with the age at onset (p = 0.120), BMI (p = 0.166), serum AQP4 antibody status (p = 0.051) or bilaterality (p = 0.096). Sex and logMAR VA at onset were also not associated with the visual outcome. The time window from onset to PE therapy was the only significant factor related to a final Snellen VA better than 20/40 (odds ratio (OR) = 0.80, 95% confidence interval (95% CI) = 0.64–0.99, p = 0.038; Table 1). Multivariate GEE analysis was further applied to determine independent predictors of treatment response in terms of visual outcome by including all affected eyes (55 eyes of 37 patients). We found that BMI at onset (OR = 0.734, 95% CI = 0.54–0.99, p = 0.044), serum AQP4 antibody-positive status (OR = 0.004, 95% CI = 0.00–0.12, p = 0.001), bilaterality (OR = 0.042, 95% CI = 0.00–0.43, p = 0.008) and time window from onset to PE therapy per 1 day (OR = 0.79, 95% CI = 0.68–0.91, p = 0.002) were the independent predictors of a good visual outcome (Snellen VA > 20/40; Table 3).
Table 3.
Predictive factors associated with good final visual acuity outcome (VA > 20/40).
| Factor | p | OR (95% CI) |
|---|---|---|
| Age at onset per 1 year | 0.142 | 0.907 (0.80–1.03) |
| Female gender versus male | 0.993 | 1.009 (0.14–7.21) |
| BMI at onset | 0.044 | 0.734 (0.54–0.99) |
| Serum AQP4-antibody positive versus negative | 0.001 | 0.004 (0.00–0.12) |
| Bilateral affected versus unilateral | 0.008 | 0.042 (0.00–0.43) |
| logMAR VA at onset | 0.467 | 0.792 (0.42–1.48) |
| Time window from onset to start of PE therapy per 1 day | 0.002 | 0.790 (0.68–0.91) |
Multivariate generalised estimating equation analyses with final visual acuity (VA) outcome (VA > 20/40 versus VA ⩽ 20/40) as dependent variable (working correlation matrix autoregressive of the first order; n = 55 eyes of 37 subjects).
Bold, p < 0.05.
CI, confidence interval; AQP4, aquaporin-4; BMI, body mass index; logMAR, log of the minimum angle of resolution; OR, odds ratio; PE, plasma exchange; VA, visual acuity.
Discussion
Patients with symptoms of severe ON possess a high risk of developing NMOSD, especially in the Chinese population.1,7 The inflammatory mediators from the bloodstream can be cleared by over 80% with respect to their initial level through a series of five exchanges of PE therapy.10,26,27 Retrospective studies have suggested that PE therapy could be beneficial for severe ON patients.28 A past history of ipsilateral ON has been shown to be significantly associated with a poor visual outcome.19 Factors including fewer prior attacks, shorter disease duration and lower pre-existing disability are associated with favourable outcomes of PE in other inflammatory demyelinating diseases.16 Better efficacy can be expected for early PE therapy administered upon the first attack of isolated ON. However, this study was the first to evaluate the visual outcomes of PE treatment in patients experiencing their first attack of severe acute isolated ON after high-dose IVMP treatment. The PE therapy in this study followed the internationally standard PE protocol, in which 1.5 plasma volumes are exchanged in five cycles over 10 days.29 However, PE is often offered as a second-line ‘rescue therapy’ for severe relapses resistant to steroids in NMOSD, and a delay of weeks is often recommended before initiating PE therapy.12,15 In our study, we recruited only patients with isolated ON, who may not fulfil the diagnostic criteria of NMOSD, and we did not limit the time window from onset to PE treatment. This time window was under 2 weeks for some of our subjects, with a mean ± SD of 27.2 ± 12.7 days (range: 6–53 days). Therefore, some of our subjects received PE earlier than the recommended time. All of our subjects tolerated the PE therapy well without any serious adverse events.
In this study, significant visual improvement began after the second PE cycle (Figure 2), consistent with the results from a previous retrospective study on nine NMO patients, which also observed significant functional improvement after the second PE cycle.30 Earlier response to treatment could be expected in our study because only those patients with isolated ON who were experiencing their first attack were recruited. Although severe vision loss was present, it should still theoretically be less severe than that in patients with relapses and severe systemic symptoms. Moreover, in most previous studies on NMO/NMOSD, the expanded disability status scale was used to assess treatment efficacy, whereas, in our study, only VA assessment was performed, which differs in nature and sensitivity. Earlier and better visual improvement is known to be associated with prior IVMP treatment.15 Although early improvement was indeed found in our study, several patients also showed gradual visual improvement from the third to fifth PE cycles. Therefore, we would still recommend that ON patients receive all five cycles of PE (Supplemental Figure 1). After the completion of PE therapy, improvement in visual outcome was observed in 73.0% (27 eyes) of the studied eyes (Supplemental Figure 2). Among the 19 eyes with NLP and three eyes with LP at the onset, 15 eyes (71.4%) showed VA improvement after the PE therapy (Supplemental Table 1). Critically, 10 (27.0%) of the 37 studied eyes achieved VA ⩾ 20/40, and five eyes (13.5%) even achieved VA of 20/20 after the five cycles of PE therapy (Table 1 and Supplemental Table 1). Our PE therapy thus achieved a considerable vision improvement as compared with the standard monotherapy with corticosteroids.7
The VA of all studied eyes at the 1-month follow-up was the same as that after the fifth PE therapy (Figure 1), indicating that the VA was sustainably maintained through the 1-month period after the PE therapy. Further investigations are needed to closely monitor the long-term efficacy of the PE therapy over a longer follow-up period.
Earlier treatment of NMO due to either optic or spinal attack is associated with better outcomes.31 Previous studies have suggested that early initiation of treatment is a predictor of good outcome.16,32 Our study found that the eyes with good visual outcomes (VA ⩾ 20/40) had a significantly shorter onset-to-PE time window of 22.4 ± 11.1 days, indicating that the time window was significantly related to the final VA (Tables 1 and 2). Our results are comparable with the response rates from a previous study on PE therapy with pooled data of severe ON patients (median treatment window of 19 days).31 Another study on various demyelinating disorders demonstrated that 83% of patients who received PE therapy showed improvement in the first 15 days, compared with 43% of those who received PE therapy more than 2 months after onset.29 Collectively, prior IVMP treatment and early PE therapy should be recommended in the clinical management of severe ON patients.
A previous study showed that the serum AQP4 antibody status was associated with better outcome after PE therapy.17 In contrast, other studies have shown that the serum AQP4 antibody status was not a predictor of better outcome.9,16 In our study, by including all affected eyes (55 eyes of 37 patients) and applying multivariate GEE analysis, the serum AQP4 antibody-positive status showed a negative association with better visual outcome (OR = 0.004, 95% CI = 0.00–0.12, p = 0.001; Table 3). In the logistic regression analysis, the association was also negative but not significant. In view of the lack of unanimity between the previous results and our results, the association of the serum AQP4 antibody status with visual outcomes would be worthy of further investigation using a larger cohort. This study was the first to discuss BMI (OR = 0.734, 95% CI = 0.54–0.99, p = 0.044) as a predictor of treatment efficacy. Bilaterality (OR = 0.042, 95% CI = 0.00–0.43, p = 0.008) also showed some predictive power, comparable with the finding of a previous study showing that unilateral ON had a significantly higher remission rate than bilateral ON.16
This study has several limitations. First, the visual outcome after the PE therapy might not have been solely a consequence of this therapy as the IVMP treatment may also have contributed to improved vision. Although our response rate (73.0%) was higher than that reported in NMO patients treated by steroids alone (40%),7 further studies should include severe ON patients treated only with IVMP so as to isolate the sole effect of the PE therapy. Second, only VA was evaluated to assess the PE therapy response. Other clinical factors, as well as the QoL, could be included in a future study for evaluating PE therapy response in severe ON patients.3,4,33 However, most of the patients in this study showed very poor initial vision and were incapable of being assessed by the VF test or retinal nerve fibre layer thickness examination by optical coherence tomography. Only eight study subjects completed the VF test with a mean deviation of −20.2 ± 10.1 dB, pattern standard deviation of 7.2 ± 3.2 dB and visual field index of 42.4 ± 35.2%. Third, the titres and variations of serum AQP4 autoantibodies were not documented during the study period. The levels of AQP4 autoantibodies might have changed during the PE therapy, with possible implications for the therapeutic effectiveness. Fourth, the MOG antibody status was not measured in the recruited patients at onset or before PE therapy, though it has been suggested to be associated with the pathology of NMOSD.34–38 Although we did test the serum MOG antibody status during follow-up, its variation during the course of disease should be monitored as a potential predictor of the efficacy of PE therapy. Whether the MOG antibody is another factor influencing the visual outcome should be established in further studies. The frequency of meningeal enhancement in inflammatory ON patients should also be further evaluated.39 Immunoadsorption (IA) in combination with PE therapy has been applied in patients with myasthenia gravis (MG) to shorten the hospital stay and reduce the MG score.40 Whether severe ON or NMOSD patients seropositive for AQP4 antibody could also benefit from IA procedures should be investigated. Furthermore, immunosuppressive medications, such as rituximab, mycophenolate, azathioprine and prednisone, are commonly prescribed to prevent relapse.14 The effectiveness of combined PE therapy with immunosuppressants14,38 or additive treatment with a complementary inhibitor41 warrants further investigations. Considering the invasiveness of PE therapy, the benefits, the treatment cost, the potential risks and the side effects need to be balanced in clinical practice. A randomised control study is necessary to further confirm the effectiveness of PE therapy.
In summary, PE therapy effectively improves the VA of patients with first attack of severe acute isolated ON after high-dose IVMP treatment. Earlier PE therapy and prior IVMP treatment lead to better visual outcomes. PE therapy can be considered as a potential treatment for severe isolated ON patients experiencing their first attack after high-dose IVMP treatment.
Supplemental Material
Supplemental material, TanS_PE_ON_paper_supp for Vision improvement in severe acute isolated optic neuritis after plasma exchange treatment in Chinese population: a prospective case series study by Shaoying Tan, Tsz Kin Ng, Quangang Xu, Mo Yang, Yuan Zhuang, Jie Zhao, Huanfen Zhou, Da Teng and Shihui Wei in Therapeutic Advances in Neurological Disorders
Acknowledgments
We are grateful to all participants in this study. We express our sincere gratitude to Dr Shuo Zhao, Dr Hao Kang, Dr Dahe Lin, Dr Chunxia Peng, Miss Mengying Lai and all optometrists and nurses at the Department of Ophthalmology in the Chinese PLA General Hospital for their assistance in this project. We would also like to thank Dr Patrick Yu-Wai-Man from Department of Clinical Neurosciences, University of Cambridge for his comments and manuscript proofreading.
Footnotes
Author contributions: Shaoying Tan: Study concept and design, acquisition of data, analysis and interpretation of data, drafting and revision of manuscript;
Tsz Kin Ng: Analysis and interpretation of data, drafting and revision of manuscript;
Quangang Xu: Study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content;
Mo Yang: Acquisition of data, revision of manuscript;
Yuan Zhuang: Study concept and design, acquisition of data;
Jie Zhao: Acquisition of data;
Huanfen Zhou: Acquisition of data;
Da Teng: Acquisition of data;
Shihui Wei: Study concept and design, study supervision, critical revision of manuscript for intellectual content.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Natural Science Foundation of China (grant number: 81800822), Special Fund for Chinese Medicine Development of Guangdong Province (grant number: 20202089), Joint Regional Basic Science and Applied Basic Science Research Fund of Guangdong Province (grant number: 2019A1515110685), National High Technology Research and Development Program of China (863 Program) (grant number: 2015AA020511), the 59th China Postdoctoral Science Foundation (grant number: 2016M592983), and Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program, China.
ORCID iD: Tsz Kin Ng  https://orcid.org/0000-0001-7863-7229
https://orcid.org/0000-0001-7863-7229
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shaoying Tan, Joint Shantou International Eye Centre of Shantou University and The Chinese University of Hong Kong, Shantou, Guangdong, China; Department of Ophthalmology, The Chinese People’s Liberation Army General Hospital, Beijing, China.
Tsz Kin Ng, Joint Shantou International Eye Centre of Shantou University and The Chinese University of Hong Kong, North Dongxia Road, Shantou, Guangdong 515041, China; Shantou University Medical College, Shantou, Guangdong, China; Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong.
Quangang Xu, Department of Ophthalmology, The Chinese People’s Liberation Army General Hospital, Beijing, China.
Mo Yang, Department of Ophthalmology, The Chinese People’s Liberation Army General Hospital, Beijing, China.
Yuan Zhuang, Department of Haematology, The Chinese People’s Liberation Army General Hospital, Beijing, China.
Jie Zhao, Department of Ophthalmology, The Chinese People’s Liberation Army General Hospital, Beijing, China.
Huanfen Zhou, Department of Ophthalmology, The Chinese People’s Liberation Army General Hospital, Beijing, China.
Da Teng, Department of Ophthalmology, The Chinese People’s Liberation Army General Hospital, Beijing, China.
Shihui Wei, Department of Ophthalmology, The Chinese People’s Liberation Army General Hospital, Beijing, 100853, China.
References
- 1. Tan CT, Mao Z, Qiu W, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2016; 86: 491–492. [DOI] [PubMed] [Google Scholar]
- 2. Beck RW, Cleary PA. Recovery from severe visual loss in optic neuritis. Arch Ophthalmol 1993; 111: 300. [DOI] [PubMed] [Google Scholar]
- 3. Beekman J, Keisler A, Pedraza O, et al. Neuromyelitis optica spectrum disorder: patient experience and quality of life. Neurol Neuroimmunol Neuroinflamm 2019; 6: e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt F, Zimmermann H, Mikolajczak J, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2017; 11: 45–50. [DOI] [PubMed] [Google Scholar]
- 5. Wingerchuk DM, Hogancamp WF, O’Brien PC, et al. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999; 53: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 6. Kremer L, Mealy M, Jacob A, et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler 2014; 20: 843–847. [DOI] [PubMed] [Google Scholar]
- 7. Merle H, Olindo S, Bonnan M, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology 2007; 114: 810–815. [DOI] [PubMed] [Google Scholar]
- 8. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004; 364: 2106–2112. [DOI] [PubMed] [Google Scholar]
- 9. Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 2014; 261: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brecher ME. Plasma exchange: why we do what we do. J Clin Apher 2002; 17: 207–211. [DOI] [PubMed] [Google Scholar]
- 11. Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the Writing Committee of the American Society for Apheresis: the eighth special issue. J Clin Apher 2019; 34: 171–354. [DOI] [PubMed] [Google Scholar]
- 12. Weinshenker BG, O’Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 1999; 46: 878–886. [DOI] [PubMed] [Google Scholar]
- 13. Weinshenker BG. Therapeutic plasma exchange for acute inflammatory demyelinating syndromes of the central nervous system. J Clin Apher 1999; 14: 144–148. [DOI] [PubMed] [Google Scholar]
- 14. Abboud H, Petrak A, Mealy M, et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler 2016; 22: 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonnan M, Valentino R, Debeugny S, et al. Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry 2018; 89: 346–351. [DOI] [PubMed] [Google Scholar]
- 16. Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol 2016; 79: 206–216. [DOI] [PubMed] [Google Scholar]
- 17. Kleiter I, Gahlen A, Borisow N, et al. Apheresis therapies for NMOSD attacks: A retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm. 2018; 5: e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stiebel-Kalish H, Hellmann MA, Mimouni M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm 2019; 6: e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deschamps R, Gueguen A, Parquet N, et al. Plasma exchange response in 34 patients with severe optic neuritis. J Neurol 2016; 263: 883–887. [DOI] [PubMed] [Google Scholar]
- 20. Beck RW, Cleary PA, Anderson MM, Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992; 326: 581–588. [DOI] [PubMed] [Google Scholar]
- 21. Gotkine M. Neuromyelitis optica and the optic neuritis treatment trial. Arch Neurol 2008; 65: 1545–1546. [DOI] [PubMed] [Google Scholar]
- 22. Wildner P, Stasiołek M, Matysiak M. Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Mult Scler Relat Disord 2019; 37: 101452. [DOI] [PubMed] [Google Scholar]
- 23. Caron-Cantin M, Cestari DM, Fortin E. Clinical and radiologic approach to ‘typical’ versus antibody-related optic neuritis. Curr Opin Ophthalmol 2019; 30: 412–417. [DOI] [PubMed] [Google Scholar]
- 24. Holladay JT. Visual acuity measurements. J Cataract Refract Surg 2004; 30: 287–290. [DOI] [PubMed] [Google Scholar]
- 25. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122: 477–485. [DOI] [PubMed] [Google Scholar]
- 26. Keegan M, Pineda AA, McClelland RL, et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology 2002; 58: 143–146. [DOI] [PubMed] [Google Scholar]
- 27. McDaneld LM, Fields JD, Bourdette DN, et al. Immunomodulatory therapies in neurologic critical care. Neurocrit Care 2010; 12: 132–143. [DOI] [PubMed] [Google Scholar]
- 28. Ruprecht K, Klinker E, Dintelmann T, et al. Plasma exchange for severe optic neuritis: treatment of 10 patients. Neurology 2004; 63: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 29. Llufriu S, Castillo J, Blanco Y, et al. Plasma exchange for acute attacks of CNS demyelination: predictors of improvement at 6 months. Neurology 2009; 73: 949–953. [DOI] [PubMed] [Google Scholar]
- 30. Bonnan M, Valentino R, Olindo S, et al. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler 2009; 15: 487–492. [DOI] [PubMed] [Google Scholar]
- 31. Bonnan M, Cabre P. Plasma exchange in severe attacks of neuromyelitis optica. Mult Scler Int 2012; 2012: 787630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weinshenker BG. Plasma exchange for acute attacks of demyelinating disease: detecting a Lazarus effect. Ther Apher 2000; 4: 187–189. [DOI] [PubMed] [Google Scholar]
- 33. Magaña SM, Keegan BM, Weinshenker BG, et al. Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol 2011; 68: 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014; 71: 276–283. [DOI] [PubMed] [Google Scholar]
- 35. Cobo-Calvo A, d’Indy H, Ruiz A, et al. Frequency of myelin oligodendrocyte glycoprotein antibody in multiple sclerosis: a multicenter cross-sectional study. Neurol Neuroimmunol Neuroinflamm 2019; 7: e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cobo-Calvo A, Ayrignac X, Kerschen P, et al. Cranial nerve involvement in patients with MOG antibody-associated disease. Neurol Neuroimmunol Neuroinflamm 2019; 6: e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borisow N, Mori M, Kuwabara S, et al. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol 2018; 9: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Narayan R, Simpson A, Fritsche K, et al. MOG antibody disease: a review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord 2018; 25: 66–72. [DOI] [PubMed] [Google Scholar]
- 39. Asgari N, Flanagan EP, Fujihara K, et al. Disruption of the leptomeningeal blood barrier in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm 2017; 4: e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneider-Gold C, Krenzer M, Klinker E, et al. Immunoadsorption versus plasma exchange versus combination for treatment of myasthenic deterioration. Ther Adv Neurol Disord 2016; 9: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levy M, Mealy MA. Purified human C1-esterase inhibitor is safe in acute relapses of neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm 2014; 1: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TanS_PE_ON_paper_supp for Vision improvement in severe acute isolated optic neuritis after plasma exchange treatment in Chinese population: a prospective case series study by Shaoying Tan, Tsz Kin Ng, Quangang Xu, Mo Yang, Yuan Zhuang, Jie Zhao, Huanfen Zhou, Da Teng and Shihui Wei in Therapeutic Advances in Neurological Disorders