Abstract
Background
Immunotherapy is a promising advance in oncology. Limited information exists regarding the interrelationship between CD47 expression and tumour-associated macrophage-related immuno-microenvironment in patients with non-small cell lung cancer (NSCLC). These factors may predict novel immunotherapy efficacy.
Patients and methods
CD47 and PD-L1 expression was retrospectively assessed in 191 resected NSCLC specimens via immunohistochemistry. Forty-six patients with pulmonary infectious diseases were enrolled as the control group. The infiltration of macrophages (M2 and M1) and CD8+ T-lymphocytes was evaluated via dual-immunofluorescence staining. Targeted DNA sequencing was performed on NSCLC specimens. Survival analysis was performed using the Cox model.
Results
Using 2+/3+ as a CD47 positive (CD47pos) expression cut-off, the prevalence of CD47pos expression in NSCLC was 33.0% (63/191), significantly higher than in pulmonary infectious diseases. CD47pos expression was significantly higher in female, non-smoking and adenocarcinoma patients (p=0.020, p<0.001 and p<0.001, respectively). Furthermore, CD47pos expression was significantly correlated with epidermal growth factor receptor mutation (p<0.001). The expression of CD47 (H-score) in NSCLC was negatively correlated with tumour PD-L1 expression (p=0.0346) and tumour mutation burden (p=0.0107). CD47pos expression was independently correlated with poor disease-free survival in patients with resected NSCLC in multivariate Cox regression analysis (p=0.035).
Conclusion
This study revealed the demographic, molecular and immuno-microenvironment characteristics of CD47 expression in NSCLC. We identified tumour CD47pos expression as an independent prognostic factor for recurrence in resected NSCLC. Our findings illustrate the potential of anti-CD47 treatment in NSCLC.
Keywords: CD47, PD-L1, immunotherapy, non-small cell lung cancer, biomarkers
Key questions.
What is already known about this subject?
Previous studies suggest that CD47 is highly expressed on non-small cell lung cancer (NSCLC) specimens and cell lines, but limited information exists regarding CD47 expression and the tumour-associated macrophage-related immuno-microenvironment in patients with NSCLC.
What does this study add?
This study extensively revealed the demographic, molecular and immuno-microenvironment characteristics of CD47 expression in NSCLC. Tumour CD47 expression significantly correlated with shorter disease-free survival of patients with resected NSCLC in our study.
How might this impact on clinical practice?
With regard to further clinical trial design and study population selection for anti-CD47 treatment, CD47 positive (CD47pos)-expressing patients with NSCLC might represent good target populations, even for those with epidermal growth factor receptor mutation. This study also illustrates the potential value of anti-CD47 treatment as a postoperative adjuvant therapy in stage IIIa CD47pos-expressing NSCLC.
Introduction
Recently, immuno-oncology has emerged as a highly promising clinical strategy in cancer therapy. The most popular approach is adaptive immune system restoration, inducing T cells to attack cancer cells.1 Established immune checkpoint inhibitors (ICIs), such as inhibitory receptor PD-1/PD-L1 targeting antibodies (pembrolizumab, nivolumab, atezolizumab, durvalumab and avelumab), exhibit impressive antitumour activity across multiple cancer types by re-correcting cancer cell recognition by the adaptive immune system.2–4
Lung cancer is the most commonly diagnosed cancer and is the leading cause of cancer-related death.5 Advanced lung cancer treatment, especially for non-small cell lung cancer (NSCLC), is representative of precision medicine, from the era of targeted therapy to immunotherapy.6 PD-1/PD-L1 axis checkpoint inhibitors dramatically altered the NSCLC treatment paradigm.2–4 Patients with advanced NSCLC receiving PD-1 inhibitor antibodies obtain long-term survival benefits,7 especially for NSCLC without biomarkers that indicate targeted treatment susceptibility.2 3 However, for patients with NSCLC harbouring targetable oncogenic driver alterations, PD-1/PD-L1 axis ICIs afford few benefits. Patients with NSCLC carrying mutant epidermal growth factor receptor (EGFR) obtain reduced survival benefits from immunotherapy compared with those harbouring wild-type EGFR.8 The objective response rate (ORR) of ICIs monotherapy is low for NSCLC patients with actionable driver alterations (EGFR mutant ORR: 12%, ALK-rearranged ORR: 0%) in the IMMUNOTARGET registry.9 The immune evasion mechanism in these patients might differ in other types of NSCLC. Thus, an urgent need exists to develop new immunotherapy strategies for NSCLC patients carrying actionable driver alterations.
CD47 is a cell-surface marker broadly expressed on normal cells and often overexpressed on solid and haematological tumours.10 This overexpression underlies the ability to evade immune surveillance. Cell-surface CD47 binding to signal regulatory protein-α (SIRPα) on phagocytic cells initiates an antiphagocytic signal cascade that inhibits macrophage phagocytosis and allows cancer cells to evade immune surveillance and phagocytic destruction.10 CD47-SIRPα axis blockade enhances macrophage phagocytic activity, leading to tumour growth impairment and inhibition of metastasis and tumour progression.11 12 As a negative checkpoint for innate and subsequent adaptive immunity, the CD47-SIRPα axis represents another promising target for immuno-oncology.13 Additionally, CD47-SIRPα axis blockade is successful in various preclinical models.14 15 Multiple anti-CD47 antibody phase I/II clinical trials are currently ongoing for solid and haematologic malignancies. Furthermore, the humanised IgG4 isotype CD47-blocking monoclonal antibody Hu5F9-G4 is well-tolerated in preliminary clinical studies.16 17 Simultaneously blocking CD47 and other cancer-specific antigens by bispecific antibodies (bsAb), including CD20-CD47 bsAb, CD19-CD47 bsAb, EGFR-CD47 bsAb18 and VEGF-CD47 bsAb induces a powerful antitumor immune response in preclinical models. Therefore, CD47-SIRPα axis blockade and anti-CD47-based combination therapy may potentially extend cancer immunotherapies to benefit patients with haematological malignancies and numerous solid tumours.
Previous studies suggest that CD47 is highly expressed on NSCLC specimens and cell lines.19 Anti-CD47/SIRPα treatment increases macrophage phagocytic and cytotoxic activity against NSCLC cell lines.19 20 However, limited information exists regarding the correlation between CD47 expression, tumour immune infiltration and clinical factors in patients with NSCLC. Additionally, whether anti-CD47/SIRPα axis blockade or anti-CD47-based combination therapy may represent another NSCLC immunotherapy option remains to be determined. Furthermore, which biomarkers indicate successful outcomes of CD47-SIRP⍺ blockade remain unclear. Here, we present a retrospective study of Chinese patients with resected NSCLC for CD47-related biomarker analysis. We aimed to (1) comprehensively profile the cellular microenvironment of NSCLC patients, especially the correlation between CD47 and related immune infiltrates; (2) explore correlations between CD47 expression and genomic characterisation in NSCLC, including actionable driver alterations; and (3) identify relevant clinical factors associated with these immune features and their prognostic value.
Methods
Study design
Patients with histologically confirmed pathological stage I and stage IIIa NSCLC who underwent curative resection at Peking Union Medical College Hospital (PUMCH, Beijing, China) between January 2009 and December 2012 were retrospectively enrolled in this study. Stage I NSCLC cases were matched with stage IIIa NSCLC according to pathological type and age at resection. The disease stage was determined according to the Union for International Cancer Control and International Association for the Study of Lung Cancer guidelines (seventh edition) released in 2009. A total of 191 patients with NSCLC were enrolled into this study, and 46 patients with pulmonary infectious diseases (36 patients with pulmonary tuberculosis or tuberculoma, 8 with pulmonary aspergilloma and 2 with pulmonary cryptococcosis) were enrolled as a control group (CONSORT diagram showed in supplemental material). The NSCLC cohort consisted of 47 patient samples with stage I lung squamous cell carcinoma (LUSC), 49 from stage I lung adenocarcinoma (LUAD), 46 from stage IIIa LUSC and 49 from stage IIIa LUAD. No patients in the stage I groups received postoperative adjuvant chemotherapy or radiotherapy, whereas all patients of the stage IIIa group received four cycles of platinum-based doublet chemotherapy. All N2 metastasis cases received adjuvant radiotherapy. Clinical data and outcomes were collected from NSCLC patients and the control group. Informed consent was obtained from each patient at the time of follow-up. The study protocol adhered to the World Medical Association Declaration of Helsinki recommendations.
esmoopen-2020-000823supp001.pdf (227.5KB, pdf)
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) tumour and pulmonary infectious disease specimens were obtained from the Department of Pathology archives. CD47 and PD-L1 expression levels were examined using immunohistochemistry (IHC). IHC studies were performed on 4 µm sections using a Lab Vision autostainer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) with mouse anti-human CD47 (1:40, clone B6H12, sc-12730; Santa Cruz, Dallas, Texas, USA) or rabbit anti-human PD-L1 (1:300, clone E1L3N, 13684; Cell Signalling Technologies, Danvers, Massachusetts, USA). Previously stained CD47-positive tissue was used as a positive control. Tris-buffered saline with Tween-20 (TBST) was used as a negative control. A total of 191 NSCLC cases and 46 pulmonary infectious disease cases were successfully analysed using IHC staining.
Two experienced blinded pathologists independently evaluated IHC samples. The pathologists were not informed regarding patient clinicopathological data. Tumour CD47 expression was evaluated based on staining intensity and membranous and/or cytoplasmic staining. Staining was scored as 0 (absent or <10% stained tumour cells), 1+ (weak in >10% of tumour cells), 2+ (moderate in >10% of tumour cells) or 3+ (strong staining in >10% of tumour cells) (figure 1A). Samples with 2+ or 3+ staining scores were considered as tumour CD47 positive (CD47pos) expression.11 21 Tumour CD47 expression was also evaluated by H-score (H-score=3×percentage of strongly staining (3+)+2×percentage of moderately staining (2+)+percentage of weakly staining (1+)).22 Pulmonary infectious disease CD47 expression was evaluated with the same interpretation method. Tumour PD-L1 expression was assessed for percent tumour cells showing membranous PD-L1 staining and percent area at any intensity.23 Cases with PD-L1 expression for tumour proportion score (TPS) ≥1% were considered positive for tumour PD-L1 expression.
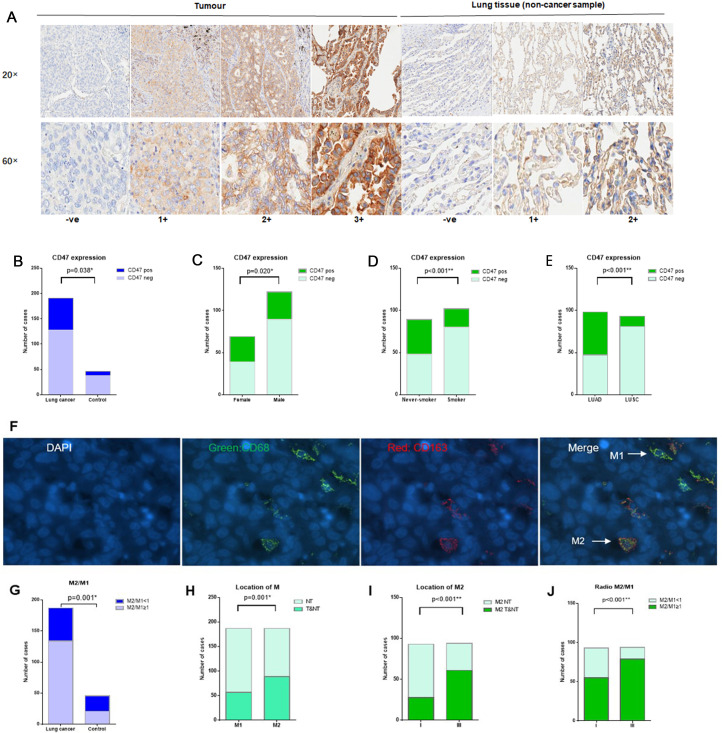
Figure 1.
CD47 expression and tumour-associated macrophage infiltration. (A) Representative IHC images of different CD47 expression level scores in tumour and lung tissue (control respiratory disease sample). (B) CD47pos expression in NSCLC is higher than in the control group (infection lung tissues) (33.0% vs 17.4%, p=0.038). (C) Tumour CD47pos expression in female patients with NSCLC was higher than in male patients with NSCLC (43.5% vs 27.0%, p=0.020). (D) Tumour CD47pos expression in never-smokers with NSCLC was higher than in smokers with NSCLC (46.1% vs 21.6%, p<0.001). (E) Tumour CD47pos expression in LUAD was higher than in LUSC (52.0% vs 12.9%, p<0.001). (F) Representative immunofluorescence images of CD68pos macrophages, CD163pos macrophages, M2-TAMs (CD8pos, CD163pos) and M1-TAMs (CD68pos, CD163neg). (G) High M2/M1 ratios were more frequently observed in NSCLC compared with the control group (71.7% vs 45.7%, p=0.001). (H) M2 infiltration in the tumour area was higher than M1 infiltration in the tumour area (89 vs 57, p=0.001). (I) M2 infiltration in the tumour area in stage III NSCLC was higher than in stage I NSCLC (64.2% vs 29.2%, p<0.001). (J) High M2/M1 ratios were more frequently observed in stage III NSCLC than in stage I NSCLC (83.1% vs 57.3%, p<0.001). *p<0.05, **p<0.001. CD47pos, cases with CD47 IHC scores of 2+ or 3+ were considered positive for tumour CD47 positive expression; CD47neg, cases with CD47 IHC scores of 1+ or ve (0) were considered negative for tumour CD47 expression; if, immunofluorescence; IHC, immunohistochemistry; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; NT, non-tumour area; TAMs, tumour-associated macrophages; T&NT, tumour area and non-tumour area.
Double immunofluorescence
Tumour-associated macrophage (TAM) (M2 macrophage and M1 macrophage) infiltration and PD-L1 and CD8+ T-lymphocytes were evaluated using dual immunostaining. The following primary antibodies were used: rabbit anti-human PD-L1 (1:300, clone E1L3N, 13684; Cell Signalling Technology), mouse anti-human CD8 (1:2, clone C8/144B, IR623, DAKO, Glostrup, Denmark), rabbit anti-human CD68 (1:1600, clone D4B9C, 76437; Cell Signalling Technology) and mouse anti-human CD163 (1:100 clone 10D6, NB110-59935; Novus Biologicals, Littleton, Colorado, USA). Secondary antibodies included goat anti-rabbit Alexa Fluor 488 (1:200, A-11008; Thermo Fisher Scientific) and donkey anti-mouse Alexa Fluor 555 (1:200, A-31570; Thermo Fisher Scientific). Tonsil specimens were used as positive controls. TBST substitution for primary antibody served as a negative control.
For dual-colour immunofluorescence, green fluorescence on cell membranes represented CD68 or PD-L1 expression, whereas CD163 or CD8 expression was identified by red fluorescence (figure 1F). The locations of CD8+ and PD-L1+ CD8+ T cells were identified in both tumour area and non-tumour areas (T&NT) or in non-tumour areas (NT); negative results were designated as neg. The locations of CD68posCD163pos (M2) and CD68posCD163neg (M1) macrophages were identified either in T&NT or only in NT. The CD68posCD163pos (M2)/CD68posCD163neg (M1) ratio was assessed as follows: low (<1) and high (≥1). Overall, 187 NSCLC and 46 pulmonary infectious disease cases were evaluated for CD68 and CD163 dual immunofluorescence.
High-throughput sequencing
Genomic DNA was isolated from 191 FFPE NSCLC tumour samples. DNA libraries were constructed using KAPA Hyper Prep Kits (KK804; Wilmington, Massachusetts, USA) and captured using customised probes. Paired-end sequencing with an average depth of 500× was performed with an Illumina HiSeq 4000 platform (San Diego, California, USA).
Briefly, data analysis was performed according to the Genome Analysis ToolKit (GATK) best practices.24 Reads were aligned to the hg19 human reference genome using the Burrows-Wheeler Aligner (V.0.7.10-r789). PCR duplicates were removed with Picard (V.1.103). Somatic variants were identified using MuTect (V.3.1–0-g72492bb). Gene fusions were detected using Clipping Reveals Structure (CREST). All somatic mutations were filtered, and suspicious variants were manually reviewed using the Integrative Genomics Viewer (V.2.3.34). Owing to the lack of control white blood cells during somatic variant identification, we performed variant calling using our in-house computational pipeline tailored for control-free variant detection. Briefly, the pipeline uses reference genomes from the 1000 Genomes Project, COSMIC, our systemic artefact knowledge base and specialised algorithms to control the false positive rate. Somatic copy number alteration (SCNA) analysis was not performed, as FFPE sample DNA was heavily fragmented due to long-term storage, rendering precise SCNA detection improbable.
Statistical analysis
The statistical association between genomic characteristics and clinicopathological phenotypes, such as somatic mutation, immune cell infiltrates and patient clinical information was tested using Pearson χ2 test or Fisher’s exact test, depending on sample size. Multiple comparisons were corrected using the Benjamini-Hochberg procedure. The association between somatic driver gene mutations and immune infiltrate abundance was investigated using the χ2 test, and contingency tables with n<5 were excluded owing to insufficient statistical power. The association between categorical and continuous characteristics, such as tumour mutation burden (TMB), was tested using non-parametric Mann-Whitney U (Wilcoxon rank-sum) test. Disease-free survival (DFS) was defined as the duration from surgical date to either the first tumour recurrence or death. After surgery, all NSCLC patients were monitored for cancer recurrence every 6 to 12 months. The last follow-up visit was on 15 November 2018. At the time of final analysis, 79 patients suffered from adverse events (disease recurrence or death). All patients not experiencing adverse events were censored at the last follow-up date. Survival analysis was performed using the Cox model. Cox proportional hazards regression analysis was used to estimate DFS hazard ratios. Kaplan-Meier plots were created to visualise differences in DFS. Statistical analyses were performed using R package (R-3.4.1), SPSS V.17.0 (SPSS Inc, Chicago, Illinois, USA) and GraphPad Prism (GraphPad Software, La Jolla, California, USA). P<0.05 was considered statistically significant.
Results
CD47 expression and TAM infiltration in NSCLC and control groups
Clinicopathological characteristics and associations between CD47 expression and macrophage immune features are presented in table 1.
Table 1.
Clinicopathological characteristics of all patients with NSCLC and associations between CD47 expression and macrophage immune features
| Characteristic | Total | CD47pos† | CD47neg† | M1 infiltration | M2 infiltration | M2/M1 | |||
| T&NT | NT | T&NT | NT | ≥1 | <1 | ||||
| (n=191) | (n=63) | (n=128) | (n=57) | (130) | (n=89) | (n=98) | (n=134) | (n=53) | |
| Age (years) | |||||||||
| <65 | 111 | 35 | 76 | 32 | 78 | 54 | 56 | 81 | 29 |
| ≥65 | 80 | 28 | 52 | 25 | 52 | 35 | 42 | 53 | 24 |
| P value | 0.615 | 0.622 | 0.624 | 0.473 | |||||
| Gender | |||||||||
| Female | 69 | 30 | 39 | 20 | 46 | 26 | 40 | 42 | 24 |
| Male | 122 | 33 | 89 | 37 | 84 | 63 | 58 | 92 | 29 |
| P value | 0.020* | 0.969 | 0.097 | 0.072 | |||||
| Smoking history | |||||||||
| Never-smoker | 89 | 41 | 48 | 27 | 60 | 39 | 48 | 61 | 26 |
| Smoker | 102 | 22 | 80 | 30 | 70 | 50 | 50 | 73 | 27 |
| P value | <0.001** | 0.878 | 0.480 | 0.662 | |||||
| Histology | |||||||||
| LUAD | 98 | 51 | 47 | 34 | 61 | 41 | 54 | 63 | 32 |
| LUSC | 93 | 12 | 81 | 23 | 69 | 48 | 44 | 71 | 21 |
| P value | <0.001** | 0.256 | 0.217 | 0.100 | |||||
| Tumour stage | |||||||||
| I | 96 | 28 | 68 | 27 | 66 | 28 | 65 | 55 | 38 |
| III | 95 | 35 | 60 | 30 | 64 | 61 | 33 | 79 | 15 |
| P value | 0.259 | 0.669 | <0.001** | <0.001** | |||||
Data are listed as n.
*p value<0.05, ** p value<0.001.
†Cases with CD 47 IHC scores of 2+ or 3+ were considered positive for tumour CD47 (CD47pos) expression. Cases with CD 47 IHC scores of 0 or 1+ were considered negative for tumour CD47 (CD47neg) expression.
CD47neg, CD47 negative; CD47pos, CD47 positive; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; NT, non-tumour area; T&NT, tumour area and non-tumour area.
Representative CD47 immunofluorescence images are shown in figure 1A. Using 2+/3+ as a CD47pos expression cut-off, NSCLC CD47pos expression was 33.0% (63/191) higher than that in non-tumour lung tissues (17.4%, 8/46, p=0.038) (figure 1B). Examination of associations between CD47pos expression and clinical characteristics in patients with NSCLC revealed significantly higher CD47pos expression in female, non-smoking and LUAD patients (χ2 test p=0.020, p<0.001 and p<0.001, respectively, table 1). CD47pos expression was not associated with any clinical characteristic in LUAD NSCLC, whereas CD47pos expression in LUSC NSCLC was more frequent in non-smoking patients (p=0.004).
Figure 1F shows representative immunofluorescence images of CD68pos macrophages, CD163pos macrophages, M2-TAMs (CD8posCD163pos) and M1-TAMs (CD68posCD163neg). High M2/M1 ratios were more frequently observed in NSCLC compared with the control group (71.7% vs 45.7%, p=0.001, figure 1G). The presence of M2-TAMs and M1-TAMs further characterised M2-TAMs with more extensive tumour area infiltration (figure 1H and 89 vs 57, p=0.001). Notably, stage III tumours had significantly higher M2-TAMs in tumour areas (figure 1I, p<0.001) and higher ratio of M2/M1 infiltration (figure 1J, p<0.001) than stage I tumours.
Correlation of CD47 expression and gene mutations in NSCLC
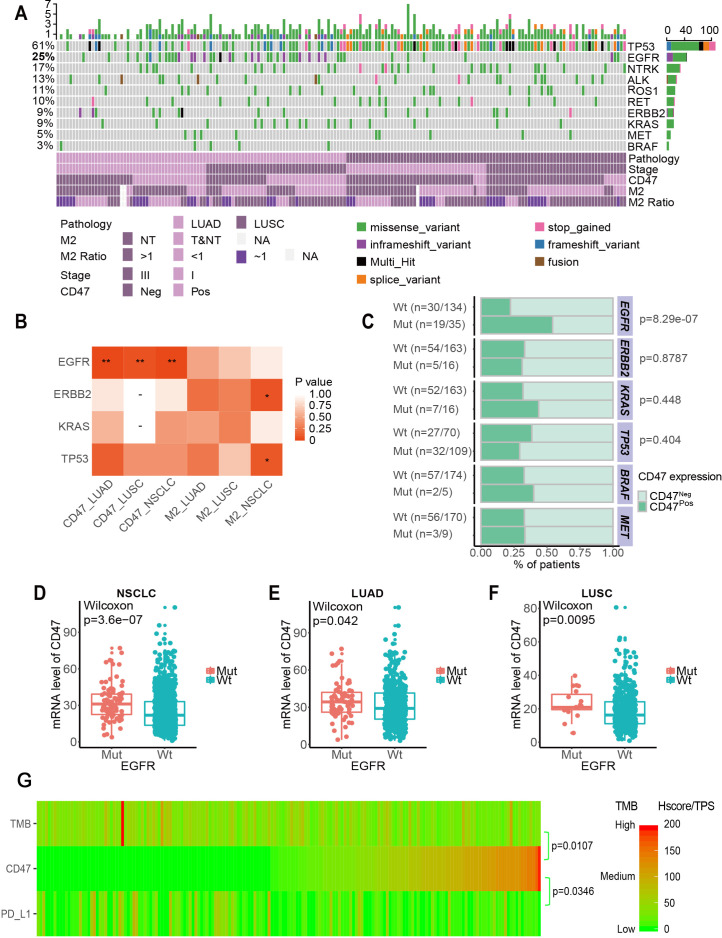
To further investigate the molecular characteristics of patients with NSCLC and correlations with CD47 expression, we performed targeted DNA sequencing. Of 191 patient samples, 12 did not pass quality control and were excluded from the analysis. Patient TMB was calculated. Consistent with previous studies, LUSC samples presented higher somatic mutations with more diverse mutational patterns than LUAD samples. Figure 2A shows the mutational landscape of top 10 well-known driver genes in NSCLC (ALK, BRAF, EGFR, ERBB2, KRAS, MET, NTRK, RET, ROS1 and TP53) among 179 patients and associated clinical characteristics. The results in figure 2B, C show that CD47pos expression correlated with EGFR mutation (p<0.001). On stratification by LUAD and LUSC, correlations remained significant in both subtypes. This correlation was later confirmed by CD47 messenger RNA expression using public data sets of The Cancer Genome Atlas (figure 2D, 2E, 2F).
Figure 2.
Correlation between CD47 expression and driver gene mutations in NSCLC. (A) Landscape of the top 10 genes involved in NSCLC (ALK, BRAF, EGFR, ERBB2, KRAS, MET, NTRK, RET, ROS1 and TP53) among 179 patients and associated clinical information. (B) Correlation between immune infiltrate expression and genomic alterations in NSCLC. Only contingency tables with n>5 were included in the test. *p<0.1, **p<0.05. (C) Correlation between CD47 expression and four well-known mutant genes in NSCLC. (D to F) The correlation between CD47 mRNA and EGFR mutation in NSCLC (D), LUAD (E) and LUSC (F) in the Cancer Genome Atlas. P values were calculated using the Wilcoxon rank-sum test. (G) CD47 expression heatmap (H-score), PD-L1 (TPS) and TMB in NSCLC. CD47pos, CD47 positive; CD47neg, CD47 negative; EGFR, epidermal growth factor receptor; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; mRNA, messenger RNA; mut, mutation; NSCLC, non-small cell lung cancer; NT, non-tumour area; T&NT, tumour area and non-tumour area; TMB, tumour mutation burden; TPS, tumour proportion score; Wt, wild-type.
Correlation of CD47 expression and the immuno-microenvironment in NSCLC
We investigated the correlation between CD47 and PD-L1, two immune-related biomarkers, and correlated these two biomarkers with TMB. All 191 samples were sorted using the CD47 H-score. CD47 H-score negatively correlated with TMB (Pearson χ2, p=0.0107) and PD-L1 expression measured by TPS (Pearson χ2, p=0.0346) (figure 2G). CD47 expression did not correlate with tumour area M2 infiltration, M1 infiltration or CD8+ T cell infiltration.
Prognostic implications of CD47 expression and TAM infiltration in NSCLC
Median NSCLC patient DFS was 92.8 months in this study. We investigated clinical factors, CD47 expression, PD-L1 expression, M2 location, M2/M1 ratio and mutant driver genes to predict DFS in NSCLC (table 2). CD47pos expression was identified as an independent prognostic factor of the NSCLC group DFS by multivariate Cox regression (p=0.035).
Table 2.
Univariate and multivariate Cox proportional hazards regression analysis for DFS prediction in patients with NSCLC
| All NSCLC group | DFS | |||
| Univariate analysis | Multivariate analysis | |||
| HR (95% CI) | P value | Relative risk (95% CI) | P value | |
| Age (years): ≥65 vs <65 | 1.417 (0.91 to 2.2) | 0.122 | – | – |
| Gender: male versus female | 1.322 (0.83 to 2.1) | 0.244 | – | – |
| Smoking history: smoker versus non-smoker | 1.323 (0.85 to 2.1) | 0.221 | – | – |
| Histology: LUAD versus LUSC | 1.218 (0.78 to 1.9) | 0.389 | – | – |
| Stage: III vs I | 1.756 (1.4 to 2.2) | <0.0001 | 1.65 (1.29 to 2.12) | <0.0001 |
| PD-L1: PD-L1+ versus PD-L | 1.366 (0.82 to 2.3) | 0.228 | – | – |
| CD47: CD47pos versus CD47neg | 1.734 (1.1 to 2.7) | 0.017 | 1.63 (1.04 to 2.58) | 0.035 |
| M2 location: T&NT versus NT | 2.036 (1.3 to 3.2) | 0.002 | 1.41 (0.86 to 2.31) | 0.17 |
| Ratio of tumour M2/M1: high versus low | 1.888 (1.1 to 3.2) | 0.021 | 1.23 (0.69 to 2.21) | 0.48 |
| EGFR: mutation versus wild type | 1.486 (0.91 to 2.4) | 0.110 | – | – |
| TP53: mutation versus wild type | 0.9975 (0.63 to 1.6) | 0.992 | – | – |
| KRAS: mutation versus wild type | 1.665 (0.85 to 3.2) | 0.134 | – | – |
| ERBB2: mutation versus wild type | 0.7146 (0.31 to 1.6) | 0.430 | – | – |
CD47neg, CD47 negative; CD47pos, CD47 positive; DFS, disease-free survival; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; NT, non-tumour area; T&NT, tumour area and non-tumour area.
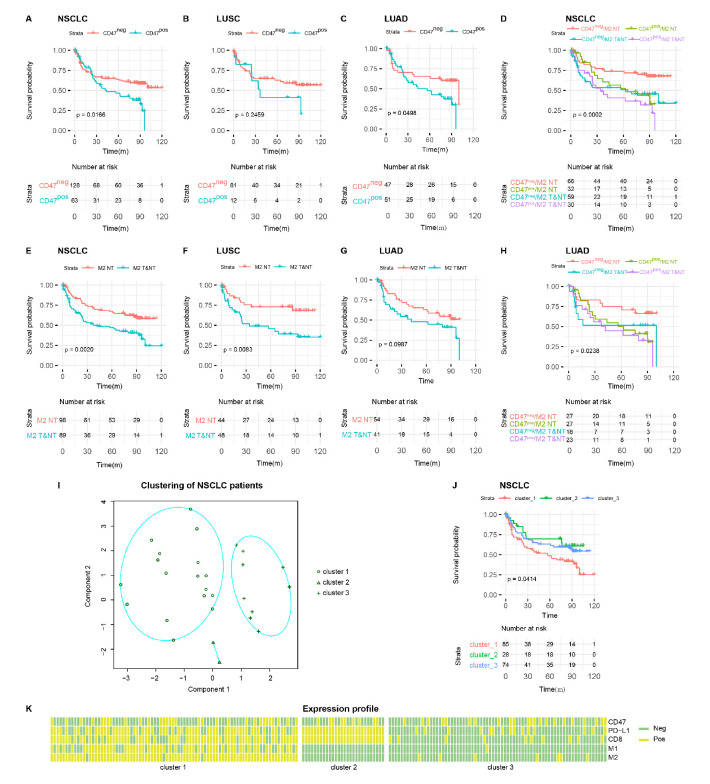
Cox model showed that CD47pos patients had poorer survival (Wald test, HR=1.734 (1.1 to 2.7), p=0.0166, table 2). Median DFS was 43.7 months in patients with CD47pos expression, significantly shorter than not arrived in the CD47neg patients (figure 3A). Similar findings were observed in the LUAD (Wald test, HR=1.861 (1 to 3.5), p=0.0498, figure 3C) but not LUSC patients (Wald test, HR=1.637 (0.71 to 3.8), p=0.246, figure 3B). Patients with M2-TAM infiltration in T&NT had poorer survival (Wald test, HR=2.036 (1.3 to 3.2), p=0.002) and exhibited shorter median DFS than patients with M2-TAMs infiltration only in NT (56.6 vs 100.2 months, figure 3E). Similar findings were observed in the LUSC patients (Wald test, HR=2.638 (1.3 to 5.4), p=0.008, figure 3F), but not in the LUAD group (figure 3G).
Figure 3.
DFS in patients with NSCLC. (A to C) DFS Kaplan-Meier curves of NSCLC with CD47pos and CD47neg expression in patients with NSCLC (A), LUSC (B) and LUAD (C). (E to G) DFS Kaplan-Meier curves of NSCLC with M2 in T&NT and M2 in NT in patients with NSCLC (E), LUSC (F), and LUAD (G). (D and H) DFS Kaplan-Meier curves of NSCLC with varying CD47 expression and M2 location in patients with NSCLC (D) and LUAD (H). (I) K-medoid clustering of patients with NSCLC. (J) DFS Kaplan-Meier curves of NSCLC with various immune component characteristics. (K) Heatmap of patients with NSCLC showing feature component expression pattern. CD47neg, CD47 negative; CD47pos, CD47 positive; DFS, disease-free survival; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; NT, non-tumour area; T&NT, tumour area and non-tumour area.
As CD47 expression and M2-TAMs location appeared to exhibit prognostic values, we asked whether CD47 expression and M2-TAMs location in combination would enhance prognostic power. Cox model showed that CD47neg patients and M2-TAMs only in NT have better survival, whereas patients with CD47pos expression and M2-TAMs in T&NT have poorer survival (Wald test, HR=1.444 (1.2 to 1.8), p=0.0002, figure 3D). These associations remained significant even when patients were stratified by LUAD (Wald test, HR=1.342 (1 to 1.7), p=0.024, figure 3H). Cox model on LUSC showed moderate significance (Wald test, HR=1.564 (1.1 to 2.1), p=0.005), possibly due to insufficient sample size.
Combinatorial effect of immune recognition pathways on patient survival
CD47/macrophages and PD-L1/CD8+ T cells are well-defined pathways that play essential roles in immune tumour cell recognition. To evaluate whether patient survival is affected when both pathways were considered, we built a K-medoid clustering model using CD47, M1, M2, PD-L1 and CD8 expression. The number of clusters was optimised to three based on silhouette width. Figure 3I shows the first two K-medoid cluster dimensions. Cox model were subsequently conducted. As the result showed in figure 3J, the DFS of each patient group differed significantly (p=0.041). To illustrate the patient characteristics in each group, we generated an expression pattern and feature component heatmap (figure 3K). Group three could be viewed as a rough representation of an immune desert. Though CD8+ T cell infiltrates were present in group 2, they were most likely counteracted by PD-L1 expression. Group 1, characterised by high M1-TAMs and M2-TAMs tumour area infiltration, exhibited the worst survival among all groups.
Discussion
To our knowledge, this is the first study to comprehensively investigate the association between CD47 expression, major immune components and genomic characteristics in patients with NSCLC. Our study indicates that: (1) tumour CD47pos expression was more frequently detected in adenocarcinoma, female and never-smoking NSCLC cases; (2) CD47pos expression correlates with EGFR mutation in patients with NSCLC; (3) the tumour CD47 expression (H-score) in NSCLC negatively correlated with tumour PD-L1 expression (TPS) or TMB; (4) CD47pos expression was identified as an independent prognostic factor in the resected NSCLC group DFS; and (5) different immune component characteristics provide greater recurrence prognostic power in resected NSCLC. These results reveal the features of tumour CD47 expression and the macrophage-related microenvironment in patients with NSCLC. Our study illustrates precision medicine by patient stratification based on immuno-microenvironment characteristics. Specifically, we emphasise the potential of phagocytosis checkpoint inhibitors for NSCLC patients, especially for patients not suited for PD-L1 inhibitors.
The definition of CD47pos in NSCLC was not well determined. As a potential immunotherapy-related biomarker, the interpretation of CD47 should be easy to perform with effective clinical significance. CD47 is a cell-surface marker broadly expressed on haematopoietic cells and other normal tissues, and often overexpressed on tumour cells.10 CD47 can detected in normal tissue, inflammatory lesions and epithelial cancer cells, but the enhanced expression of CD47 has been reported on tumour cells.11 25 Therefore, low-to-undetectable CD47 expression was defined as CD47 negative and intermediate-to-high CD47 expression was defined as CD47 positive in previous studies, which could effectively make differentiation between tumour and non-tumour tissues.11 21 Our data showed that CD47 staining (1+/2+/3+) was observed in 53.4% (102/191) of NSCLC and 80.4% (37/46) of pulmonary infectious samples (p=0.001), while using 2+/3+ as a CD47pos expression cut-off, NSCLC CD47pos expression was 33.0% (63/191) higher than that in non-tumour lung tissues (17.4%, 8/46, p=0.038). The results of CD47 expression in NSCLC in our study was consistent with previous studies, and highly supporting the 2+/3+ as a CD47pos expression cut-off for definition of CD47pos in future clinical trials.
Several previous studies reported widespread CD47 expression in lung cancer, and significant associations between CD47 expression, tumour stage and metastasis.19 26 Until now, the clinicopathological characteristics of patients with NSCLC positive for CD47 expression remained unclear. Our analysis revealed NSCLC subpopulations that are predisposed to CD47pos expression; that is, never-smoking, female and LUAD patients, who also exhibit high EGFR mutation frequency. This finding led to the possible association between CD47pos expression and EGFR mutation in NSCLC. We further analysed associations between oncogenic alterations and major immune infiltrates. As expected, CD47pos expression indeed correlated with EGFR mutation. Arrieta et al22 also found that high CD47 expression (using a cut-off value of 150 based on staining index scores) was associated with presence of EGFR (+) mutations (66.7% vs 33.3%, p=0.04). However, the biological association between EGFR mutation and CD47pos expression remains unclear. Currently, no relevant translational medicine or basic medical research exists to clarify the mechanism for this phenomenon. Liu et al27 revealed that Akt phosphorylation increases after CD47 overexpression and PI3K suppressors impair CD47-overexpressing cell invasion. Kaur et al28 showed that anti-CD47 (B6H12) treatment decreases EGFR expression and inhibits EGF-induced EGFR tyrosine phosphorylation in breast cancer stem cells. Nigro et al29 found that CD47 expression became up-regulated following in vitro drug resistance development, and blocking this protein by a specific monoclonal antibody increased the clearance of EGFR tyrosine kinase inhibitor (TKI)-resistant cells by phagocytes. We hypothesise that CD47/SIRPα axis inhibition is a potential antitumour immunotherapy strategy for EGFR mutant NSCLC with acquired resistance to EGFR-TKI. Further research is urgently needed to clarify this hypothesis.
CD47 and PD-L1 are involved in two distinct immune-tumour recognition pathways. PD-L1 expression is enriched in LUSC patients with smoking history,30 31 as opposed to patients who are non-smoking and female and in LUAD subpopulations characterised by CD47 expression. Few studies have evaluated CD47 and PD-L1 expression, and TAM infiltration simultaneously in NSCLC tissues. Our analysis showed that CD47 H-score negatively correlated with PD-L1 expression and TMB. A possible explanation of this moderate mutual exclusion is that for some NSCLC cases, one compromised immune-tumour recognition pathway coincident with other cancer hallmarks is sufficient to initiate tumourigenesis. Such patient stratification provides an important clue that patients who do not respond or become resistant to checkpoint inhibitors may benefit from alternative immunotherapy targeting other checkpoint signalling pathways. The idea of ‘precision medicine’ through the stratification of patients with NSCLC by immune-related characteristics should be emphasised. For patients with NSCLC exhibiting CD47pos expression, anti-CD47-axis treatment might be a better choice than PD-1/PD-L1 axis ICIs. Increasing efforts have been directed towards developing CD47-specific inhibitors. Multiple phase I clinical trials are on-going to evaluate potential clinical benefits of CD47-specific inhibitors.16 32 33 With regard to further clinical trial design and study population selection for anti-CD47 treatment, CD47pos expressing patients with NSCLC might represent good target populations, even for those with EGFR mutation. The Hu5F9-G4 antibody with rituximab revealed that simultaneously blocking CD47 and other cancer-specific antigens might be a good choice for cancer immunotherapy.17 For CD47pos NSCLC with EGFR mutation, EGFR-CD47 bsAb18 might represent another option. Further exploration of preclinical models and preliminary clinical studies is urgently needed. However, immune-related adverse events caused by combinatorial therapy must also be considered.
Tumour CD47 expression serves as an adverse prognostic factor in multiple cancer types.21 34–38 Barrera et al39 found that CD47 expression on the surface of neutrophils is associated with lower overall survival (OS) of patients with NSCLC. However, tumour CD47 expression was not evaluated in this study. Arrieta et al22 revealed that high expression of CD47 were associated with shortened OS in EGFR mutant patients. Tumour CD47pos expression significantly correlated with shorter DFS of patients with NSCLC in our study. These observations indicate that CD47pos expression might be associated with more aggressive tumour progression, rendering it a potential NSCLC therapeutic target. Chung et al40 revealed that high TAM count is related to poor progression-free survival in advanced NSCLC treated with EGFR-TKI. Our study also revealed that CD47 and M2 combined can enhance prognostic power. To understand the essential role of CD47/macrophage and PD-L1/CD8+ T cell pathways in immune tumour cell recognition, we built a K-medoid clustering model using CD47, M1, M2, PD-L1 and CD8 expression. In our study, high M1 and M2 infiltration in tumour areas presented the worst survival. One interpretation is that a tumour-favourable microenvironment created by TAMs imposes a strong adverse impact on patient survival. The unsupervised clustering approach clearly separated CD8/PD-L1 but not CD47/TAM expression. This implies that CD47 is not a unique promoter that impacts macrophage infiltration as an immune escape mechanism similar to PD-L1 and CD8.
The main limitation of our study is the lack of white blood cells as controls during somatic variant identification. We, therefore, limited our mutational analysis to well-annotated driver genes, wherein somatic mutations can be identified with high confidence through a customised computational pipeline. Furthermore, the core role of CD47 expression in NSCLC needs to be validated in vitro, shedding light on the detailed mechanism of CD47 as a macrophage checkpoint in tumour growth and progression. Moreover, some clinical trials focussing on anti-CD47 treatment or anti-CD47-based combination therapy should be designed for NSCLC patients, especially for EGFR-mutant patients.
In conclusion, we extensively investigated the demographic, molecular and immuno-microenvironment characteristics of CD47 expression in NSCLC. We further identified tumour CD47pos expression as an independent prognostic factor for NSCLC recurrence. This study illustrates the potential value of anti-CD47 treatment in NSCLC.
Acknowledgments
The authors thank the patients for providing their information in this study. The authors acknowledge the contributions of Hulin Han, Haihua Fu and Tianwei Zhang (Dizal Pharmaceutical Co, Ltd) for technical support with immunohistochemistry and dual-immunofluorescence staining. The authors also acknowledge the contributions of Shuang Wang (Genetron Health (Beijing) Co, Ltd) for bioinformatics analysis.
Footnotes
YX, JL, BT and MC contributed equally.
Contributors: Study supervision: MW. Conception and design: MW and YX. Collection and assembly of data: YX, BT, JL, XL, WZ, JZ, MC and MW. Data analysis and interpretation: YX, BT, JL, MC and MW. Manuscript writing: YX, JL, BT, MC and MW. Final approval of manuscript: All authors.
Funding: This study was supported by the ‘13th Five-Year’ National Science and Technology Major Project for New Drugs (No: 2019Z×09734001-002), CAMS Innovation Fund for Medical Sciences (to MZW) (No: 2018-I2M-1-003) and the National Natural Science Foundation of Beijing (to YX) (No: 7194311). This study was funded in part by Innovent Biologics (Suzhou) Co, Ltd. The funders had no role in study design, data collection, analysis, decision to publish or manuscript preparation.
Competing interests: MZW received partial research funding from Innovent Biologics (Suzhou) Co, Ltd, and Dizal Pharmaceutical Co, Ltd.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Ethical Committee of Peking Union Medical College Hospital (S-K146).
Provenance and peer review: Not commissioned; externally peer-reviewed.
Data availability statement: Data are available upon reasonable request. The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced Nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med 2017;377:849–61. 10.1056/NEJMra1703413 [DOI] [PubMed] [Google Scholar]
- 7.Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-Year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 2019;20:1395–408. 10.1016/S1470-2045(19)30407-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Q, Zhang H, Hai J, et al. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: a meta-analysis of randomized trials. Oncoimmunology 2018;7:e1396403. 10.1080/2162402X.2017.1396403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321–8. 10.1093/annonc/mdz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matlung HL, Szilagyi K, Barclay NA, et al. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev 2017;276:145–64. 10.1111/imr.12527 [DOI] [PubMed] [Google Scholar]
- 11.Cioffi M, Trabulo S, Hidalgo M, et al. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin Cancer Res 2015;21:2325–37. 10.1158/1078-0432.CCR-14-1399 [DOI] [PubMed] [Google Scholar]
- 12.Vaeteewoottacharn K, Kariya R, Pothipan P, et al. Attenuation of CD47-SIRPα signal in cholangiocarcinoma potentiates tumor-associated macrophage-mediated phagocytosis and suppresses intrahepatic metastasis. Transl Oncol 2019;12:217–25. 10.1016/j.tranon.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Pu Y, Cron K, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015;21:1209–15. 10.1038/nm.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Lv B, Liu Y, et al. Blocking the CD47-SIRPα axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology 2018;7:e1391973. 10.1080/2162402X.2017.1391973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F, Feng B, Yu H, et al. Tumor Microenvironment-Activatable prodrug vesicles for Nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater 2019;31:1805888. 10.1002/adma.201805888 [DOI] [PubMed] [Google Scholar]
- 16.Sikic BI, Lakhani N, Patnaik A, et al. First-In-Human, first-in-class phase I trial of the Anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J Clin Oncol 2019;37:946–53. 10.1200/JCO.18.02018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Advani R, Flinn I, Popplewell L, et al. Cd47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med 2018;379:1711–21. 10.1056/NEJMoa1807315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Guo R, Chen Q, et al. A novel bispecific antibody fusion protein co-targeting EGFR and CD47 with enhanced therapeutic index. Biotechnol Lett 2018;40:789–95. 10.1007/s10529-018-2535-2 [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Wang J, Kong X, et al. Cd47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci Rep 2016;6:29719. 10.1038/srep29719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Fan J, Wang S, et al. Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res 2017;5:363–75. 10.1158/2326-6066.CIR-16-0398 [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K, Tsujimoto H, Matsumura K, et al. Cd47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med 2015;4:1322–33. 10.1002/cam4.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrieta O, Aviles-Salas A, Orozco-Morales M, et al. Association between CD47 expression, clinical characteristics and prognosis in patients with advanced non-small cell lung cancer. Cancer Med 2020;9:2390–402. 10.1002/cam4.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsao M-S, Le Teuff G, Shepherd FA, et al. Pd-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol 2017;28:882–9. 10.1093/annonc/mdx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somatic short variant discovery (SNVs + indels). Available: https://gatk.broadinstitute.org/hc/en-us/articles/360035894731-Somatic-short-variant-discovery-SNVs-Indels-
- 25.Willingham SB, Volkmer J-P, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662–7. 10.1073/pnas.1121623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiskopf K, Jahchan NS, Schnorr PJ, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest 2016;126:2610–20. 10.1172/JCI81603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Wu X, Wang Y, et al. Cd47 promotes human glioblastoma invasion through activation of the PI3K/Akt pathway. Oncol Res 2019;27:415–22. 10.3727/096504018X15155538502359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur S, Elkahloun AG, Singh SP, et al. A function-blocking CD47 antibody suppresses stem cell and EGF signaling in triple-negative breast cancer. Oncotarget 2016;7:10133–52. 10.18632/oncotarget.7100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nigro A, Ricciardi L, Salvato I, et al. Enhanced expression of CD47 is associated with off-target resistance to tyrosine kinase inhibitor gefitinib in NSCLC. Front Immunol 2019;10:3135. 10.3389/fimmu.2019.03135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper WA, Tran T, Vilain RE, et al. Pd-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181–8. 10.1016/j.lungcan.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 31.Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 2015;41:450–6. 10.1016/j.ejso.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 32.Takimoto CH, Chao MP, Gibbs C, et al. The Macrophage 'Do not eat me' signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol 2019;30:486–9. 10.1093/annonc/mdz006 [DOI] [PubMed] [Google Scholar]
- 33.Gholamin S, Mitra SS, Feroze AH, et al. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 2017;9:eaaf2968. 10.1126/scitranslmed.aaf2968 [DOI] [PubMed] [Google Scholar]
- 34.Nagahara M, Mimori K, Kataoka A, et al. Correlated expression of CD47 and SIRPa in bone marrow and in peripheral blood predicts recurrence in breast cancer patients. Clin Cancer Res 2010;16:4625–35. 10.1158/1078-0432.CCR-10-0349 [DOI] [PubMed] [Google Scholar]
- 35.Fujiwara-Tani R, Sasaki T, Ohmori H, et al. Concurrent expression of CD47 and CD44 in colorectal cancer promotes malignancy. Pathobiology 2019;86:182–9. 10.1159/000496027 [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Lu H, Xiang L, et al. Hif-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A 2015;112:E6215–23. 10.1073/pnas.1520032112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brightwell RM, Grzankowski KS, Lele S, et al. The CD47 "don't eat me signal" is highly expressed in human ovarian cancer. Gynecol Oncol 2016;143:393–7. 10.1016/j.ygyno.2016.08.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Lu S, Xu Y, et al. Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res 2017;9:2901–10. [PMC free article] [PubMed] [Google Scholar]
- 39.Barrera L, Montes-Servín E, Hernandez-Martinez J-M, et al. Cd47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer 2017;117:385–97. 10.1038/bjc.2017.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung F-T, Lee K-Y, Wang C-W, et al. Tumor-Associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer 2012;131:E227–35. 10.1002/ijc.27403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000823supp001.pdf (227.5KB, pdf)