Abstract
Background
There is no information on acute kidney injury (AKI) and continuous renal replacement therapy (CRRT) among invasively ventilated coronavirus disease 2019 (COVID-19) patients in Western healthcare systems.
Objective
To study the prevalence, characteristics, risk factors and outcome of AKI and CRRT among invasively ventilated COVID-19 patients.
Methods
Observational study in a tertiary care hospital in Milan, Italy.
Results
Among 99 patients, 72 (75.0%) developed AKI and 17 (17.7%) received CRRT. Most of the patients developed stage 1 AKI (33 [45.8%]), while 15 (20.8%) developed stage 2 AKI and 24 (33.4%) a stage 3 AKI. Patients who developed AKI or needed CRRT at latest follow-up were older, and among CRRT treated patients a greater proportion had preexisting CKD. Hospital mortality was 38.9% for AKI and 52.9% for CRRT patients.
Conclusions
Among invasively ventilated COVID-19 patients, AKI is very common and CRRT use is common. Both carry a high risk of in-hospital mortality.
Keywords: Coronavirus disease 2019, Acute kidney injury, Renal replacement therapy, Critical care, Coronavirus
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic infection caused by a virus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Older patients with comorbid conditions, including hypertension, and diabetes are at higher risk of death, suggesting particularly susceptible populations.
The main feature of COVID-19 is lung involvement with patients mainly presenting with acute respiratory failure and acute respiratory distress syndrome [1, 2, 3]. However, the involvement of other organs in this setting is poorly understood [4]. After primary infection, the virus may accumulate in kidney and cause damage to renal cells. Data from 2002–2004 SARS epidemic revealed that 6.7% of patients with SARS developed acute kidney injury (AKI) and the mortality of SARS patients with AKI was 91.7% [5]. In addition, special algorithms for COVID-19 spreading in the dialysis population, recommendations for isolation, and preventive measures in positive dialytic patients are necessary [6].
Activation of macrophage, cytokine storm, and other common features associated with COVID-19 can result in release of tissue factor and activation of coagulation factors that create a predisposition to hypercoagulability [7]. In addition, recent data observed strong complement C5b-9 deposition in renal tubules in patients with SARS-CoV-2 infection [7, 8]. Also, inflammation-induced erythrocyte aggregation and heme-mediated pathology may worsen oxidative stress, inflammation, and complement activation, to aggravate microvascular injury [7, 9]. Further, organ cross talk between the injured lung, the heart, and the kidney can worsen pathology [10].
A recent report in patients with COVID-19 admitted to a Chinese hospital showed that the prevalence of kidney disease on admission and the development of AKI during hospitalization were high and associated with in-hospital mortality [11]. On the other hand, another study in a similar population concluded that AKI was uncommon in COVID-19 [12]. However, information on AKI development in critically ill patients with COVID-19 and on the use of renal replacement therapy (RRT) in this population in a Western healthcare setting is not known. The present study aimed to assess the prevalence, patient characteristics clinical outcomes, and predictors of AKI and need of RRT in patients with COVID-19 receiving mechanical ventilation in the intensive care unit (ICU).
Methods
Study Design
The COVID-BioB study is an observational study performed at a 1,350-bed university hospital in Milan, Italy (Istituto di Ricovero e Cura a Carattere Scientifico [IRCCS] San Raffaele Scientific Institute). The study was registered on ClinicalTrials.gov (NCT04318366) and approved by the Hospital Ethics Committee (protocol No. 34/int/2020). Full description of patient management and clinical protocols were previously published [13, 14].
Enrollment Criteria
All patients aged ≥18 years admitted to an ICU at IRCCS San Raffaele Scientific Institute with confirmed SARS-CoV-2 infection were consecutively enrolled. We defined confirmed infection as positive Reverse transcriptase-polymerase chain reaction (RT-PCR) from a nasal and/or throat swab together with symptoms, signs, and radiological findings suggestive of COVID-19 pneumonia. Patients on chronic RRT were excluded from this analysis.
Data Collection
Data were collected from medical records by trained investigators independent from clinical teams. We obtained data on contact exposure, onset of symptoms and presenting symptoms, medical history and current medications at time of symptoms onset, daily clinical and laboratory data, treatment data, and outcome data. We performed an extensive round of data cleaning to check for data accuracy and outliers before analysis.
Definitions
AKI was identified according to Kidney Disease: Improving Global Outcomes (KDIGO) [15]. The standard definition of AKI in adults is one of the following: an increase in serum creatinine (Cr) by ≥0.3 mg/dL within 48 h, or an increase in Cr to >1.5 times baseline within the previous 7 days, or urine volume <0.5 mL/kg/h for >6 h. The value of Cr at hospital admission was used as the baseline of renal function. The staging of AKI was also defined according to the KDIGO criteria. The use of RRT was determined according to physician criteria.
Outcomes
The primary outcome was hospital mortality until the latest follow-up available. Other secondary outcomes are as follows: (1) ICU mortality until the latest follow-up available, (2) duration of mechanical ventilation until the latest follow-up available, (3) ICU length of stay until the latest follow-up available, and (4) hospital length of stay until the latest follow-up available. The latest follow-up available for all data is April 20, 2020.
Statistical Analysis
A convenience sample was considered for this analysis, with consecutively patients included until the latest follow-up. No missing data for any of the outcomes is present in the dataset; thus, all analyses were complete case analyses. Continuous variables are presented as medians (quartile 25–75%) and categorical variables as number and percentages. Patients were divided in groups according to development of AKI (AKI vs. no AKI) and need of RRT (RRT vs. no RRT) until the latest follow-up.
Baseline and clinical characteristics of the patients were compared among the groups using Fisher's exact tests and Wilcoxon rank sum tests. Daily data are compared using mixed-effect quantile models accounting for repeated measures, with day as a continuous variable and with day and group (and an interaction among them) as the fixed effect. Quantile models considered a = 0.50, an asymmetric Laplace distribution, and p values were extracted after 1,000 bootstrap samplings. Overall p values from this analysis represent the overall difference among groups over time and p values from interaction represent if the trend over time differs among the groups.
To account for the different follow-up times for the hospital and ICU mortality, a survival model with a Cox proportional hazard model was considered. The assumption of proportional hazards was assessed with Schoenfeld residuals. ICU and hospital length of stay and duration of mechanical ventilation were assessed using sub-distribution hazard ratio derived from a Fine-Gray competing risk model with death before the event treated as competing risk and presented in cumulative incidence plots.
To account for potential confounders, propensity scores for the development of AKI or need of RRT were calculated using generalized linear models with a Binomial distribution. The probability of a patient having AKI or receiving RRT was estimated as a function of relevant covariates (namely age, baseline SpO2, presence of hypertension, and CKD). The results of these logistic propensity models were used for inverse probability weighting of observations within the final models described above. This allowed patients to be weighted based on how likely the observed development of AKI or need of RRT was on the basis of the observed covariates. In general, such inverse probability of treatment weighting yields estimates in the final model that represent the average effect of having AKI or needing RRT on outcomes.
Due to the nature of the study, the small sample size, and the low number of events, all analyses should be considered exploratory and hypothesis generating only. In addition, all analyses for the secondary outcomes and complications were not adjusted for confounders. All analyses were conducted in R v.3.6.3 (R Foundation) [16].
Results
Population
From February 25, 2020 to April 20, 2020, 112 patients receiving invasive mechanical ventilation in the ICU and 99 with COVID-19 receiving mechanical ventilation and complete follow-up were included in the study. Pre-admission demographic and clinical characteristics are shown in Table 1. In total, 72 patients (75.0%) developed AKI and 17 patients (17.7%) needed continuous RRT by latest follow-up. Most of the patients developed stage 1 AKI (33 [45.8%]), while 15 (20.8%) developed stage 2 AKI and 24 (33.4%) a stage 3 AKI. Three patients, for whom baseline Cr was not available and the KDIGO criteria were not applied, were excluded from the AKI analysis.
Table 1.
Baseline characteristics of the patients according to development of AKI or need of RRT
| AKI (n = 72) | No AKI (n = 24) | p value | RRT (n = 17) | No RRT (n = 82) | p value | |
|---|---|---|---|---|---|---|
| Age, years | 63.0 (58.5−70.0) | 54.5 (46.0−66.2) | 0.004 | 67.0 (63.0−71.0) | 61.0 (53.0−67.0) | 0.003 |
| Male gender, n (%) | 59 (81.9) | 21 (87.5) | 0.752 | 13 (76.5) | 70 (85.4) | 0.586 |
| BMIa, kg/m2 | 27.8 (26.0−30.9) | 27.5 (24.6−34.4) | 0.934 | 26.5 (26.2−31.2) | 27.9 (24.8−31.0) | 0.920 |
| Normal, n (%) | 8 (17.0) | 3 (20.0) | 0.475 | 1 (7.1) | 10 (20.8) | 0.735 |
| Overweight, n (%) | 23 (48.9) | 6 (40.0) | 8 (57.1) | 21 (43.8) | ||
| Obesity class 1, n (%) | 11 (23.4) | 3 (20.0) | 3 (21.4) | 11 (22.9) | ||
| Obesity class 2, n (%) | 5 (10.6) | 2 (13.3) | 2 (14.3) | 5 (10.4) | ||
| Obesity class 3, n (%) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (2.1) | ||
| Healthcare worker, n (%) | 3 (8.6) | 0 (0.0) | 0.929 | 1 (12.5) | 2 (5.7) | 0.999 |
| Coexisting disorder, n (%) | ||||||
| Hypertension | 34 (53.1) | 8 (36.4) | 0.267 | 10 (66.7) | 32 (44.4) | 0.200 |
| Diabetes | 12 (19.7) | 4 (18.2) | 0.999 | 2 (13.3) | 14 (20.3) | 0.796 |
| Coronary artery disease | 5 (8.1) | 1 (4.3) | 0.906 | 1 (7.1) | 5 (6.9) | 0.999 |
| Cardiac arrhythmias | 7 (11.1) | 0 (0.0) | 0.237 | 3 (21.4) | 4 (5.6) | 0.146 |
| Cerebrovascular disease | 2 (3.2) | 0 (0.0) | 0.969 | 1 (7.1) | 1 (1.4) | 0.742 |
| Chronic respiratory disease* | 0 (0.0) | 1 (4.5) | 0.586 | 0 (0.0) | 1 (1.4) | 0.999 |
| Asthma | 3 (4.8) | 1 (4.5) | 0.999 | 1 (7.1) | 3 (4.2) | 0.999 |
| COPD | 1 (1.6) | 1 (4.5) | 0.999 | 0 (0.0) | 2 (2.8) | 0.999 |
| Chronic neurological disease** | 2 (3.3) | 0 (0.0) | 0.961 | 1 (7.1) | 1 (1.4) | 0.749 |
| Moderate/severe CKDb | 6 (10.0) | 0 (0.0) | 0.288 | 4 (26.7) | 2 (2.9) | 0.008 |
| Solid tumor | 3 (5.2) | 0 (0.0) | 0.668 | 2 (14.3) | 1 (1.5) | 0.127 |
| Tobacco smoker | 0.819 | 0.652 | ||||
| Current | 1 (2.2) | 1 (5.0) | 0 (0.0) | 2 (3.5) | ||
| Former | 2 (4.3) | 1 (5.0) | 0 (0.0) | 3 (5.3) | ||
| Medications on chronic use, n (%) | ||||||
| ACEI | 11 (17.7) | 1 (4.5) | 0.244 | 1 (7.7) | 11 (15.3) | 0.772 |
| ARB | 10 (16.1) | 2 (9.1) | 0.648 | 2 (15.4) | 10 (13.9) | 0.999 |
| Calcium channel blockers | 8 (12.9) | 0 (0.0) | 0.177 | 2 (15.4) | 6 (8.3) | 0.775 |
| Beta-blockers | 12 (19.4) | 2 (9.1) | 0.437 | 1 (7.7) | 13 (18.1) | 0.602 |
| Vitamin-K antagonists | 1 (1.6) | 0 (0.0) | 0.999 | 0 (0.0) | 1 (1.4) | 0.999 |
| Novel oral anticoagulants | 1 (1.6) | 0 (0.0) | 0.999 | 0 (0.0) | 1 (1.4) | 0.999 |
| Anti-arrhythmic | 5 (7.9) | 0 (0.0) | 0.403 | 2 (14.3) | 3 (4.2) | 0.392 |
| Aspirin | 14 (21.5) | 1 (4.5) | 0.134 | 3 (20.0) | 12 (16.4) | 0.999 |
| Other anti-aggregant | 3 (4.8) | 0 (0.0) | 0.711 | 2 (14.3) | 1 (1.4) | 0.107 |
| Statins | 10 (15.6) | 0 (0.0) | 0.113 | 3 (20.0) | 7 (9.7) | 0.490 |
| Corticosteroids | 3 (4.7) | 0 (0.0) | 0.719 | 0 (0.0) | 3 (4.1) | 0.999 |
Data are median (quartile 25–75%) or n (%). Percentages may not total 100 because of rounding. ACEI, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; AKI, acute kidney injury; COPD, chronic obstructive pulmonary disease; RRT, renal replacement therapy.
Excluding asthma and COPD.
Excluding dementia and cerebrovascular disease.
BMI is the weight in kilograms divided by the square of the height in meters.
On dialysis, post kidney transplant, uremia, or Cr >3 mg/dL.
Most patients were male, median age was 61 years, and >25% were obese. The most frequent comorbidities were hypertension (52.9%) and diabetes (13.6%). Angiotensin-converting enzyme inhibitors were used by 12.9% and angiotensin II receptor blockers (ARBs) in 17.7%. Patients who developed AKI or needed RRT at latest follow-up were older, and among RRT-treated patients, a greater proportion had premorbid CKD (Table 1). All patients treated with RRT received continuous therapy (CRRT).
Time from symptoms to hospital admission and ICU admission was 7.0 (4.0–10.0) days and 10.0 (7.0–14.0) days, respectively (online suppl. Table 1; see www.karger.com/doi/10.1159/000508657 for all online suppl. material). At hospital admission, fever was present in 56.4% of patients, median SpO2 was 92 (84–96) %, and median respiratory rate was 30 (25–36) breaths per minute. Patients with AKI had higher heart rate, lactate levels, and WBC count than patients without AKI. Compared to patients not receiving RRT at latest follow-up, patients receiving RRT had a shorter time from symptoms and ICU admission, and higher urea and Cr at admission.
Daily Data
Daily clinical and laboratory variables in the first 7 days according to AKI or need of RRT are shown in online suppl. Tables 2–5. Urine output and oxygenation was similar among the groups assessed (online suppl. Fig. 1, 2). Over time, urea, Cr, and C-reactive protein were higher in patients with AKI (online suppl. Fig. 3, 4).
Primary Outcome
At latest follow-up on April 20, 2020, patients were followed up for a median period of 34.0 (28.0–42.0) days. The impact of confounders considered for adjustment of the models on hospital mortality is shown in online suppl. Table 6 and Fig. 5. In the unadjusted analysis, neither development of AKI (HR, 2.17 [95% CI, 0.76–6.22]; p = 0.149) nor needed of RRT (HR, 1.73 [95% CI, 0.77–3.85]; p = 0.184) were significantly associated with hospital mortality. The results after adjustment for confounders are shown in Tables 2 and 3 and in Figures 1 and 2.
Table 2.
Primary and secondary outcomes according to the development of AKI at the latest follow-up*
| AKI (n = 72) | No AKI (n = 24) | Effect estimate (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Hospital mortality | 28 (38.9) | 4 (16.7) | 1.32 (0.48−3.63)a | 0.597 |
| Secondary outcomes | ||||
| ICU mortality | 26 (36.1) | 3 (12.5) | 1.26 (0.40−4.00)a | 0.690 |
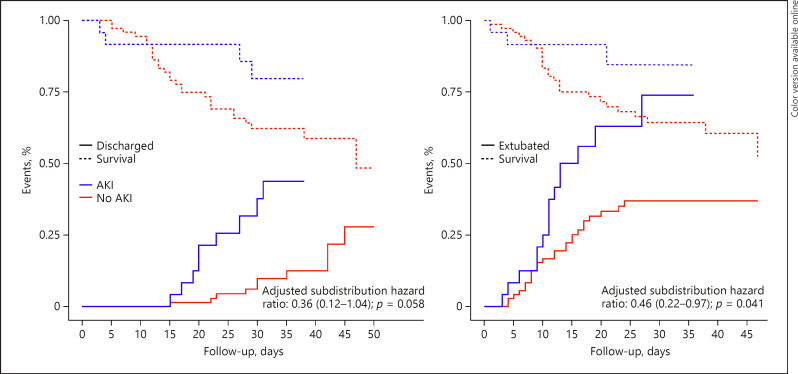
| Duration of ventilation, days | 15.1 (9.8−24.0) | 12.6 (9.1−16.6) | 0.46 (0.22−0.97)b | 0.041 |
| In survivors | 16.6 (10.6−27.5) | 13.1 (10.1−16.1) | ||
| ICU length of stay, days | 16.0 (10.0−24.2) | 13.5 (10.0−17.2) | 0.46 (0.22−0.97)b | 0.041 |
| In survivors | 17.0 (11.5−27.5) | 14.0 (11.0−17.0) | ||
| Hospital length of stay | 27.5 (16.8−35.0) | 25.0 (19.8−31.2) | 0.36 (0.12−1.04)b | 0.058 |
| In survivors | 32.0 (27.0−38.2) | 25.0 (20.0−32.0) |
Data are median (quartile 25–75%) or n (%). Percentages may not total 100 because of rounding. ICU, intensive care unit; CI, confidence interval; AKI, acute kidney injury.
Latest follow-up at April 20, 2020.
Effect estimate is hazard ratio from a Cox proportional hazard model with inverse probability of treatment weighting.
Effect estimate is sub-distribution hazard ratio from an unadjusted Fine-Gray competing risk model with death before the event as the competing risk and with inverse probability of treatment weighting.
Table 3.
Primary and secondary outcomes according to the development of RRT at the latest follow-up*
| RRT (n = 17) | No RRT (n = 82) | Effect estimate (95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Hospital mortality | 9 (52.9) | 24 (29.3) | 0.68 (0.21−2.15)a | 0.514 |
| Secondary outcomes | ||||
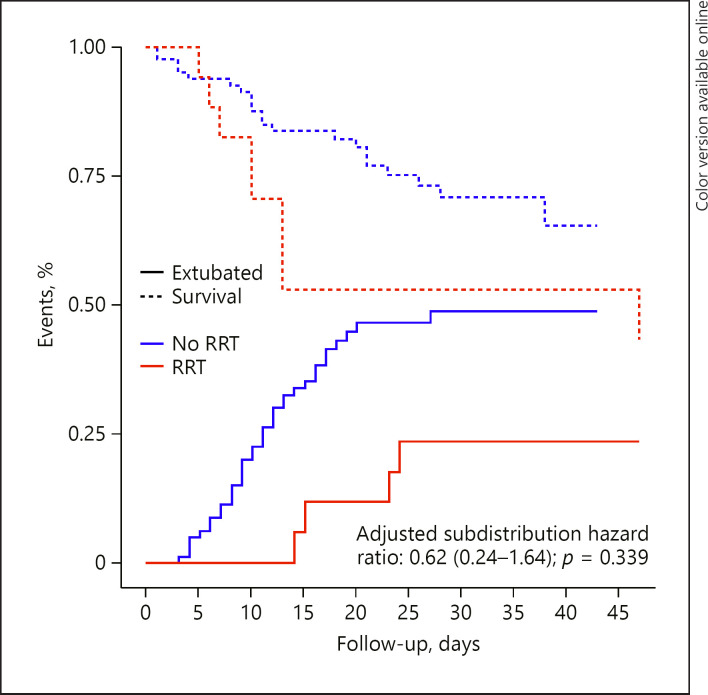
| ICU mortality | 9 (52.9) | 21 (25.6) | 0.56 (0.17−1.82)a | 0.338 |
| Duration of ventilation, days | 14.1 (10.0−33.0) | 14.0 (9.1−20.0) | 0.62 (0.24−1.64)b | 0.339 |
| In survivors | 28.6 (21.1−37.2) | 15.0 (9.1−19.6) | ||
| ICU length of stay, days | 15.0 (10.0−33.0) | 14.0 (10.0−20.2) | 0.62 (0.24−1.64)b | 0.339 |
| In survivors | 29.0 (22.0−37.2) | 15.0 (10.0−20.0) | ||
| Hospital length of stay | 27.0 (13.0−42.0) | 27.0 (19.0−32.0) | −c | − |
| In survivors | 39.0 (31.8−44.8) | 30.0 (23.0−34.8) |
Data are median (quartile 25–75%) or n (%). Percentages may not total 100 because of rounding. ICU, intensive care unit; CI, confidence interval; RRT, renal replacement therapy.
Latest follow-up at April 20, 2020.
Effect estimate is hazard ratio from a Cox proportional hazard model with inverse probability of treatment weighting.
Effect estimate is sub-distribution hazard ratio from an unadjusted Fine-Gray competing risk model with death before the event as the competing risk and with inverse probability of treatment weighting.
Not estimated because there is no hospital discharge in the RRT group until the latest follow-up.
Fig. 1.
Cumulative incidence function of hospital discharge and extubation according to development of AKI. Cumulative incidence function considering death before the event as competing risk. AKI, acute kidney infection.
Fig. 2.
Cumulative incidence function of hospital discharge and extubation according to need of RRT. Cumulative incidence function considering death before the event as competing risk. RRT, renal replacement therapy.
Secondary Outcomes
At the latest follow-up, the only difference in secondary outcomes according to the development of AKI was longer time to ICU discharge and longer duration of mechanical ventilation in patients developing AKI (Table 2; Fig. 1). There was no difference in any of the secondary outcomes according to the need of RRT (Table 3; Fig. 2).
Discussion
Key Findings
In a large cohort of adult invasively ventilated COVID-19 patients, we assessed the prevalence, patient characteristics, risk factors, and outcome of AKI and RRT-treated patients. We found that 3 out of 4 patients developed AKI and 1 in 6 patients was treated with CRRT. Older age was a risk factor for AKI and CRRT and premorbid CKD was a risk factor for CRRT. Patients with AKI had a mortality of approximately 40% and patients treated with CRRT had a mortality of above 50%.
Relationship with Previous Studies
Several studies have provided information on the prevalence of AKI in COVID-19 patients in Wuhan, China. A study by Cheng et al. [11] reported a prevalence of AKI of 5.1% among patients admitted to hospital in Wuhan, and identified AKI as a risk factor for mortality, but did not provide data on ventilated patients. In another study by Wang et al. [12] and in another hospital in Wuhan, investigators reported that AKI was very uncommon to absent in a cohort of 116 hospitalized patients. In a further study of 113 deceased patients in Wuhan [17], AKI was reported in 25% of cases but without data on RRT. In a retrospective study by Zhou et al. [18] involving 191 patients, 28.8% had AKI, and RRT use was reported in 10.5% of patients, in a population with a mean age of 56 years. In a small study of eight patients admitted to ICU in 3 hospitals in Hong Kong, 2 required RRT [19]. Finally, in a recent study of postmortem findings, Su et al. [20] investigated the histology of the kidneys in 26 patients with a mean age of 69 years. Tubular injury and capillaries obstruction by erythrocyte aggregation were reported in nine patients, a finding in keeping with the prothrombotic features of COVID-19 [21]. In addition, they also report clusters of coronaviruses in the tubular epithelium and podocytes implying direct viral invasion-induced renal damage [21]. The relevance of these studies to Western healthcare systems, however, remains unclear. This is because, regrettably, despite multiple calls for the early use of extracorporeal support technologies including CRRT [22, 23, 24], even the most recent and largest study of 1591 ICU patients in the Lombardy region of Italy provided no data on AKI or RRT [25]. Despite such lack of primary data, a variety of mechanisms are being proposed as responsible for AKI in the setting of COVID-19 infection [7].
Implications of Study Findings
Our findings imply that in patients admitted to the ICU of a university hospital and treated with invasive ventilation for COVID-19 pneumonia within a Western healthcare system, the incidence of AKI was very high in 3 out of 4 patients, 3 patients out of 4 developed AKI, while 1 in 6 required CRRT. Moreover, they imply that, in such patients, older age is a major risk factor for AKI and CRRT use and that premorbid CKD is a major risk factor for CRRT use. Finally, they imply that the development of AKI is associated with a hospital mortality of close to 40% and that use of CRRT is associated with a mortality of greater than 50%.
Strengths and Limitations
This is the first report on the prevalence, patient characteristics, risk factors for, and outcome of AKI among COVID-19 patients receiving invasive ventilation in the ICU of a large tertiary hospital in a Western healthcare system. As such, it demonstrates clear differences in the prevalence of AKI and CRRT use compared with Chinese hospitals and highlights the need to have relevant information for different healthcare systems. Moreover, it focused on the sickest patients (those receiving invasive ventilation) where renal complications are particularly important. In addition, it provides data on one of the largest cohorts to date in the world. Finally, by presenting the high mortality of patients receiving CRRT, and even of patients with AKI, it refocuses the attention of intensive care and nephrology specialists on the kidney as well as the lungs.
The limitations of this study relate to its observational nature. Accordingly, no inferences can be drawn on whether the use of CRRT affected the outcome. Despite being large in terms of patient numbers and in comparison, with the literature, it is single center in design. Thus, the external validity of the study findings is open to challenge. However, the study center has all the characteristics of similar tertiary hospitals in the Western world, making it likely that these findings have a degree of generalizability similar centers elsewhere in Western-style healthcare systems. We do not have detailed data on the duration and intensity of CRRT in this cohort. However, we provide information on the general dose applied and the technique. Finally, we do not have detailed data on fluid management, fluid balance, and the use of potentially nephrotoxic drugs. However, collection of such information was problematic in the midst of dramatic and high-risk clinical environment.
Conclusion
In a Western healthcare system setting and in invasively ventilated COVID-19 patients, 3 out of 4 patients develop AKI and 1 in 6 patients is treated with CRRT. Older age is a risk factor for AKI and CRRT, and premorbid CKD is a risk factor for CRRT. Patients with AKI have a mortality of approximately 40% and patients treated with CRRT have a mortality of above 50%. This information has important prognostic value and can help clinicians with decision-making in the setting of the current COVID-19 pandemic.
Statement of Ethics
The study was registered on ClinicalTrials.gov (NCT04318366) and approved by the Hospital Ethics Committee (Protocol No. 34/int/2020). Given the de-identified retrospective use of data normally collected as part of daily patient care and the non-interventional nature of the study, the need for informed consent was waived.
Disclosure Statement
The authors have no conflict of interest in relation to this study. They also have no other nonfinancial relationship which may influence the writing of the manuscript. Other forms of support which took place in the 3 preceding years and that have no relevance to the study are listed below.
Prof. Lorenzo Dagna received consultation honoraria from Roche, Sanofi-Genzyme, and SOBI outside of the submitted work. Prof. Ary Serpa Neto received speaking fees from Dräger outside of the submitted work.
Funding Source
The authors did not receive any funding.
Author Contributions
Design of the study and biobank: Fomininskiy E.V., Scandroglio A.M., Monti G., Calabrò M.G., Landoni G., Dell'Acqua A., Beretta L., Moizo E., Ravizza A., Monaco F., Campochiaro C., Pieri M., Azzolini M.L., Borghi G., Crivellari M., Conte C., Mattioli C., Silvani P., Mucci M., Turi S., Tentori S., Baiardo Radaelli M., Sartorelli M., Angelillo P., Belletti A., Nardelli P., Nisi F.G., Valsecchi G., Barberio C., Ciceri F., and Zangrillo A. Data collection: Fomininskiy E.V., Scandroglio A.M., Monti G., Calabrò M.G., Landoni G., Dell'Acqua A., Beretta L., Moizo E., Ravizza A., Monaco F., Campochiaro C., Pieri M., Azzolini M.L., Borghi G., Crivellari M., Conte C., Mattioli C., Silvani P., Mucci M., Turi S., Tentori S., Radaelli M.B., Sartorelli M., Angelillo P., Belletti A., Nardelli P., Nisi F.G., Valsecchi G., Barberio C., and Ciceri F. Statistical analysis: Landoni G., Belletti A., and Serpa Neto A. Manuscript draft and critical review: Fomininskiy E.V., Scandroglio A.M., Monti G., Calabrò M.G., Landoni G., Dell'Acqua A., Beretta L., Moizo E., Ravizza A., Monaco F., Campochiaro C., Pieri M., Azzolini M.L., Borghi G., Crivellari M., Conte C., Mattioli C., Silvani P., Mucci M., Turi S., Tentori S., Radaelli M.B., Sartorelli M., Angelillo P., Belletti A., Nardelli P., Nisi F.G., Valsecchi G., Barberio C., Ciceri F., Serpa Neto A., Bellomo R., and Zangrillo A. Administrative support: Landonni G., Bellomo R., and Zangrillo A.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323((13)):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382((18)):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazory A, Ronco C, McCullough PA. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc (Bayl Univ Med Cent) 2020;0((0)):1–6. doi: 10.1080/08998280.2020.1754700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67((2)):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijers B, Messa P, Ronco C. Safeguarding the maintenance hemodialysis patient population during the coronavirus disease 19 pandemic. Blood Purif. 2020;49((3)):259–64. doi: 10.1159/000507537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020:ASN.2020040419. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diao B, Wang WH, Wang RS, Feng ZQ, Tan YJ, Wang HM, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection [preprint posted online April 10, 2020] medRxiv. doi:10.1101/2020.03.04.20031120. [Google Scholar]
- 9.Frimat M, Tabarin F, Dimitrov JD, Poitou C, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, et al. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood. 2013;122((2)):282–92. doi: 10.1182/blood-2013-03-489245. [DOI] [PubMed] [Google Scholar]
- 10.Ronco C, Bellasi A, Di Lullo L. Implication of acute kidney injury in heart failure. Heart Fail Clin. 2019;15((4)):463–76. doi: 10.1016/j.hfc.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97((5)):829–38. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from wuhan, China. Am J Nephrol. 2020;51((5)):343–348. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, Colombo S, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc Forthcoming. 2020 doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zangrillo A, Beretta L, Silvani P, Colombo S, Scandroglio AM, Dell'Acqua A, et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc Forthcoming. 2020 doi: 10.51893/2020.2.pov1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KDIGO AKI Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;17:1–138. [Google Scholar]
- 16.R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. URL https://www.R-project.org/ [Google Scholar]
- 17.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling L, So C, Shum HP, Chan PKS, Lai CKC, Kandamby DH, et al. Critically ill patients with COVID-19 in Hong Kong: a multicenter retrospective observational cohort study. Crit Care Resusc. 2020 April 6; doi: 10.51893/2020.2.oa1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 April 9; doi: 10.1016/j.kint.2020.04.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, et al. Microvascular COVID-19 lung vessel obstructive thromboinflammatory syndrome (MicrroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc Forthcoming. 2020 doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020;8((3)):240–1. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronco C, Reis T, De Rosa S. Coronavirus Epidemic and Extracorporeal Therapies in Intensive Care: si vis pacem para bellum. Blood Purif. 2020;49((3)):255–8. doi: 10.1159/000507039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol Forthcoming. 2020 doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patient infected with SARS-coV-2 admitted to ICUs in the Lombardy region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]