Abstract
Introduction
The severe acute respiratory syndrome coronavirus 2 caused a pandemic of coronavirus disease 2019 (COVID-19). Unprecedented public health actions were introduced, including social distancing, travel restrictions and quarantine. The Belgian government announced a national emergency plan, thereby postponing all non-urgent medical consultations and operations. This report analyses the impact of these measures on cancer screening, through assessment of the workload of a laboratory for histopathology and cytopathology.
Methods
Data on monthly numbers of histological and cytological samples, immunohistochemistry and molecular tests were extracted from the laboratory information management system.
Results
The global histopathological and cytological workload was substantially reduced. The impact on oncology-related surgical procedures was rather limited. The anti-COVID-19 measures significantly diminished all screening-related samples, such as colon biopsies, breast biopsies and cervical cytology, and strongly reduced the number of samples related to “functional” pathology, such as thyroidectomies and gastric biopsies.
Conclusions
Since many health care interventions are reflected in the workload of a pathology laboratory, this study enabled us to identify areas for “deconfinement” health care actions. Our findings indicate that various areas in medicine were affected, but the impact seemed largest for cancer screening. Health care professionals should assure that consultations related to cancer screening are postponed instead of cancelled.
Keywords: COVID-19, SARS-CoV-2 infection, Cancer screening, Histopathology, Cervical cytology
Introduction
The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), started in Wuhan (Hubei Province, People's Republic of China) [1]. On December 31, 2019, twenty-seven cases of pneumonia of unknown cause were reported to the World Health Organisation [1]. COVID-19 was identified as the underlying cause [2]. The first Chinese COVID-19-related death was reported on January 9, 2020. SARS-CoV-2 quickly spread to other Chinese provinces and neighbouring countries [1]. The French authorities confirmed the first “imported” COVID-19 case on January 24, 2020, which represented the official arrival in Europe [3]. The number of COVID-19 patients quickly rose within Europe, and in Italy in particular, where the so-called “patient zero” remains unknown [4]. On March 11, 2020, the outbreak qualified as a pandemic [5].
In Belgium, the first imported case was confirmed on February 4, 2020, represented by a businessman returning from China [6]. The number of Belgian patients quickly rose after the February holidays, as many winter sport tourists returned from COVID-19-affected areas in Austria and Italy [7]. The first Belgian COVID-19-related death was reported on March 11, 2020, and along with the spread of the infection, mortality rates quickly increased [8]. In response to this pandemic, unprecedented public health actions were proclaimed, including social distancing, hygienic measures, travel restrictions and quarantine [9]. All measures had to protect the hospitals and their intensive care units from being flooded by a sudden peak of COVID-19 patients, a process which was designated as “flattening the curve.” The Belgian National Security Council declared a national state of emergency on March 12, 2020: schools, universities and non-food shops were closed, and teleworking was recommended wherever possible [10]. A relative confinement started, allowing people to leave the house only for grocery shopping, visiting pharmacies and medical doctors for urgent matters, and commuting (if employed in essential food- and health care-associated businesses) [10].
The Belgian government obliged all hospitals to announce a “Hospital Emergency Plan” (HEP) on March 14, 2020, thereby postponing all non-urgent consultations and operations. This measure had to prevent non-COVID-19 patients from being infected with SARS-CoV-2. Although this measure was supported by the majority of the medical staff, critical voices put forward that the collateral damage caused by this health care delay might transcend the COVID-19-related morbidity and mortality [11, 12, 13]. The HEP lasted for 7 weeks and was abrogated on May 4, 2020.
In this report, we investigated the impact of the governmental anti-COVID-19 measures on the workload of a Belgian academic laboratory for histopathology and cytopathology. By comparing the organ-specific number of biopsies, cytological samples and surgical specimens between 2020 and the previous years, we aimed to identify which health care domains were most severely affected by postponed care. We attempted to provide recommendations for future “deconfinement” health care actions, thereby minimizing the potential negative effects of the anti-COVID-19 measures.
Materials and Methods
This study was performed in the Department of Pathology of the Cliniques universitaires Saint-Luc (Brussels, Belgium). Overall sample numbers were retrieved from the software for electronic histopathological reports (LIS DaVinci, MIPS, Ghent, Belgium). Monthly amounts were compared for the first trimester of 2017–2020 for a representative selection of sample types and immunohistochemical stains. The following samples were selected: colon biopsies, gastric biopsies, cervical cytology, cervical biopsies, skin biopsies, pulmonary biopsies performed by endobronchial ultrasound (EBUS), bone marrow biopsies, mammary surgical specimens (comprising lumpectomies, quadrantectomies and mastectomies), breast biopsies, surgical thyroid specimens, prostate biopsies, appendectomy specimens, resections of the lower digestive tract and neuropathological biopsies. The following immunohistochemical stains were selected: CD3, CD34, cytokeratin-7 (CK-7), CK-20, oestrogen receptor, HER2, Helicobacter pylori, Ki67, p16, p53, p63 and S100. This selection was based on the number of pathologists requesting a particular stain, as well as the request frequency, to ensure that all subspecialties were represented by this selection. For instance, immunohistochemistry for H. pylori is the most frequently requested stain in our laboratory, and S100 is regularly requested by pathologists practicing soft tissue, head and neck, dermato- and neuropathology. The number of tests for dual-probe silver-enhanced in situ hybridisations for HER2/CEP17 were retrieved. The number of demands from external laboratories for any type of molecular analysis, comprising in situ hybridisation, human papillomavirus typing and targeted next-generation sequencing, was determined. The monthly total number of paraffin blocks and glass slides was retrieved using Ventana Vantage (Roche Diagnostics, Basel, Switzerland), for the first trimester of 2017–2020.
All data were exported to Microsoft Excel (Redmond, WA, USA). The number of slides, blocks, samples, stains or molecular tests per month per year was compared by using contingency tables and χ2 tests. Statistical analyses were performed using the online χ2 contingency table calculator of the Physics Department of the College of Saint Benedict and Saint John's University (St. Joseph and Collegeville, MN, USA) [14]. The significance level was set at <0.05. This study did not involve experiments on human subjects or animals nor any experiments requiring residual tissue material. This study is therefore exempt from approval by the local ethics committee, in accordance with institutional guidelines.
Results
Overall Impact of COVID-19 on the Pathology Workload and Staff
In March 2020, the total number of samples received by our laboratory was reduced by 35% when compared with January and February 2020, which equalled a reduction of 40–45% in comparison with the 3 previous years. The number of paraffin blocks was reduced by 27–35% and the number of slides by 16–17% when compared with March of the 3 previous years (Table 1). In April 2020, the total number of samples decreased by 72% when compared with January and February 2020, and by around 70% when compared with the same month of the previous years. The number of paraffin blocks diminished by 54–59% and the number of slides by 47–53% in comparison with April of the previous years. The absolute number of complaints by technical staff concerning issues with paraffin blocks, such as presence of staplers, insufficient decalcification or insufficient formalin fixation, diminished by 47% in April 2020. Such technical issues are less likely to occur with biopsies than with surgical resection specimens. This explains why the relative number of complaints (i.e., the percentage of paraffin blocks with a complaint concerning a technical issue) rose by 84% in April 2020: surgical resection specimens took a larger share of the total number of samples, as the number of biopsies was strongly reduced.
Table 1.
Overview of the monthly total number of samples, tissue blocks and slides per year for the first trimester
| Jan | Feb | Mar | Apr | p | |
|---|---|---|---|---|---|
| Total number of samples | <0.001 | ||||
| 2017 | 6,042 | 6,098 | 6,835 | 5,317 | |
| 2018 | 6,305 | 5,733 | 6,658 | 5,616 | |
| 2019 | 6,287 | 6,290 | 6,011 | 5,448 | |
| 2020 | 5,856 | 5,374 | 3,723 | 1,585 | |
| Total number of tissue blocks | <0.001 | ||||
| 2017 | 8,577 | 8,198 | 9,016 | 7,133 | |
| 2018 | 7,622 | 6,577 | 7,966 | 6,533 | |
| 2019 | 7,176 | 7,266 | 7,957 | 7,512 | |
| 2020 | 6,698 | 7,444 | 5,806 | 3,069 | |
| Total number of slides | <0.001 | ||||
| 2017 | 18,397 | 17,720 | 19,755 | 16,348 | |
| 2018 | 18,926 | 15,921 | 19,952 | 16,470 | |
| 2019 | 18,950 | 18,080 | 19,403 | 18,160 | |
| 2020 | 19,161 | 18,423 | 16,467 | 8,676 | |
| Foetal and adult autopsies | 0.759 | ||||
| 2017 | 8 | 4 | 5 | 9 | |
| 2018 | 5 | 5 | 6 | 6 | |
| 2019 | 12 | 7 | 9 | 5 | |
| 2020 | 10 | 9 | 7 | 14 | |
Since our laboratory also receives histological and cytological samples from external non-academic hospitals, the total number of samples could have been influenced by a change of contracts with these external hospitals during the period 2017–2020. We therefore investigated whether the amount of internal samples had been affected in a similar way. For January and February 2020, a respective 6% and an 8% increase in internal samples were noted upon comparison with 2019. After comparison of March and April 2020 versus March and April 2019, we noted an 8.5 and 57% reduction of internal samples, respectively. Despite a slight increase in April 2020, the number of autopsies was not statistically significantly different throughout the investigated time period. In the first trimester of 2020, thirteen autopsies were SARS-CoV-2-related.
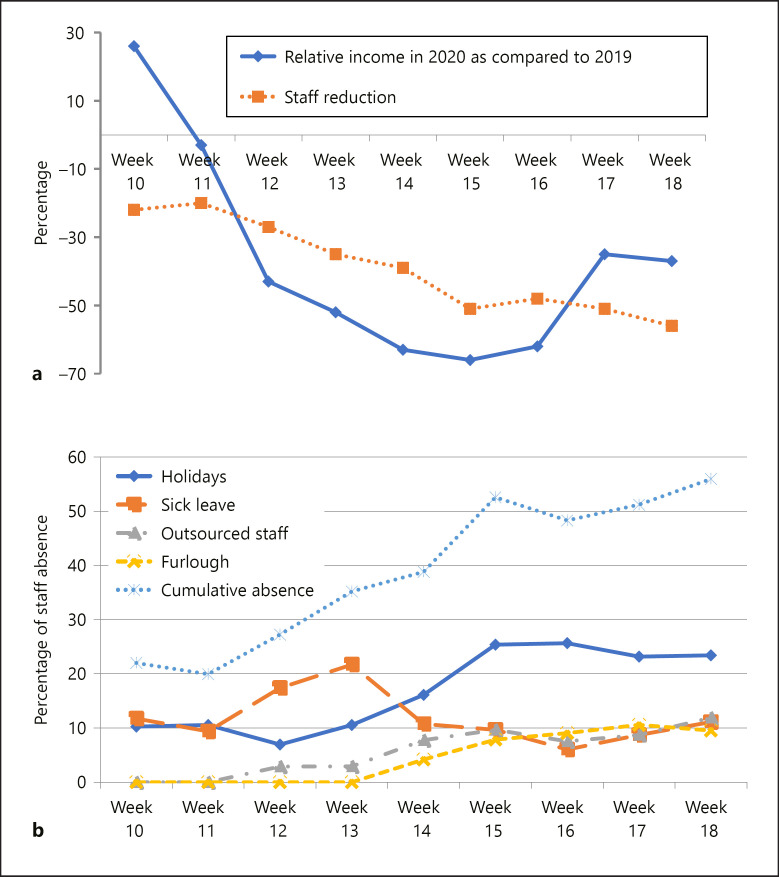
We analysed the financial impact of the sample reduction during the national HEP (i.e., weeks 12–18 of 2020), including the 2 weeks before (Fig. 1a). When the HEP was announced, the income of the laboratory decreased by 43% as compared with the same week in 2019. During the fourth week of the HEP, this decrease amounted to 66% and was slowly restored thereafter. The reduced income forced us to introduce furlough for 5–10% of technical and administrative staff (Fig. 1b). Simultaneously, around 8–12% of technical staff were outsourced to the virology laboratory during weeks 3–7 of the HEP, as this department faced a substantially increased workload due to SARS-CoV-2 testing. Sick leave amounted to 17–22% in the first 2 weeks of the HEP, and slowly decreased in the weeks thereafter. Technical and administrative staff were encouraged to take up a part of their annual holidays during this period, to prevent being sent on furlough, and to prevent a lack of staff during the summer holidays, as we foresee a potential rebound effect on the laboratory workload during the “deconfinement period.”
Fig. 1.
a Graph illustrating the reduced income in 2020 as compared to 2019 during the period of the Belgian Hospital Emergency Plan (HEP) in correlation with the reduced technical and administrative staff of the Department of Pathology. The HEP started in week 12 of 2020. b Graph illustrating the causes of absent technical staff as a function of time during the HEP (weeks 12–18 of 2020).
COVID-19 Impact on the Number of Samples
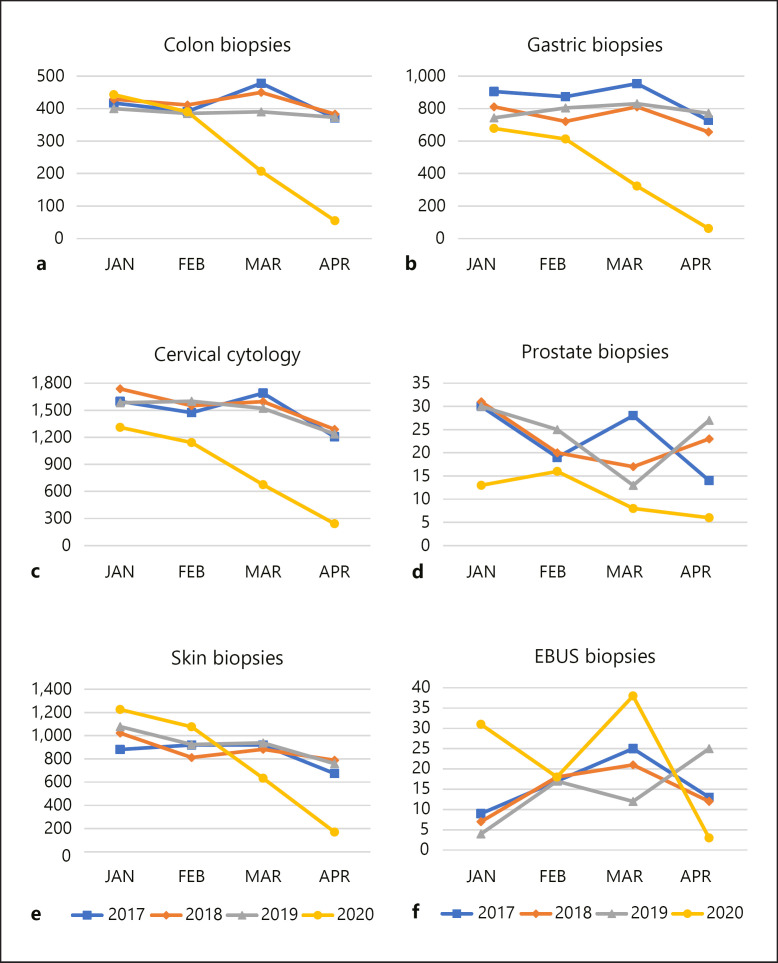
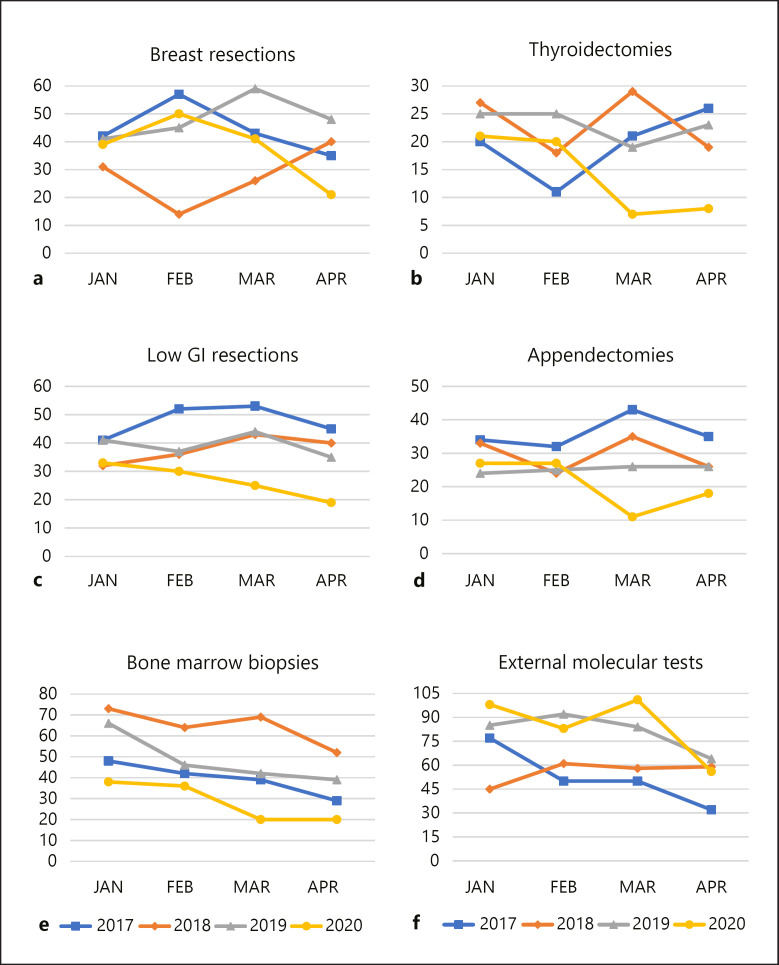
Figures 2 and 3 illustrate the samples per month per year for a particular sample type. Since the COVID-19 pandemic covers the first trimester of 2020 so far, all data are limited to this 4-month-period for the years 2017 till 2020. The exact numbers per sample type are provided in Tables 2 and 3. For each sample type, a substantial reduction was noted in March and April 2020, although this decrease was not statistically significant for the prostate biopsies, appendectomies, low gastrointestinal tract resection specimens, bone marrow biopsies and central nervous system samples. Oncological surgery was considered urgent and was therefore allowed to continue. Nevertheless, breast surgical activities were halved in April 2020. For pulmonary EBUS biopsies, a 100% increase was noted in March 2020 as compared with the same month of the previous years, followed by a reduction of around 82% in April 2020. Surgical interventions related to the central nervous system remained relatively stable over time. Non-gynaecological cytology was reduced by 45% in March and 61% in April.
Fig. 2.
Graphs illustrating the decline of the number of samples per month, for colon biopsies (a), gastric biopsies (b), cervical cytology (c), prostate biopsies (d), skin biopsies (e) and EBUS biopsies (f).
Fig. 3.
Graphs illustrating the decline of the number of samples per month, for breast resections (a), thyroidectomies (b), resections of the lower gastrointestinal (GI) tract (c), appendectomy specimens (d), bone marrow biopsies (e) and external demands for any type of molecular test (f).
Table 2.
Monthly number of samples per year for the first trimester
| Jan | Feb | Mar | Apr | p | ||
|---|---|---|---|---|---|---|
| Colon biopsies | <0.001 | |||||
| 2017 | 417 | 391 | 478 | 371 | ||
| 2018 | 429 | 411 | 450 | 383 | ||
| 2019 | 400 | 385 | 390 | 373 | ||
| 2020 | 443 | 390 | 207 | 55 | ||
| Gastric biopsies | <0.001 | |||||
| 2017 | 905 | 873 | 954 | 727 | ||
| 2018 | 811 | 721 | 811 | 656 | ||
| 2019 | 743 | 804 | 830 | 771 | ||
| 2020 | 678 | 613 | 323 | 62 | ||
| Non-gynaecological cytology | <0.001 | |||||
| 2017 | 538 | 560 | 579 | 530 | ||
| 2018 | 592 | 516 | 654 | 579 | ||
| 2019 | 552 | 526 | 565 | 568 | ||
| 2020 | 518 | 486 | 327 | 220 | ||
| Cervical cytology | <0.001 | |||||
| 2017 | 1,598 | 1,473 | 1,690 | 1,206 | ||
| 2018 | 1,738 | 1,551 | 1,595 | 1,288 | ||
| 2019 | 1,583 | 1,600 | 1,521 | 1,237 | ||
| 2020 | 1,310 | 1,142 | 675 | 243 | ||
| Cervical biopsies | <0.001 | |||||
| 2017 | 42 | 38 | 67 | 45 | ||
| 2018 | 55 | 42 | 49 | 43 | ||
| 2019 | 51 | 56 | 47 | 45 | ||
| 2020 | 68 | 48 | 34 | 15 | ||
| Skin biopsies | <0.001 | |||||
| 2017 | 881 | 920 | 920 | 674 | ||
| 2018 | 1,023 | 812 | 883 | 789 | ||
| 2019 | 1,079 | 924 | 938 | 760 | ||
| 2020 | 1,226 | 1,077 | 634 | 170 | ||
| Thyroidectomies | 0.008 | |||||
| 2017 | 20 | 11 | 21 | 26 | ||
| 2018 | 27 | 18 | 29 | 19 | ||
| 2019 | 25 | 25 | 19 | 23 | ||
| 2020 | 21 | 20 | 7 | 8 | ||
| Prostate biopsies | 0.065 | |||||
| 2017 | 30 | 19 | 28 | 14 | ||
| 2018 | 31 | 20 | 17 | 23 | ||
| 2019 | 30 | 25 | 13 | 27 | ||
| 2020 | 13 | 16 | 8 | 6 | ||
Table 3.
Monthly number of samples per year for the first trimester
| Jan | Feb | Mar | Apr | p | |
|---|---|---|---|---|---|
| Breast resections | <0.001 | ||||
| 2017 | 42 | 57 | 43 | 35 | |
| 2018 | 31 | 14 | 26 | 40 | |
| 2019 | 41 | 45 | 59 | 48 | |
| 2020 | 39 | 50 | 41 | 21 | |
| Breast biopsies | <0.001 | ||||
| 2017 | 41 | 50 | 57 | 35 | |
| 2018 | 41 | 51 | 52 | 45 | |
| 2019 | 60 | 89 | 57 | 45 | |
| 2020 | 33 | 31 | 30 | 10 | |
| Appendectomies | 0.188 | ||||
| 2017 | 34 | 32 | 43 | 35 | |
| 2018 | 33 | 24 | 35 | 26 | |
| 2019 | 24 | 25 | 26 | 26 | |
| 2020 | 27 | 27 | 11 | 18 | |
| Lower digestive tract resections | 0.628 | ||||
| 2017 | 41 | 52 | 53 | 45 | |
| 2018 | 32 | 36 | 43 | 40 | |
| 2019 | 41 | 37 | 44 | 35 | |
| 2020 | 33 | 30 | 25 | 19 | |
| EBUS biopsies | <0.001 | ||||
| 2017 | 9 | 17 | 25 | 13 | |
| 2018 | 7 | 18 | 21 | 12 | |
| 2019 | 4 | 17 | 12 | 25 | |
| 2020 | 31 | 18 | 38 | 3 | |
| CNS biopsies and resections | 0.749 | ||||
| 2017 | 46 | 35 | 51 | 40 | |
| 2018 | 39 | 35 | 43 | 42 | |
| 2019 | 25 | 27 | 31 | 28 | |
| 2020 | 29 | 37 | 28 | 26 | |
| Bone marrow biopsies | 0.639 | ||||
| 2017 | 48 | 42 | 39 | 29 | |
| 2018 | 73 | 64 | 69 | 52 | |
| 2019 | 66 | 46 | 42 | 39 | |
| 2020 | 38 | 36 | 20 | 20 | |
CNS, central nervous system; EBUS, endobronchial ultrasound.
COVID-19 Impact on the Number of Immunohistochemical and Molecular Tests
We selected a panel of 12 immunohistochemical stains to investigate the impact of the anti-COVID-19 measures (Table 4). In March 2020, there was no impact on the amount of immunohistochemistry, except for p53 and H. pylori, which were halved when compared with March 2019 and March 2018. In April 2020, we noted a significant decrease for most immunohistochemical stains when compared with April of the previous years, with the exception of CK-7, CK-20, Ki67, p16 and S100. Some stains (such as CK-7 and p16) presented with a significantly altered request frequency since 2017, which we can only explain by differences in pathologists' individual request behaviour (Fig. 3); this Figure illustrates the number of external demands for any type of molecular test. This number was lower in April 2020 than in March 2020 but did not substantially differ from the amount of external demands in the previous years. The number of HER2 silver-enhanced in situ hybridisations, a technique used for the investigation of the amplification status of the HER2 gene in breast cancer and gastric cancer, almost halved in April 2020 in comparison with April 2019.
Table 4.
Monthly number of tests per year for the first trimester
| Jan | Feb | Mar | Apr | p | |
|---|---|---|---|---|---|
| CD3 | <0.001 | ||||
| 2017 | 79 | 78 | 89 | 100 | |
| 2018 | 191 | 177 | 204 | 160 | |
| 2019 | 175 | 199 | 186 | 177 | |
| 2020 | 225 | 236 | 221 | 106 | |
| CD34 | <0.001 | ||||
| 2017 | 43 | 47 | 39 | 30 | |
| 2018 | 49 | 28 | 53 | 47 | |
| 2019 | 48 | 41 | 60 | 42 | |
| 2020 | 55 | 69 | 32 | 29 | |
| CK-7 | 0.013 | ||||
| 2017 | 148 | 136 | 152 | 106 | |
| 2018 | 80 | 50 | 86 | 71 | |
| 2019 | 51 | 52 | 59 | 54 | |
| 2020 | 61 | 67 | 45 | 66 | |
| CK-20 | 0.057 | ||||
| 2017 | 30 | 31 | 23 | 21 | |
| 2018 | 24 | 13 | 26 | 28 | |
| 2019 | 22 | 32 | 22 | 19 | |
| 2020 | 32 | 20 | 22 | 15 | |
| ER | 0.022 | ||||
| 2017 | 75 | 79 | 75 | 53 | |
| 2018 | 103 | 101 | 111 | 99 | |
| 2019 | 95 | 139 | 145 | 104 | |
| 2020 | 116 | 92 | 116 | 69 | |
| HER2 | 0.003 | ||||
| 2017 | 78 | 71 | 71 | 40 | |
| 2018 | 57 | 71 | 51 | 65 | |
| 2019 | 57 | 82 | 85 | 56 | |
| 2020 | 53 | 36 | 54 | 30 | |
| HP | <0.001 | ||||
| 2017 | 511 | 590 | 611 | 461 | |
| 2018 | 465 | 481 | 544 | 425 | |
| 2019 | 439 | 493 | 497 | 479 | |
| 2020 | 438 | 371 | 270 | 37 | |
| Ki67 | 0.054 | ||||
| 2017 | 175 | 176 | 193 | 159 | |
| 2018 | 147 | 171 | 185 | 176 | |
| 2019 | 169 | 194 | 206 | 163 | |
| 2020 | 163 | 158 | 152 | 103 | |
| p16 | 0.004 | ||||
| 2017 | 113 | 80 | 111 | 85 | |
| 2018 | 98 | 103 | 73 | 81 | |
| 2019 | 65 | 88 | 88 | 63 | |
| 2020 | 78 | 90 | 65 | 46 | |
| p53 | <0.001 | ||||
| 2017 | 77 | 78 | 72 | 77 | |
| 2018 | 78 | 78 | 138 | 82 | |
| 2019 | 79 | 81 | 119 | 73 | |
| 2020 | 100 | 85 | 62 | 50 | |
| p63 | 0.004 | ||||
| 2017 | 17 | 16 | 8 | 11 | |
| 2018 | 14 | 7 | 26 | 16 | |
| 2019 | 23 | 24 | 16 | 6 | |
| 2020 | 10 | 10 | 16 | 7 | |
| S100 | 0.151 | ||||
| 2017 | 67 | 49 | 47 | 44 | |
| 2018 | 50 | 37 | 34 | 39 | |
| 2019 | 30 | 38 | 50 | 29 | |
| 2020 | 45 | 42 | 53 | 38 | |
| External demands for molecular tests | 0.005 | ||||
| 2017 | 76 | 48 | 49 | 32 | |
| 2018 | 45 | 61 | 58 | 59 | |
| 2019 | 85 | 92 | 84 | 64 | |
| 2020 | 98 | 83 | 101 | 56 | |
| HER2 SISH | <0.001 | ||||
| 2017 | 73 | 54 | 68 | 44 | |
| 2018 | 87 | 87 | 106 | 111 | |
| 2019 | 72 | 79 | 77 | 71 | |
| 2020 | 92 | 37 | 68 | 39 | |
CK, cytokeratin; ER, oestrogen receptor; HP, Helicobacter pylori; SISH, silver-enhanced in situ hybridisation.
Discussion
The unprecedented announcement of a 7-week-lasting national HEP in Belgium to cope with the COVID-19 pandemic offered a unique opportunity to investigate the impact of these measures on the workload of an academic laboratory for histo- and cytopathology. As this workload indirectly reflects various medical interventions, this study enabled us to identify areas for “deconfinement” health care actions. Overall, laboratory activities in the first trimester of the year are higher in March than in April. This is explained by the annual 2-week Easter holidays that reduce overall medical and laboratory activities. The national HEP started mid-March and lasted till May 4, 2020. The workload reduction is therefore most pronounced in April. Although urgent medical consultations and operations were allowed to continue as long as the intensive care units could cope with the number of COVID-19 patients [15, 16, 17], we noted a significant decrease in the number of breast resection specimens and pulmonary EBUS biopsies. However, the number of external demands for molecular tests did not decline in our centralised laboratory. This reflects the continued care for cancer patients. Although Malapelle et al. [18]described a substantial shift from next-generation sequencing towards fully automated molecular pathology, a similar shift was not observed in our laboratory. We do not perform germline mutation testing, but Minucci et al. [19] previously described a COVID-19-related decline in genomic diagnostics. As many countries start to ease the COVID-19-related confinement measures and cancer screening programmes restart, an incoming wave of undetected malignancies is expected [20]. Our study indirectly supports this assumption, as cancer screening-related samples were severely affected by the HEP.
The temporary annulation of the national breast cancer screening programme for women aged 50–70 explains the diminished number of breast biopsies and surgeries. Symptomatic patients, presenting with a palpable lump and/or nipple discharge and/or skin retraction were further investigated, but during the HEP, no breasts were biopsied for suspicious clusters of calcifications on screening mammograms. All aesthetic breast surgery was postponed. Similarly, no colonoscopies were performed to investigate positive immunochemical faecal occult blood tests (iFOBT). Since 2013, all Belgian men and women aged 56–74 are invited for a biannual immunochemical faecal occult blood test [21, 22]. Since 2020, screening starts at the age of 50 [23, 24, 25]. A positive immunochemical faecal occult blood test is generally followed by colonoscopy, to exclude colorectal polyps and carcinomas [26]. These colonoscopies were considered as non-urgent, which is reflected in the reduction of colon biopsies.
A third Belgian (pre)cancer screening programme consists of triannual cervical smears for women aged 25–64 [27]. Both cervical and non-gynaecological cytology rates dramatically collapsed during the HEP. A similar COVID-19-related reduction in cytology samples was observed in Italy [28].
At present, there is no organised prostate cancer screening programme in Belgium. However, many general practitioners screen for prostate cancer by determining the prostate-specific antigen level, which can result in further investigation by medical imaging and prostate biopsies [29, 30]. Although not statistically significant, we observed a decline in prostate biopsies, which may reflect the reduced prostate-specific antigen screening activities. Similarly, patients at high risk for dysplastic naevi and melanoma are generally followed up by a dermatologist. Since most skin conditions were considered non-urgent, the activities of many dermatologists dropped to zero, which was reflected in the strongly reduced number of skin biopsies. Biopsies, cytology and resection specimens that are commonly related to functional pathology also diminished.
Overall, the total number of slides and tissue blocks, as well as the number of immunohistochemical stains, was not affected as severely as the overall sample number. This reflects the complexity of the samples we continued to receive: in the first place, the number of cytological samples and biopsies diminished, whereas oncology-related resection specimens were continued. Since a surgical resection specimen requires more tissue blocks than a single biopsy specimen, the total number of blocks did not extremely diminish. Oncology-related histopathological samples often require multiple immunohistochemical stains to establish a final diagnosis. Therefore, the overall immunohistochemical load was less affected than the total workload or the total number of samples. The relative overrepresentation of surgical specimens was also reflected in the relative increase in complaints made by technical staff concerning technical issues with tissue blocks: although the absolute number of complaints decreased along with the decreased number of samples, the relative number of complaints rose by 84% in April, because the ratio of biopsies versus specimens altered. All data reported here were repetitively discussed during biweekly staff meetings, for which conferencing software was implemented [31].
Lastly, we did not observe a substantially increased number of autopsies in the first trimester of 2020. The COVID-19 outbreak poses new challenges for the safety of the operators [32, 33]. In the absence of a positive COVID-19 test, all deceased patients were still considered as potentially infected, and all post-mortem studies were performed in accordance with international guidelines concerning biosafety measures for autopsies on SARS-CoV-2-positive deceased patients [34, 35].
Conclusion
The national emergency state announced by the Belgian government to contend the COVID-19 pandemic provided an unrivalled opportunity to investigate the impact of a viral pandemic on the management of cancer screening programmes. Several areas in medicine were affected by the anti-COVID-19 measures. Although the numbers of a single laboratory are too small to generate robust conclusions for an entire nation, we feel that they do give an impression of the general impact of the national anti-COVID-19 measures. Sample types issued from cancer screening programmes seem most severely decreased. Health care actions during the deconfinement period should focus on restarting cancer screening programmes. Health care professionals should assure that patient contacts related to cancer screening are postponed instead of cancelled, to limit the number of patients that slips through the cracks of the screening network.
Statement of Ethics
This study report was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Ethics approval and informed consent were not required, as this study did not involve human studies or experiments involving animals.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive funding for this study.
Author Contributions
Conceptualisation and supervision: C.G., M.R.V.B. Acquisition of the data: M.-C.D.P., Y.G., J.V. Analysis of the data and statistics: M.-C.D.P., M.R.V.B. Interpretation of the data: all authors. Software and resources: Y.G., J.V. Arrangement of the figures: M.-C.D.P., Y.G. Writing − original draft of the manuscript: M.-C.D.P. Writing − review and editing and final approval: all authors.
Acknowledgement
The authors gratefully acknowledge the help of Mr. G. De Bruyker, senior consultant IT & Data Solutions at Roche Diagnostics Belgium, for his assistance with the Vantage software.
References
- 1.Chen ZL, Zhang Q, Lu Y, Guo ZM, Zhang X, Zhang WJ, et al. Distribution of the COVID-19 epidemic and correlation with population emigration from Wuhan, China. Chin Med J (Engl) 2020 May;133((9)):1044–50. doi: 10.1097/CM9.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb;382((8)):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard Stoecklin S, Rolland P, Silue Y, Mailles A, Campese C, Simondon A, et al. Investigation Team First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020 Feb;25((6)) doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carinci F. Covid-19: preparedness, decentralisation, and the hunt for patient zero. BMJ. 2020 Feb;368:m799. doi: 10.1136/bmj.m799. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation Coronavirus disease. 2019. (COVID-19) - Situation report 51. www.who.int.
- 6.Sciensano One repatriated Belgian has tested positive for the novel coronavirus. https://www.info-coronavirus.be/en/news/. Accessed on 12 May 2020.
- 7.Sciensano 10 new patients contaminated by the Covid-19 virus in our country out of 495 tests performed in the reference laboratory. https://www.info-coronavirus.be/en/news/. Accessed on 12 May 2020.
- 8.Sciensano 1702 new Covid-19 infections. https://www.info-coronavirus.be/en/news/. Accessed on 12 May 2020.
- 9.Sjödin H, Wilder-Smith A, Osman S, Farooq Z, Rocklöv J. Only strict quarantine measures can curb the coronavirus disease (COVID-19) outbreak in Italy, 2020. Euro Surveill. 2020 Apr;25((13)):1–6. doi: 10.2807/1560-7917.ES.2020.25.13.2000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sciensano Coronavirus : Phase 2 maintained, transition to the federal phase and additional measures. https://www.info-coronavirus.be/en/news/. Accessed on 12 May 2020.
- 11.Søreide K, Hallet J, Matthews JB, Schnitzbauer AA, Line PD, Lai PB, et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg Forthcoming. 2020 Apr; doi: 10.1002/bjs.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood S. The Mystery of the Missing STEMIs During the COVID-19 Pandemic. https://www.tctmd.com/news/. Accessed on 12 May 2020.
- 13.Tejera-Vaquerizo A, Nagore E. Estimated effect of COVID-19 lockdown on melanoma thickness and prognosis: a rate of growth model. J Eur Acad Dermatol Venereol. 2020 May;:jdv.16555. doi: 10.1111/jdv.16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Physics Department, College of Saint Benedict and Saint John's University (St. Joseph and Collegeville, Minnesota U. r×c Contingency Table: How many rows? Columns? http://www.physics.csbsju.edu/stats/contingency_NROW_NCOLUMN_form.html. Accessed on 6 May 2020.
- 15.Dietz JR, Moran MS, Isakoff SJ, Kurtzman SH, Willey SC, Burstein HJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020 Jun;181((3)):487–97. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denys A, Guiu B, Chevallier P, Digklia A, de Kerviler E, de Baere T. Interventional oncology at the time of COVID-19 pandemic: problems and solutions. Diagn Interv Imaging. 2020 Jun;101((6)):347–53. doi: 10.1016/j.diii.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curigliano G, Cardoso MJ, Poortmans P, Gentilini O, Pravettoni G, Mazzocco K, et al. editorial board of The Breast Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020 Aug;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malapelle U, De Luca C, Iaccarino A, Pepe F, Pisapia P, Russo M, et al. Predictive molecular pathology in the time of COVID-19. J Clin Pathol. 2020 May;:jclinpath-2020-206711. doi: 10.1136/jclinpath-2020-206711. [DOI] [PubMed] [Google Scholar]
- 19.Minucci A, Scambia G, Santonocito C, Concolino P, Urbani A. BRCA testing in a genomic diagnostics referral center during the COVID-19 pandemic. Mol Biol Rep. 2020 Jun;47((6)):4857–60. doi: 10.1007/s11033-020-05479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troncone G, Hofman P. Pathologists and the coronavirus distraction effect. J Clin Pathol. 2020 Jun;:jclinpath-2020-206807. doi: 10.1136/jclinpath-2020-206807. [DOI] [PubMed] [Google Scholar]
- 21.Guo F, De Brabander I, Francart J, Candeur M, Polus M, Van Eycken L, et al. Benefits of switching from guaiac-based faecal occult blood to faecal immunochemical testing: experience from the Wallonia-Brussels colorectal cancer screening programme. Br J Cancer. 2020 Mar;122((7)):1109–17. doi: 10.1038/s41416-020-0754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toes-Zoutendijk E, Portillo I, Hoeck S, de Brabander I, Perrin P, Dubois C, et al. Participation in faecal immunochemical testing-based colorectal cancer screening programmes in the northwest of Europe. J Med Screen. 2020 Jun;27((2)):68–76. doi: 10.1177/0969141319879712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. https://dikkedarmkanker.bevolkingsonderzoek.be/en/welcome-0. Accessed on 10 May 2020.
- 24. https://www.ccref.org/particulier/colorectal.php. Accessed on 10 May 2020.
- 25.van de Veerdonk W, Van Hal G, Peeters M, Hoeck S. Should Flanders consider lowering its target age for colorectal cancer screening to 45-49? Cancer Epidemiol. 2019 Aug;61:172–5. doi: 10.1016/j.canep.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 26.van de Veerdonk W, Van Hal G, Peeters M, De Brabander I, Silversmit G, Hoeck S. Risk stratification for colorectal neoplasia detection in the Flemish colorectal cancer screening programme. Cancer Epidemiol. 2018 Oct;56:90–6. doi: 10.1016/j.canep.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 27.De Prez V, Jolidon V, Willems B, Cullati S, Burton-Jeangros C, Bracke P. Cervical cancer (over)screening in Belgium and Switzerland: trends and social inequalities. Eur J Public Health. 2020 Jun;30((3)):410–5. doi: 10.1093/eurpub/ckaa041. [DOI] [PubMed] [Google Scholar]
- 28.Vigliar E, Iaccarino A, Bruzzese D, Malapelle U, Bellevicine C, Troncone G. Cytology in the time of coronavirus disease (covid-19): an Italian perspective. J Clin Pathol. 2020 Apr;:jclinpath-2020-206614. doi: 10.1136/jclinpath-2020-206614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlsson SV, de Carvalho TM, Roobol MJ, Hugosson J, Auvinen A, Kwiatkowski M, et al. Estimating the harms and benefits of prostate cancer screening as used in common practice versus recommended good practice: A microsimulation screening analysis. Cancer. 2016 Nov;122((21)):3386–93. doi: 10.1002/cncr.30192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandaglia G, Albers P, Abrahamsson PA, Briganti A, Catto JW, Chapple CR, et al. Structured Population-based Prostate-specific Antigen Screening for Prostate Cancer: The European Association of Urology Position in 2019. Eur Urol. 2019 Aug;76((2)):142–50. doi: 10.1016/j.eururo.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 31.Gosney JR, Hofman P, Troncone G, Lopez-Rios F. Cellular pathology in the COVID-19 era: a European perspective on maintaining quality and safety. J Clin Pathol. 2020 Jun;:jclinpath-2020-206789. doi: 10.1136/jclinpath-2020-206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 May;:his.14134. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020 Jun;33((6)):1007–14. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley B, Lucas SB, Youd E, Swift B, Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020 May;73((5)):239–42. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 35.Sapino A, Facchetti F, Bonoldi E, Gianatti A, Barbareschi M, Società Italiana di Anatomia Patologica e Citologia - SIAPEC The autopsy debate during the COVID-19 emergency: the Italian experience. Virchows Arch. 2020 Jun;476((6)):821–3. doi: 10.1007/s00428-020-02828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]