Abstract
Background
The COVID-19 outbreak is currently the major public health concern worldwide. This infection, caused by the novel coronavirus Sars Cov2, primarily affects respiratory system, but there is increasing evidence of neurologic involvement and cerebrovascular accidents.
Case Report
We present a case of stroke in a 62-year-old COVID-19-positive patient, with multiple vascular risk factors. The patient arrived 1 h after onset of symptoms, was treated with recombinant tissue plasminogen activator (rtPA) with improvement of neurologic deficits, and later developed right foot arterial ischemia (recanalized by balloon catheter angioplasty) and left arm superficial venous thrombosis. A control computed tomography (CT) scan 7 days after onset showed hemorrhagic transformation of ischemic lesion without mass effect. However, respiratory and neurologic conditions improved so that the patient was discharged to rehabilitation.
Discussion
Until now, few cases of stroke in COVID-19 have been described, mainly in severe forms. This patient had ischemic injuries in different sites as well as venous thrombosis; hence, we speculate that Sars Cov2 could have a direct role in promoting vascular accidents since its receptor ACE2 is a surface protein also expressed by endothelial cells. This case suggests that COVID-19 can favor strokes and in general vascular complications, even in milder cases, and the presence of preexisting risk factors could play a determinant role.
Keywords: COVID-19, Acute stroke thrombolysis, Acute stroke management, Angioplasty
Background
On June 7, 2020, COVID-19, the infection caused by the novel coronavirus Sars Cov19, represents a global emergency, affecting more than 6.7 million patients in the world [1]. Sars Cov2, as well as the previous SARS Cov of 2003, penetrates the cells through ACE2, which is a surface protein expressed mainly in the lungs, but it is also present in other organs and tissues, including endothelial cells and nervous system [2]. COVID-19 mainly affects the respiratory system, but there is increasing evidence of neurologic involvement. However, the mechanisms through which Sars Cov2 attacks the neurologic system or can cause strokes are matter of debate [3, 4]. We present a case of a COVID-19-positive patient with stroke and thromboses in different locations.
Case Report
F.C., 62-year-old male, with history of hypertension, diabetes, previous smoker, and myocardial infarction treated with PTCA + stent 3 years before, referred to our hospital on March 30, 2020, for speech disturbance and right limbs weakness. At arrival in the emergency room, 1 h after onset, neurologic exam showed right hemiplegia, expressive aphasia with partial sparing of comprehension, and right neglect (NIHSS 18). Computed tomography (CT) scan and CT angiography were performed in urgency and showed no parenchymal lesions (ASPECT score 10) and no vascular intracranial or extracranial occlusions. ECG showed sinus rhythm with Q wave on V1–V2. Blood pressure was 170/70.
The patient underwent rtPA therapy (0.9 mg/kg), with subsequent improvement of right leg strength and sensation (NIHSS 14). Anamnestic inquiry showed that the patient presented with fever (38°C maximum) and cough since 10 days before stroke onset, but at his arrival temperature was normal (36°C). For this reason, nasopharyngeal swab was done, and PCR rapid assay resulted positive for COVID-19 (genes E, RdRP, N, all detected). A second swab, 1 day later, gave the same result.
The patient underwent lung CT scan (Fig. 1), which revealed bilateral (right more than left) inhomogeneous hyperdensity of basal lobes with areas of consolidation mixed with ground glass pattern. Hemogasanalysis showed mild hypoxemia (pO2 72 mm Hg), while other values were normal.
Fig. 1.
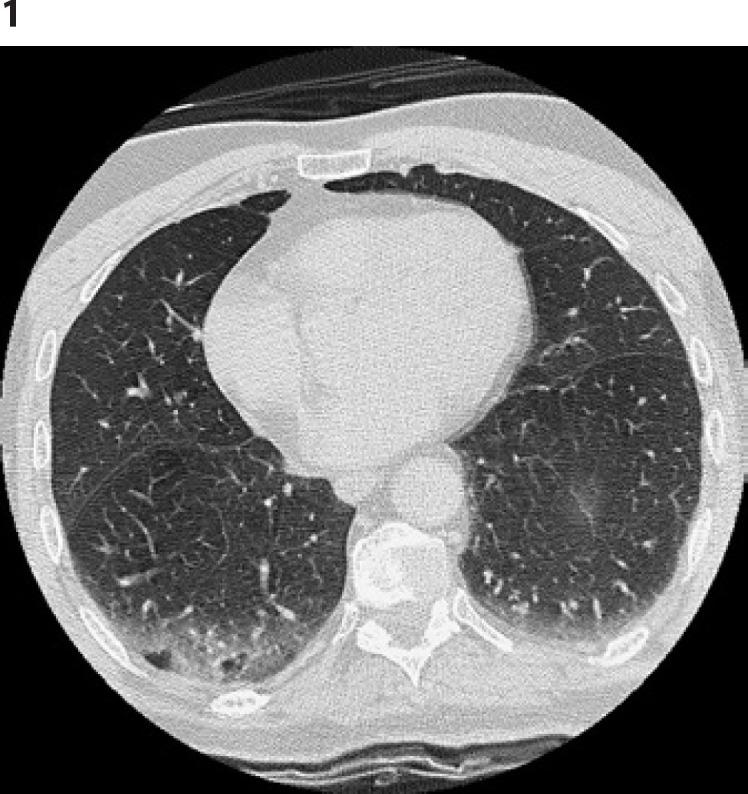
Thorax CT scan: bilateral (right more than left) inhomogeneous hyperdensity of basal lobes with areas of consolidation mixed with ground glass pattern. CT, computed tomography.
Blood count test showed increased total leucocytes (14.5 × 103/mmc, n.v. 4–10), increased neutrophils (10.8 × 103 Ul, n.v. 1.8–7.0), normal lymphocytes (1.9 × 103/mmc), normal platelets (263 × 103/mmc), normal hemoglobin (13.0 g/dL). D-dimer (1,510 ng/mL, n.v. < 278) and C-reactive protein levels were also increased (53.8 mg/L, n.v. 0.0–5.0). Transthoracic echocardiography showed basal hypokinesia with normal global function (EF 55%). The patient was isolated and accepted in the stroke unit. Therapy with oxygen (4 L/min), clopidogrel (75 mg q.d.), atorvastatin (40 mg q.d.), hydroxychloroquine (200 mg b.i.d.), azythromycin (500 mg q.d.), and enoxaparin (4,000 UI q.d.) was started. Subsequently, amlodipine (5 mg q.d.) and ramipril (5 mg q.d.) were added to control the blood pressure.
A control CT scan, 24 h after onset, showed left frontotemporal hypodense area consistent with acute ischemia. Plasmatic levels of IL 6 were tested at baseline, after 48 and 96 h, and always resulted in the normal range. Anti-cardiolipin antibodies (IgM and IgG), anti-beta2 glycoprotein 1 antibodies (IgM and IgG) and lupus-like anticoagulant were tested and resulted negative, as well as the thrombophilia panel.
The patient never developed dyspnea, and hemogasanalysis values remained stable. Continuous electrocardiographic monitoring (72 h) did not show relevant rhythm disorder, especially atrial fibrillation was excluded.
On day 6, right first toe cyanosis and right distal leg and foot hypothermia were observed. Ultrasound exam showed absent blood flow in right dorsalis pedis artery, and the patient underwent urgent angiography that revealed right dorsalis pedis and distal lateral plantar artery occlusion that were recanalized during the exam with balloon catheter (Fig. 2).
Fig. 2.
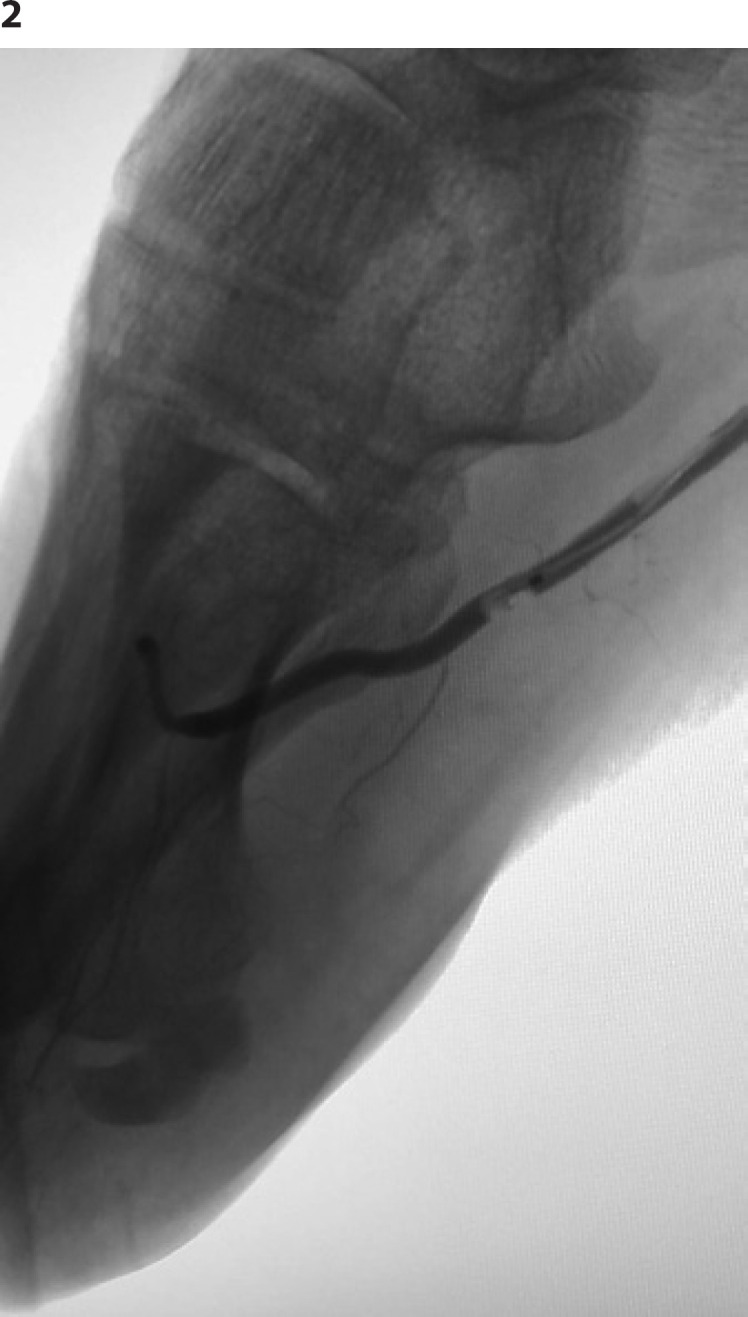
Angiography: intra-procedural recanalization with balloon catheter of dorsalis pedis artery and distal lateral plantar artery occlusion.
On day 7, edema of left forearm was observed and ultrasound exam showed superficial thrombophlebytis. D-dimer dosage was repeated, and it was further raised (2,145 ng/mL).
The same day, a third cerebral CT scan was performed and showed hemorrhagic transformation in the context of the ischemic lesion, without signs of compression (HI 2) (Fig. 3). Neurologic exam result was the same.
Fig. 3.
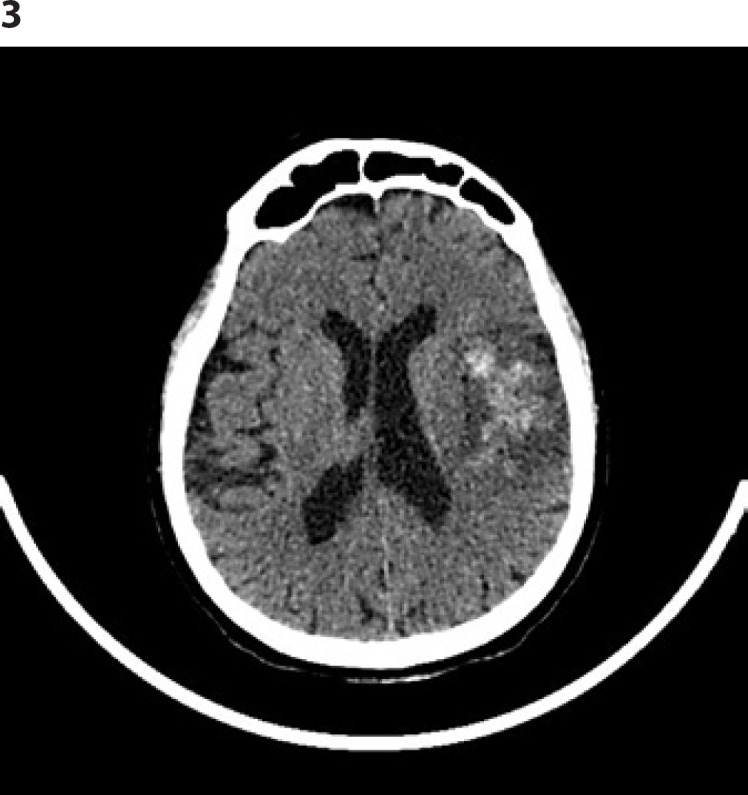
Head CT scan: hemorrhagic transformation in the context of the left frontotemporal ischemic lesion (HI 2). CT, computed tomography.
On days 7 and 8, third and fourth swabs were performed, respectively, and resulted negative for COVID-19; hence, the therapy with azythromycin and hydroxychloroquine was stopped. On day 14, thorax CT scan was repeated and showed disappearance of basal hyperdensity and ground glass pattern, while cerebral CT scan showed no progression of hemorrhagic transformation. D-dimer dosage was reduced (409 ng/mL). Neurologic exam gradually improved (at discharge NIHSS 10), so that on day 16, the patient was transferred to rehabilitation hospital.
Discussion
COVID-19 often affects aged patients with multiple vascular risk factors [5], as in the case of our patient; hence, it is relevant to question if there is a link between these two conditions. Several viruses can induce stroke with different mechanisms, such as varicella-zoster which can cause a direct infection of cerebral arteries and citomegalovirus which can accelerate atherosclerosis [6].
Sars Cov2 may penetrate the central nervous system through the olfactory nervous terminations [7] since many patients refer smell impairment as a prodromal symptom of COVID-19, or alternatively through the hematogenous route. Furthermore, it has been reported that COVID-19 is associated with coaguloapathy and antiphospholipid antibodies [8]. Stroke cases, as well as other neurologic complications, have been mainly reported in severe forms of COVID-19 [4, 9].
Our patient did not present a severe form of COVID-19, he did not present an increase in cytokines such as IL6, and antiphospholipid antibodies were also absent. On the other hand, he presented a multifocal vascular syndrome affecting arterial and venous vessels; it is known that Sars Cov2 penetrates the cells through ACE2, also expressed by endothelial cells.
It is possible to speculate on different ways for Sars Cov2 to affect the vessels, both with an inflammatory mechanism and accelerating atherosclerosis. This patient had several vascular risk factors and a history of myocardial infarction, so it is possible that Sars Cov2 promoted clot formation in different sites in a thrombosis-prone patient.
Despite hemorrhagic transformation of the ischemic cerebral infarction, we confirmed therapy with clopidogrel and enoxaparin, since in the presence of multiple arterial and venous involvement, we considered the risk of thrombosis higher than that of hemorrhagic lesion worsening. Neurologic involvement in this patient was the main clinical issue; thus, the warning is about the fact that COVID-19 patients may arrive to physicians' attention due to a neurologic symptom.
This case suggests that COVID-19 can favor strokes and in general vascular complications, even in milder cases; the presence of preexisting risk factors could play a determinant role. Further evidence is needed, and surveillance for cerebrovascular complications of COVID-19 is crucial to investigate a potential role in stroke pathogenesis.
Statement of Ethics
The patient gave his informed consent for the publication of this case. The authors have no conflicts of interest to declare. The authors received no fund for the preparation of the manuscript.
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
Author Contributions
Chiara del Bianco, Maria Rita Di Ruzza, Maria Magarelli, Roberto Gandini, and Maurzio Plocco substantially contributed to the collection of the data and to the preparation of the manuscript. Roberto Gandini substantially contributed to the collection and interpretation of the angiographic images. Maurizio Plocco substantially contributed to the ultimate supervision of the manuscript.
Acknowledgement
We thank Dr. Tamzin Hackett and Dr. Olga Pavlova for their support in text revision.
References
- 1.World Health Organization Novel coronavirus (2019-nCoV): situation report-139. 2020. [cited 2020 Jun 7]. Available from: https://www.who.int/docs/defaultsource/coronaviruse/situationreports/20200607-sitrep-139-ncov.pdf.
- 2.Hamming I, Timens W, Bulthius MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal Pathol. 2004;203((2)):631–7. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus 2019 in Wuhan, China. JAMA Neurol. 2020;77((6)):683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395((10223)):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel M, Mahalingam R, Cohrs RJ, Gilden D. Virus vasculopathy and stroke: an under recognized cause and treatment target. Infect Disord Drug Targets. 2010;10((2)):105–11. doi: 10.2174/187152610790963537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao X, Jin W. The Covid-19 pandemic: consideration for brain infection. Neuroscience. 2020 Jun 1;437:130–1. doi: 10.1016/j.neuroscience.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Xiao M, Zhang S, Xia P, Cao W, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid 19. N Engl J Med. 2020 Apr 23;382((17)):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Meziani F. Neurologic features in severe SARS-Cov-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]


