Abstract
Background
Human immunodeficiency virus (HIV) remains a major global public health issue, particularly in Africa. In resource-limited settings like Ethiopia, regular weight measurement and monitoring is useful in the examination of patient response to antiretroviral therapy and in clinical decision-making. However, there is a paucity of evidence on factors that affect longitudinal weight change. Therefore, the present study was intended to identify predictors of weight change among people living with HIV (PLWH) in West Hararghe, Ethiopia.
Methods
An institutional-based retrospective cohort study was conducted among 558 PLWH aged 18 years and above from September 2013 to January 2019 at Chiro Zonal Hospital and Gelemso General Hospital in West Hararghe zone, Ethiopia. Data were entered in Epi info 7 and analyzed in R software. The linear mixed effect regression model was used to identify predictors of longitudinal change in weight. Regression coefficients with their 95% confidence intervals were used to indicate the strength and significance of the association.
Results
Weight showed improvement in follow-up periods. In this study, age of respondent (beta=0.136, 95% CI, 0.044:0.227), time since the initiation of antiretroviral therapy (ART) (beta=0.089, 95% CI, 0.075:0.104), primary educational status (beta=2.403, 95% CI, 0.540:4.266), secondary educational status (beta=4.035, 95% CI, 1.666:6.404), tertiary and above educational status (beta=3.444, 95% CI, 0.330:6.558), sex (beta= −5.514, 95% CI, -7.260:-3.768), ambulatory functional status (beta= −3.419, 95% CI, −6.169:-0.668) and baseline CD4 count (≤200) (beta=2.205, 95% CI, 0.593, 3.817) were significant predictors of longitudinal weight change.
Conclusion
We observed an increment in weight among PLWH who were on ART in Ethiopia. Educational status, time since the beginning of ART, age and having CD4 count above 200 have contributed positively to the change in weight, while ambulatory functional status and being female are negatively associated with longitudinal change in weight. Close monitoring is recommended for patients with ambulatory baseline functional status and for patients with baseline CD4 count ≤200.
Keywords: weight change, PLWH, ART, linear mixed effect model, Ethiopia
Introduction
Human Immunodeficiency Virus (HIV) remains a major global public health issue, responsible for 770 000 deaths in 2018, of which more than two-thirds (25.7 million) are from the World Health Organization (WHO) African Region. Around 37.9 million people are living with HIV in 2019 and more than half of them live in Africa.1 In Ethiopia, 690,000 were living with HIV and 11,000 people died of acquired immunodeficiency syndrome (AIDS) related illness, according to the UNAIDS report in 2019.2
People Living with HIV (PLWH) had a persistently lower subcutaneous adipose tissue compared to HIV-free participants. Most PLWH gain weight after antiretroviral therapy (ART) has started in the current ART era, despite relatively normal immune function and minimal pre-ART weight loss.3,4 The most pronounced increase in weight occurs in the first year after the initiation of ART. A small proportion of patients may also be underweight. Developing countries are experiencing a double burden of underweight and overweight, which may increase the risk of cardiovascular disease among PLWH.5,6
Weight is one of the clinical measurements used to assess the efficacy of ART. It can be used as an open index for the monitoring and evaluation of PLWH, among other diagnostic indices, particularly in developing countries. Ensuring normal Body Mass Index (BMI) for PLWH in clinical settings is crucial in providing important insights and identifying opportunities for interventions.6,7
In resource-limited settings like Ethiopia, regular weight measurement and monitoring is useful for clinicians to examine the response of patients to ART and predict the disease stage as well as clinical decision-making. Studies are needed to determine whether weight improvement prior to or at ART initiation will result in improved ART outcomes.
Despite this, there is little evidence in Ethiopia of factors affecting longitudinal weight change. Therefore, the present study was intended to identify predictors of weight change among PLWH on ART in West Hararghe, Eastern Ethiopia. The finding would be helpful for monitoring the effectiveness of ART and reducing the complication that may arise as a result of over and underweight. In addition, it would help for clinical decision-making and improving therapeutic care.
Methods
Study Design and Settings
An institutional-based retrospective cohort study was conducted among PLWH who are on ART between September 2013 and January 2019 at public hospitals in the West Hararghe zone of Oromia, Eastern Ethiopia. Among the five hospitals in the area, the study was conducted in Chiro Zonal Hospital and Gelemso General Hospital due to the availability of ART data. The two hospitals are providing services to approximately 2,300,000 people in the area. At the time of the study, a total of 1120 patients from Chiro Zonal Hospital and 521 patients from Gelemso General Hospitals were on ART.
Study Population and Sample Size
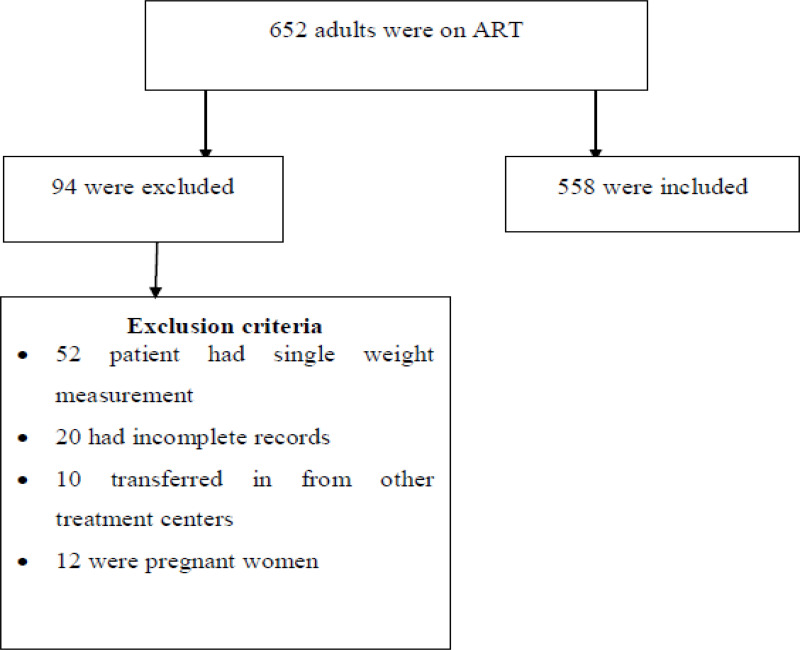
The study included all PLWH aged 18 years and above who started ART between 2013 and 2019 and registered in the ART Registry of Chiro and Gelemso Hospitals. Patients who had at least two weight measurements were included in the study. Patients who had single body weight measurement, those with incomplete records, transferred in from other treatment center and pregnant women were excluded from the study. Figure 1 flow chart shows how the study participants were selected. Out of PLWH who started Highly Active Anti-Retroviral Therapy (HAART) in the two selected hospitals between September 2013 and January 2019, 558 patients who met the inclusion criteria were selected.
Figure 1.
Flowchart depicting selection process of study participants.
Data Collection Procedures
The data for this study were obtained from secondary data and collected using a data extraction checklist. A baseline and follow-up weight data were identified and collected from the registration logbook of HAART attendants. In addition, socio-demographic variables, visiting times and clinical data were collected from the registration documents of patients. The data were collected by health professionals after they had been given adequate orientation.
Measurement of Study Variables
The outcome variable for this study was longitudinal weight change. Weight was measured using a standard weighing scale with graduation 0.1 kg and measuring range up to 150 kg. The scale pointer was calibrated at zero before taking measurement. Measurement of weight was recorded to the nearest 0.1 kg. Weight change refers to the difference between weight (kg) in the current visit time and weight (kg) in the visit time immediately prior to the current response.
Socio-demographic variables, baseline clinical variables (WHO Retroviral Infection (RVI) stage, baseline CD4, initial ART regimen, tuberculosis (TB) co-infection, opportunistic infection prophylaxis (OIP), baseline functional status and baseline adherence) were independent variables.
Data Quality Assurance
The preliminary assessment of the adequacy of the checklist was carried out and the variables on which the data were not available were excluded from the checklist. Trained health professionals have been assigned as data collectors. In addition, to ensure that the data quality of the completed checklist was checked for consistency and completeness. Strict supervision has been applied by supervisors during data collection.
Data Processing and Analysis
Data entry was done using Epi Info 7 and exported to R statistical software 3.6.2 for further analysis. Descriptive statistics such as mean, median, standard deviations (SDs) and tables have been used to investigate the characteristics of the study participant. The Linear Mixed Effect Model (LMM) was used to identify predictors of weight change. Both the random intercept and slope-intercept models were examined to determine the fitness of the model. The model comparison was made using likelihood ratio test.
The predictors significantly associated with longitudinal weight change in the bi-variable analysis at p-values below 0.2 were included in the multivariable linear mixed effect model. Regression coefficients of the final model and their 95% confidence intervals were used as a measure of association between the predictors and the outcome variable. A p-value of less than or equal to 0.05 was considered to be statistically significant.
Ethical Consideration
Ethical clearance and letter of cooperation for selected hospitals were obtained from the Institutional Review Board of the University of Gondar. Support letter was obtained from medical director of the hospitals in order to access medical records of patients. Data were fully anonymized and no personal identifiers, such as name and private information were not collected. Confidentiality during all phases of research activities was kept and data were held on secured password-protected system.
Results
A total of 558 PLWH were included in the study. The median age of the subject was 32 years IQR: (27.0–40.0). The majority of study subjects 354 (63.4%) were female, 350 (62.7%) were from urban area and more than half 303 (54.3%) were married. Of the total study participants, 470 (84.2%) had a working baseline functional status, 459 (82.3%) had TB co-infection and a majority of 358 (64.2%) had CD4 baseline status above 200. More than three-quarters (88.2%) of patients received OIP, 57 (28.1%) and 24 (4.3%) were at WHO stage III and IV, respectively (Table 1).
Table 1.
Baseline Characteristics of PLWH in West Hararghe Zone, Eastern Ethiopia, 2013 to 2019
| Variables | Category | Frequency (n=558) | Percent (%) |
|---|---|---|---|
| Sex | Male | 204 | 36.6 |
| Female | 354 | 63.4 | |
| Marital status | Single | 71 | 12.7 |
| Married | 303 | 54.3 | |
| Divorced | 87 | 15.6 | |
| Widowed | 62 | 11.1 | |
| Separated | 35 | 6.3 | |
| Occupational status | Government employed | 58 | 10.4 |
| Non-government employed | 138 | 24.7 | |
| Farmer | 106 | 19.0 | |
| Jobless | 162 | 29 | |
| Others | 94 | 16.8 | |
| Place of residence | Urban | 350 | 62.7 |
| Rural | 208 | 37.3 | |
| Educational status | Uneducated | 167 | 29.9 |
| Primary | 204 | 36.6 | |
| Secondary | 110 | 19.7 | |
| Tertiary and above | 77 | 13.8 | |
| RVI stage | Stage I | 285 | 51.1 |
| Stage II | 92 | 16.5 | |
| Stage III | 157 | 28.1 | |
| Stage IV | 24 | 4.3 | |
| Functional | Working | 470 | 84.2 |
| Ambulatory | 70 | 12.5 | |
| Bedridden | 18 | 3.2 | |
| Adherence | Good | 482 | 86.4 |
| Fair | 30 | 5.4 | |
| Poor | 46 | 8.2 | |
| TB co-infection | Yes | 459 | 82.3 |
| No | 99 | 17.7 | |
| OIP | Yes | 492 | 88.2 |
| No | 66 | 11.8 | |
| CD4 Count | Less than or equal to 200 | 200 | 35.8 |
| Greater than 200 | 358 | 64.2 | |
| ART regimen | AZT, 3TC, NVP | 30 | 5.4 |
| AZT, 3TC, EFV | 24 | 4.3 | |
| TDF, 3TC, NVP | 18 | 3.2 | |
| TDF, 3TC, EFV | 486 | 87.1 |
Note: Others = Daily laborer, Driver, Merchant.
Abbreviations: AZT, zidovudine; 3TC, lamivudine; NVP, nevirapine; EFV, efavirenz; PLWH, people Living with HIV; RVI, retroviral infection; TB, tuberculosis; OIP, opportunistic infection prophylaxis; ART, antiretroviral therapy.
Exploring Longitudinal Weight Changes
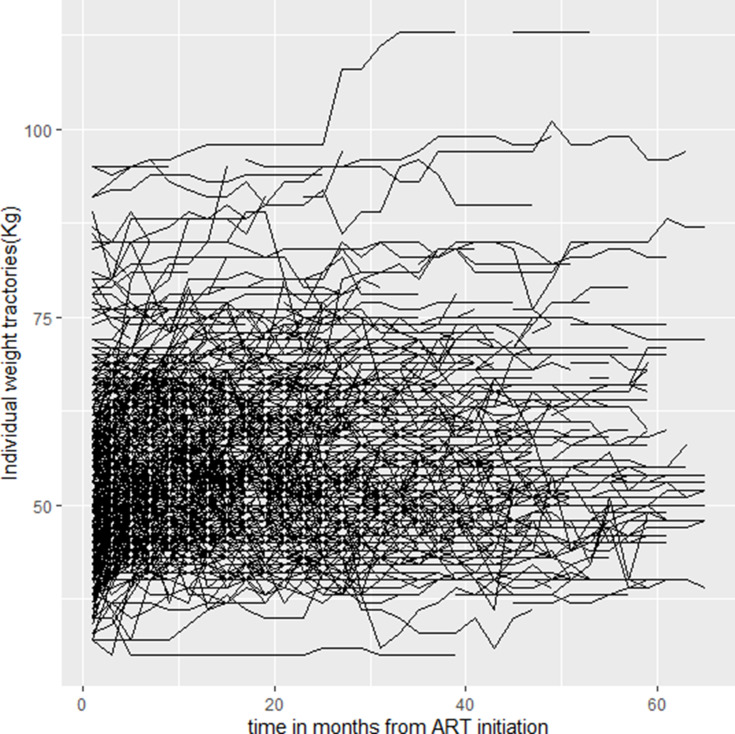
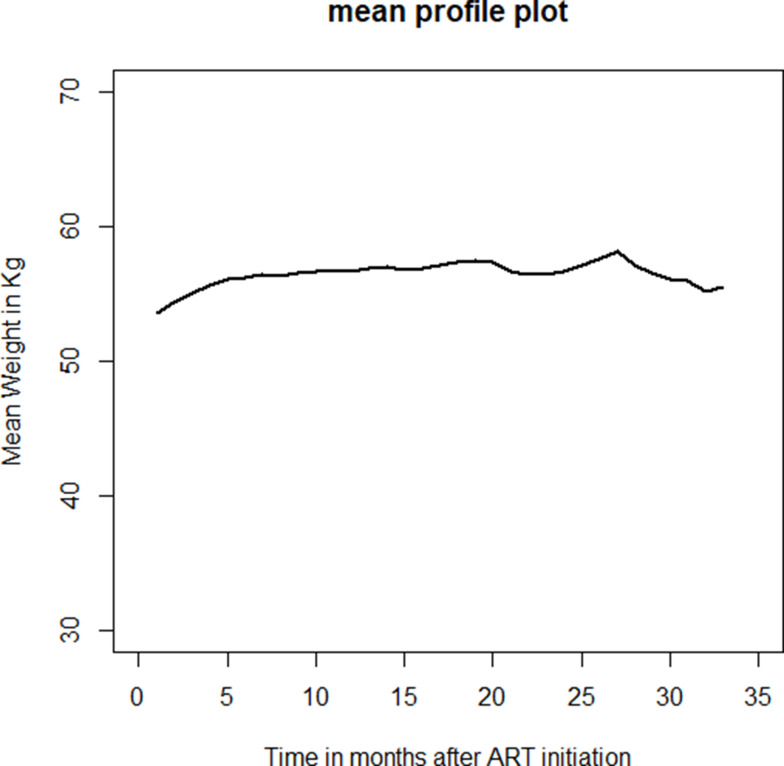
A minimum of two and a maximum of 33 weight measurements were taken for ART patients during the study period. Figures 2 and 3 presents individual profile plots and mean profile plot indicating that there is variability of weight within and between patients. At baseline, patients had different starting points for weight, suggesting a random intercept model. As it can be shown from Figure 2, trajectories of weight over time show considerable heterogeneity which suggests to consider a mixed model with random slopes. The mean weight and the number of patients analyzed at each time point are shown in Table 2.
Figure 2.
Individual profile plot for longitudinal weight trajectory among PLWH at public hospitals in west Hararghe zone, Eastern Ethiopia, 2013 to 2019.
Figure 3.
Overall mean weights for PLWH in West Hararghe Zone, Eastern Ethiopia 2013 to 2019.
Table 2.
Average Baseline and Follow-Up Values of Weight for PLWH in West Hararghe Zone, Eastern Ethiopia 2013 to 2019
| Follow-Up Period | Weight in Kilograms | |
|---|---|---|
| N | Mean (±SD) | |
| Baseline | 558 | 53.54 (10.30) |
| 3rd month | 558 | 54.40 (9.89) |
| 5th month | 550 | 55.08 (9.86) |
| 7th month | 531 | 55.59 (9.88) |
| 9th month | 510 | 56.04 (9.86) |
| 11th month | 493 | 56.16 (9.78) |
| 13th month | 471 | 56.38 (9.94) |
| 15th month | 458 | 56.28 (10.14) |
| 17th month | 439 | 56.57 (10.20) |
| 19th month | 417 | 56.68 (10.35) |
| 21st month | 406 | 56.67 (10.39) |
| 23rd month | 376 | 56.70 (10.56) |
| 25th month | 356 | 56.89 (10.99) |
| 27th month | 327 | 56.97 (11.39) |
| 29th month | 301 | 56.75 (11.28) |
| 31st month | 281 | 56.84 (11.37) |
| 33rd month | 269 | 57.11 (11.62) |
| 35th month | 259 | 57.39 (11.53) |
| 37th month | 235 | 57.44 (11.91) |
| 39th month | 210 | 57.41 (12.18) |
| 41st month | 194 | 56.69 (11.46) |
| 43rd month | 181 | 56.48 (11.68) |
| 45th month | 169 | 56.46 (11.49) |
| 47th month | 149 | 56.64 (11.78) |
| 49th month | 121 | 57.07 (13.20) |
| 51st month | 99 | 57.61 (13.68) |
| 53rd month | 89 | 58.14 (14.09) |
| 55th month | 78 | 57.14 (11.96) |
| 57th month | 62 | 56.60 (12.50) |
| 59th month | 52 | 56.08 (12.13) |
| 61st month | 36 | 55.94 (13.14) |
| 63rd month | 21 | 55.19 (13.91) |
| 65th month | 10 | 55.50 (13.79) |
Model Comparison
The model comparison was made for the two nested models (mixed model with random intercept and mixed model with both random intercept and slope) using the likelihood ratio test and the result shows that the linear mixed effect model with both random intercept and slope was a more parsimonious model consistent with the exploratory analysis result (Table 3). Variance covariance structure showed correlation between repeated measurements diminishes over time. Therefore, a model with autoregressive (AR1) covariance structure was selected as a better model for this data.
Table 3.
Model Comparison for Longitudinal Models Using Likelihood Ratio Test
| Model | Log-Likelihood | LR | P-value |
|---|---|---|---|
| Random intercept model | −24585.04 | ||
| Random intercept and slope model | −23410.89 | 2348.316 | < 0.001 |
Abbreviation: LR, likelihood ratio.
Predictors of Longitudinal Weight Change
The Linear Mixed Effect Model was designed to identify predictors of longitudinal change in weight. Respondent sex, age, time after ART initiation, educational status, occupational status, baseline CD4 count, WHO RVI stage, baseline adherence, TB co-infection and functional status showed significant association in bi-variable analysis whereas marital status, residence and OIP had no significant association with weight change at p-value of 0.2 and therefore dropped from multi-variable model.
In multivariable analysis time since the beginning of ART (beta=0.089, 95% CI, 0.075:0.104), age of respondent (beta=0.136, 95% CI, 0.044:0.227), primary educational status (beta=2.403, 95% CI, 0.540:4.266), secondary educational status (beta=4.035, 95% CI, 1.666:6.404), tertiary and above educational status (beta=3.444, 95% CI, 0.330:6.558), sex (beta= −5.514, 95% CI, -7.260:-3.768), ambulatory functional status (beta= −3.419, 95% CI, −6.169:-0.668) and baseline CD4 count (≤200) (beta=2.205, 95% CI, 0.593, 3.817) were significant predictors of longitudinal weight change (Table 4).
Table 4.
Linear Mixed Effect Model for Predictors of Longitudinal Changes in Weight Over Time for PLWH, 2013 to 2019
| Variables | Category | Beta | P-value | 95% CI |
|---|---|---|---|---|
| Intercept | – | 51.036 | <0.001 | 44.748, 57.323 |
| Time | – | 0.089 | <0.001 | 0.075, 0.104 |
| Sex | Male | Ref. | Ref. | Ref. |
| Female | −5.514 | <0.001 | −7.260, −3.768 | |
| Age | 0.136 | 0.004 | 0.044, 0.227 | |
| Educational status | Uneducated | Ref. | Ref. | Ref. |
| Primary | 2.403 | 0.012 | 0.540, 4.266 | |
| Secondary | 4.035 | 0.001 | 1.666, 6.404 | |
| Tertiary and above | 3.444 | 0.030 | −0.330, 6.558 | |
| Occupational status | Government employed | Ref. | Ref. | Ref. |
| Non-government employed | 0.023 | 0.989 | −3.202, 3.248 | |
| Farmer | −0.435 | 0.809 | −3.958, 3.089 | |
| Jobless | −1.281 | 0.439 | −4.527, 1.965 | |
| Others | 2.548 | 0.159 | −1.000, 6.096 | |
| RVI stage | Stage I | Ref. | Ref. | Ref. |
| Stage II | −1.393 | 0.194 | −3.497, 0.712 | |
| Stage III | −1.325 | 0.199 | −3.349, 0.700 | |
| Stage IV | −0.626 | 0.778 | −4.989, 3.737 | |
| Functional | Working | Ref. | Ref. | Ref. |
| Ambulatory | −3.419 | 0.015 | −6.169, −0.668 | |
| Bedridden | −2.656 | 0.285 | −7.531, 2.219 | |
| Adherence | Good | Ref. | Ref. | Ref. |
| Fair | −2.144 | 0.223 | −5.593, 1.305 | |
| Poor | −1.247 | 0.432 | −4.364, 1.870 | |
| TB co-infection | No | Ref. | Ref. | Ref. |
| Yes | −1.922 | 0.093 | −4.164, 0.319 | |
| Baseline CD4 | ≤200 | Ref. | Ref. | Ref. |
| >200 | 2.205 | 0.007 | 0.593, 3.817 | |
| ART regimen | AZT, 3TC, NVP | Ref. | Ref. | Ref. |
| AZT, 3TC, EFZ | 3.324 | 0.177 | −1.509, 8.157 | |
| TDF, 3TC, NVP | −1.502 | 0.574 | −6.743, 3.739 | |
| TDF, 3TC, EFZ | 0.473 | 0.783 | −2.893, 3.814 | |
| Variance component | ||||
| SD of intercept | 8.455 | |||
| SD of time | 0.115 | |||
| Residual SD | 3.004 | |||
Note: Others=Daily worker, Drivers, Merchant. Bold indicates that variables are significantly associated with the outcome.
Abbreviations: Ref, reference category; SD, standard deviation; RVI, retroviral infection.
Discussion
This longitudinal study examined weight change and its predictors among PLWH. The expected weight for all patients at baseline is 8.455 but shows variation from one patient to another with a standard deviation of 0.115. The study found that sex, educational status, age, time since ART initiation, functional status and baseline CD4 count were significantly associated with weight change.
Time since the start of ART is one of the factors that is significantly associated with weight change. For a unit increase in time spent on ART, the expected weight of PLWH is increased by 0.089. This finding is consistent with the study in Ethiopia8 and may be due to the effect of combined antiretroviral therapy which may result in changes in body fat composition overtime.9
In our study female sex is an independent predictor of weight change. People Living with HIV who are female are expected to have 5.514 lower mean weight as compared to male patients. This finding is supported by a low-resource setting study which reported that after initiation of ART, the mean adjusted body weight change was higher in males than in females.10 This could be due to females have less ability to bear traumatic life stressors like HIV/AIDS and in addition biological (hormonal) difference of female with their counterparts make them more likely to experience psychological problems like anxiety and depression which is negatively related to weight.
Age is another significant predictor of weight change. Expected weight of PLWH is increased by 13.6% for a unit increase in age of the patient in years. This finding is in line with previous study in USA which indicated as age of patient increases PLWH gain weight due to increment in lean body mass related to effect of combined ART.
Educational status is another variable that has shown significant association with weight change. Patients with primary, secondary and tertiary and above educational status are expected to have 2.403, 4.305 and 3.444 times higher mean weights compared to uneducated patients, respectively. The possible explanation for this could be that literate patients have a better awareness on the importance of continuous intake of drugs in order to have better adherence, and that literate persons may have a better socio-economic status and be able to eat balanced and adequate food.
In the current study, the mean weight of patients with ambulatory baseline functional status is expected to be 3.419 lower compared to those with working baseline functional status and this might be due to patients with ambulatory baseline functional status may be more susceptible and likely to have to opportunistic infection and complications which, in turn, have a negative effect on weight.
This study found that PLWH with baseline CD4 counts greater than 200 are expected to have 2.205 higher mean weights than those with baseline CD4 counts less than or equal to 200. This finding is in line with previous studies8,11 which reported a significant positive association between weight change and CD4 count. This can be explained by the fact that patients with baseline CD4 counts above 200 have a lower risk of developing opportunistic infections and malignancies, which could contribute to higher mean weight.
The limitation of this study was that it was based on secondary data from patient records that could not be obtained for some important variables such as nutritional status and substance use. In the conclusion, we observed an increment in weight among PLWH who were on ART in Ethiopia. Educational status, time since the beginning of ART, age and having CD4 count above 200 have contributed positively to the change in weight, while ambulatory functional status and being female are negatively associated with longitudinal change in weight. Close monitoring is recommended for patients with ambulatory baseline functional status and for patients with baseline CD4 count ≤200.
Acknowledgments
We thank the University of Gondar for funding and giving ethical clearance to conduct this research. Our special thanks also go to data collectors and supervisors for their support during data collection period.
Funding Statement
The study was funded by the University of Gondar. The research was designed, conducted, analyzed and interpreted by the authors entirely independently of the funding source. The funder has no role in the publication process.
Abbreviations
AIC, Akaike Information Criteria; AIDS, acquired immunodeficiency syndrome; ART, anti-retroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; LMM, linear mixed model; OIP, opportunistic infection prophylaxis; PLWH, people living with HIV; RVI, retroviral infection; SD, standard deviation; SE, standard error; TB, tuberculosis; WHO, World Health Organization.
Data Sharing Statement
The datasets supporting the conclusions of this article are available upon request to the corresponding author.
Author Contributions
ABW conceptualized the study, participated in proposal writing, data collection, formal analysis, investigation, interpretation, and critical revision of the manuscript. TAA, MMS, BST, SAK participated in formal analysis, investigation, interpretation and critical revision of the manuscript. All authors have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.WHO. Global HIV/AIDS statistics; 2019. Available from: http://aidsinfo.unaids.org/. Accessed August3, 2020.
- 2.UNAIDS. HIV and AIDS updates in Ethiopia; 2019. Available from: www.unaids.org. Accessed August3, 2020.
- 3.Grunfeld C, Saag M, Cofrancesco J Jr., et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. Aids J. 2010;24(11):1717–1726. doi: 10.1097/QAD.0b013e32833ac7a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasse B, Iff M, Ledergerber B, et al. Obesity trends and body mass index changes after starting antiretroviral treatment: the Swiss HIV Cohort Study. J Open Forum Infect Dis. 2014;1(2). doi: 10.1093/ofid/ofu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suastika K, Dwipayana P, Saraswati MR, et al. Underweight is an important risk factor for coronary heart disease in the population of Ceningan Island, Bali. J Diabetes Vasc Dis Res. 2012;9(1):75–77. doi: 10.1177/1479164111422828 [DOI] [PubMed] [Google Scholar]
- 6.Mustapha K, Ehianeta T, Kirim R, Osungwu F, Oladepo D. Highly active antiretroviral therapy (HAART) and body mass index (BMI) relationship in people living with HIV/AIDS (PLWHA) in the Federal Capital Territory, Nigeria and the neighbouring states. J AIDS HIV Res. 2011;3(3):57–62. [Google Scholar]
- 7.EMH. Standard Treatment Guideline for Primary Hospital: Drug Administration and Control Authority of Ethiopia Contents Ethiopia. Ethiopian Minstry of Health; 2010. [Google Scholar]
- 8.Reda AA, Biadgilign S, Deribew A, Gebre B, Deribe K. Predictors of change in CD4 lymphocyte count and weight among HIV infected patients on anti-retroviral treatment in Ethiopia: a retrospective longitudinal study. PLoS One. 2013;8(4):e58595. doi: 10.1371/journal.pone.0058595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrin M, Tate JP, Akgün KM, et al. Weight gain and incident diabetes among HIV infected-veterans initiating antiretroviral therapy compared to uninfected individuals. J Acquir Immune Defic Syndr. 2016;73(2):228. doi: 10.1097/QAI.0000000000001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huis D. Determinants of weight evolution among HIV-positive patients initiating antiretroviral treatment in low resource settings. J Acquir Immune Defic Syndr. 2015;70(2):146. doi: 10.1097/QAI.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangili A, Murman D, Zampini A, Wanke C, Mayer KHJCID. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. J Clin Infect Dis. 2006;42(6):836–842. doi: 10.1086/500398 [DOI] [PubMed] [Google Scholar]