Abstract
The immune-suppressive effects of cannabidiol (CBD) are attributed to the modulation of essential immunological signaling pathways and receptors. Mechanistic understanding of the pharmacological effects of CBD emphasizes the therapeutic potential of CBD as a novel immune modulator. Studies have observed that the antagonists of CB1 and CB2 receptors and transient receptor potential vanilloid 1 reverse the immunomodulatory effects of CBD. CBD also inhibits critical activators of the Janus kinase/signal transducer and activator of transcription signaling pathway, as well as the nucleotide-binding oligomerization domain-like receptor signaling pathway, in turn decreasing pro-inflammatory cytokine production. Furthermore, CBD protects against cellular damage incurred during immune responses by modulating adenosine signaling. Ultimately, the data overwhelmingly support the immunosuppressive effects of CBD and this timely review draws attention to the prospective development of CBD as an effective immune modulatory therapeutic.
Keywords: cannabidiol, CBD, immune modulation, CB1 and CB2 receptors, TRPV1, JAK/STAT, inflammasome
Cannabidiol (CBD)
Cannabidiol (CBD) is one of the biochemical compounds, referred to as cannabinoids, isolated from the Cannabis plant (Figure 1).1,2 CBD is the second most prominent cannabinoid within the plant and comprises nearly 40% of the plant extricate.3,4 In recent years, CBD has become one of the most widely studied cannabinoids for its anti-inflammatory and immunomodulatory effects.5,6 These anti-inflammatory and immunomodulatory pharmacological properties of CBD have set the precedent for its use as a natural compound for autoimmune disorders such as multiple sclerosis (MS), type 1 diabetes, and rheumatoid arthritis.7,8 To date, however, the FDA has approved only one CBD prescription drug, Epidiolex, for treatment of two rare and otherwise drug-resistant forms of epilepsy, Dravet Syndrome and Lennox-Gastaut Syndrome.9,10 An overview of elemental receptor and signaling pathway followed by a critical review of CBD’s involvement in the modulation of the receptors and immunological signaling cascades encourage further development of CBD as a state-of-the-art immunomodulatory therapy.8,11
Figure 1.
Chemical structure of cannabidiol (CBD).
CB1 and CB2 Receptors
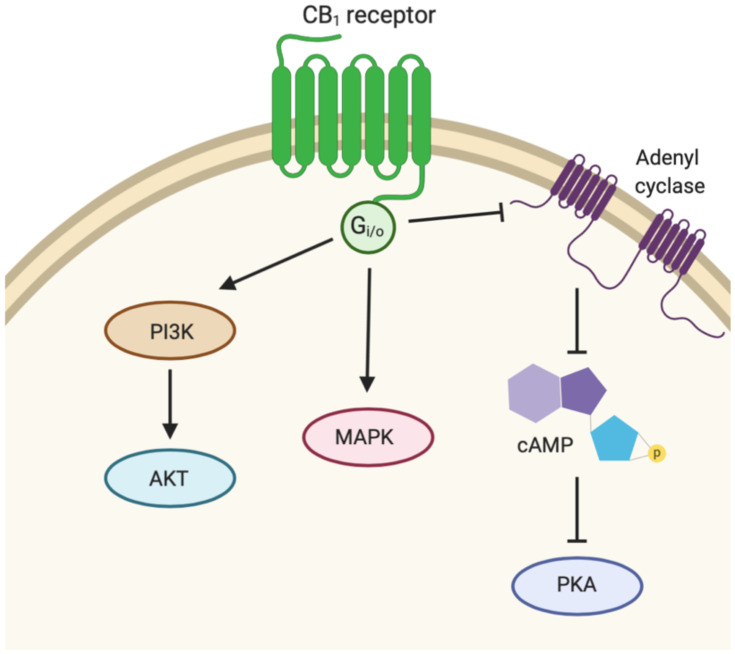
CB1 and CB2 are members of the G-protein coupled receptor family and are encoded by the CNR1 and CNR2 genes, respectively.12 CB1 receptors have a prominent presence in the central nervous system (CNS) while CB2 receptors are more localized in immune cells.13–15 CB1 receptor activation initiates down-stream modulation of three main signaling pathways (Figure 2).12 Activated CB1 receptor coupled to Gi/o inhibits adenyl cyclase (AC) activity, in turn inhibiting cyclic adenosine monophosphate (cAMP) formation. As a result, intracellular signal transduction, and protein kinase A (PKA) activity is hindered.16–18 The cAMP-PKA signal transduction is crucial for many cellular responses including enzyme activation and gene expression.19 Studies have also shown that activation of the CB1 receptor also leads to regulation of mitogen-activated protein kinases (MAPKs) that are vital to inflammatory and immune cellular responses, including extracellular signal-regulated protein kinase 1/2 (ERK1/2), p38, and c-jun terminal kinase (JNK).20,21 The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) is a third pathway involved in cell growth and survival that may also be activated by CB1 receptor.22,23 Similarly, CB2 receptor agonism modulates and inhibits AC and cAMP leading to the inactivation of PKA by Gi/o protein coupling.24,25
Figure 2.
Signaling pathway activity downstream of CB1. Activated CB1 receptor couples with Gi/o to inhibit adenyl cyclase (AC) activity, thus inhibiting cyclic adenosine monophosphate (cAMP) production and protein kinase A (PKA) activity. CB1 receptor activation also regulates mitogen-activated protein kinases (MAPK) and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathways.
CBD and CB1 and CB2 Receptors
The low affinity of CBD for both CB1 and CB2 receptors (Ki > 2000 nM) suggests the pharmacodynamics of CBD to be independent of these receptors.26–29 For example, CBD suppressed cytokine production in CB1 and CB2 receptor knockout (CNR1−/−/CNR2−/−) mice, suggesting the effects of CBD are independent of CB1 and CB2 receptors.30 Specifically, Interleukin-2 (IL-2), a T-cell-derived cytokine, was significantly reduced in splenocytes of CNR1−/−/CNR2−/− mice upon treatment with CBD (1 μM). CBD (10 μM) also significantly decreased adaptive immune response cytokine production of Interferon gamma (IFN-γ). However, CBD has been shown to function as an inverse agonist of CB1 and CB2 receptors and can antagonize the effects of CB1 and CB2 receptor agonists.4 The use of CB1 and CB2 receptor antagonists reverses the anti-inflammatory and immune-suppressive effects of CBD.31,32 For instance, while treatment with CBD (10 mg/kg) was shown to reduce gastrointestinal motility in a bacterial lipopolysaccharide (LPS) sepsis mouse model, the effects were reversed upon treatment with CB1 receptor antagonist, AM251 (1 mg/kg).31 Similar reversal of CBD-induced neuroprotective effects was observed in a hypoxic-ischemic brain injury model in newborn pigs.32 In this model, CBD (1 mg/kg) inhibited inflammatory cytokine interleukin-1 (IL-1) production in hypoxic-ischemic pigs, and co-administration with CB2 receptor antagonist, AM630 (1 mg/kg), reversed this CBD-mediated neuroprotective effect. Conclusively, the immune-protective effects of CBD are pharmacologically supported to be independent of CB1 and CB2 receptors as well as by inverse agonism of CB1 and CB2.
Transient Receptor Potential Vanilloid 1 (TRPV1)
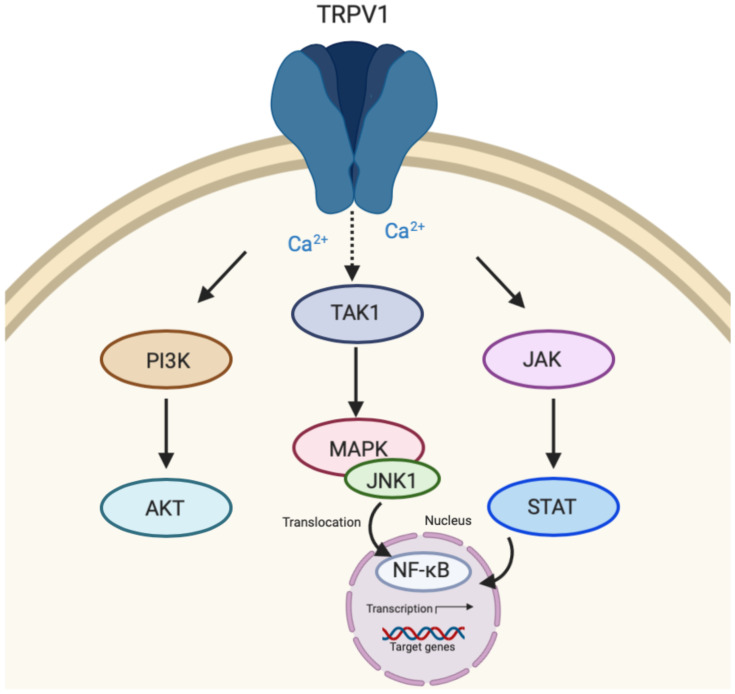
The transient receptor potential vanilloid 1 (TRPV1) is a homotetrameric membrane protein and is the most well-studied nonselective ion channel of the transient receptor potential cationic family.33 TRPV1 is expressed in various immune cells including lymphocytes, microglia, and astrocytes and is involved in pathophysiological responses of the immune system.34 Numerous exogenous and endogenous stimuli activate TRPV1 with capsaicin as the most notable agonist.35 TRPV1 is responsible for detecting stimuli to maintain and regulate cellular homeostasis by promoting Ca2+ influx as a secondary messenger for the induction of pro-inflammatory cytokines and chemokines.36 Similar to CB1 and CB2 receptors described above, activation of TRPV1 may lead to varying downstream signaling such as PI3K/Akt and ERK1/2 signaling pathways (Figure 3).37 TRPV1 may also lead to transforming growth factor-activated kinase 1 (TAK-1) dependent JNK/MAPK cascade that leads to pro-inflammatory nuclear factor-κB (NF-κB) activation.38 Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway may also be activated downstream of TRPV1 activation leading to increased expression of pro-inflammatory cytokines.39
Figure 3.
Transient receptor potential vanilloid 1 (TRPV1) signaling. TRPV1 activation initiates down-stream signaling of three major pathways including PI3K/AKT. Transforming growth factor-activated kinase 1 (TAK-1) dependent c-jun terminal kinase (JNK)/MAPK and Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling cascades may also be activated as a result of TRPV1 activation leading to nuclear factor-κB (NF-κB) activation within the nucleus and transcription of target genes.
CBD and TRPV1
As TRPV1 activation induces pro-inflammatory and immune mediators, TRPV1 channels are a potential target for novel immune modulatory therapeutics.40–42 Vanilloid receptor knockout (TRPV1−/−) mice treated with CBD dose-dependently (5 mg/kg – 50 mg/kg) inhibited induction of myeloid-derived suppressor cells (MDSCs), suggesting that TRPV1 receptors are critical for induction of MDSCs by CBD.43 CBD (10 mg/kg) was also shown to be an effective therapeutic in a rodent model of thermal hyperalgesia which is associated with immune activation.44,45 In contrast, co-administration of CBD (10 mg/kg) with TRPV1 antagonist, capsazepine (CPZ) (2 mg/kg), partially reversed the anti-hyperalgesia effects of CBD. Treatment with CPZ (10 mg/kg) fully reversed the anti-hyperalgesia effects, signifying that CBD modulates TRPV1 for immune suppression.45 Similarly, CBD (10 μM) increased phagocytosis of CNS immune response microglial cells and treatment with CPZ (10 μM) abolished this enhanced phagocytosis, attributing the increased phagocytic effects to modulation of TRPV1.46 The role of TRPV1 in the immunomodulatory effects of CBD was also apparent in CBD-induced endometrial cancer cell apoptosis. The apoptotic effects of CBD (5 μM) on the endometrial cancer cell line were dependent on TRPV1 modulation as these effects were reversed upon co-administration with TRPV1 antagonist, iRTX (20 nM), hence signifying that CBD may be a potential candidate for immune modulation of TRPV1.47
Janus Kinase (JAK) and Signal Transducer and Activator of Transcription 3 (STAT3)
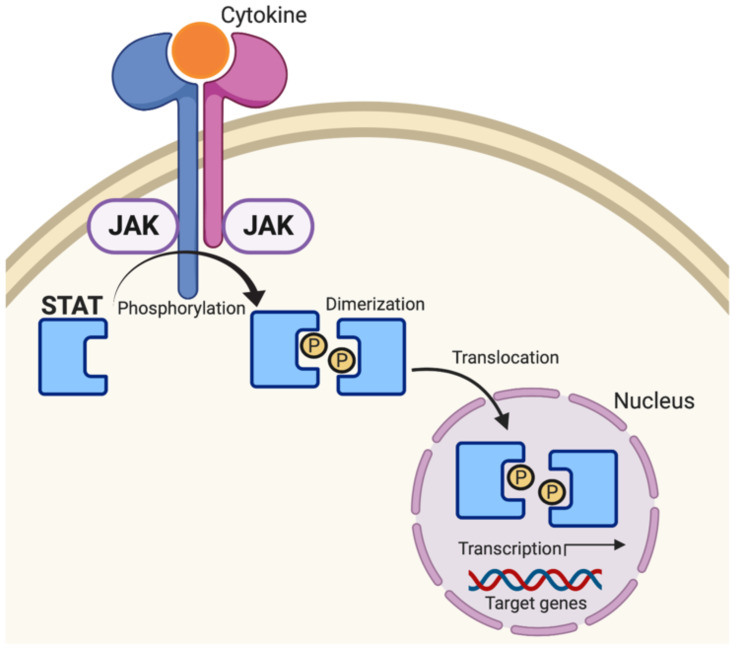
Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway is an evolutionary conserved cascade fundamental to T cell-mediated immunity and adaptive immune responses.48–50 Broadly, cytokines or growth factors activate their respective transmembrane receptors, facilitating activation of receptor-bound JAKS51,52 (Figure 4). Activated JAKs (JAK1, JAK2, JAK3, TYK2) proceed to phosphorylate latent STAT monomers (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, STAT6) leading to STAT dimerization, translocation to the nucleus, binding to specific DNA promoter sequences, and ultimately transcription of the target gene.53,54 Signal transducer and activator of transcription 3 (STAT3) is one of the seven STAT proteins involved in the regulation of critical cellular gene transcriptions including cell differentiation, proliferation, metastasis, as well as immune responses.55 Constitutive activation of JAK-STAT3 signaling is the basis for the development and progression of many cancers; thus, it is not surprising that JAK-STAT3 remains a principal anticancer target.56 However, as STAT3 is a transcription factor that remains generally confined to the nucleus, the drug-ability of STAT3 remains low, posing an unresolved scientific and clinical challenge.57
Figure 4.
Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway. Cytokines facilitate activation of receptor-bound JAKS leading to phosphorylation, dimerization, and translocation of STAT to the nucleus to promote transcription of target genes.
CBD and JAK/STAT
The use of CBD, to modulate of JAK/STAT is one novel approach towards immunosuppression. Transcriptomic profiles of CBD-treated human Gingival Mesenchymal Stem Cells (hGMSCs) revealed down-regulation of JAK/STAT pathways, particularly complete down-regulation of Interleukin-6 (IL-6), a potent but not exclusive activator of JAK/STAT3 signaling.58,59 Similarly, treatment with CBD (ranging from 0.5 μM to 20 μM) has been shown to significantly inhibit IL-2 and IFN-γ production in mouse splenocytes.30,60 Both IL-2 and IFN-γ are activators of JAK/STAT. IL-2 is also a stimulator of PI3K-Akt and MAPK signaling cascades critical in regulating lymphocyte proliferation. CBD also inhibits another anti-inflammatory and immune regulatory cytokine involved in the modulation of JAK/STAT, Interleukin-10 (IL-10), in human leukemic T-cells.61,62
CBD also has been shown to inhibit JAK/STAT activators, including IFN-γ and inflammatory and immune response tumor necrosis factor (TNF) cytokine in a rheumatoid arthritis model.63–65 This study revealed that CBD (5 mg/kg per day intraperitoneally (ip)) reduced TNF and IFN-γ production in arthritic mice. The anti-arthritic and immunosuppressive effects of CBD were further underlined by concentration-dependent (0–10 μg/mL) inhibition of lymphocyte proliferation.65 CBD (5 mg/kg) is also effective in the suppression of T-cell proliferation and microglial activation, as well as reduction of axon damage within the spinal cord of in an encephalomyelitis murine model of Multiple Sclerosis (MS) by suppression of fundamental JAK/STAT initiators including IL-2, TNF-α, and IFN-γ.11 Notably, T cells express many IL receptors that can lead to activation of JAK/STAT; therefore, suppression of T cells by CBD may inhibit activation of this signaling pathway. High concentration of CBD (20 μg/mL) also attenuates TNF-α and IFN-γ expression in the intestinal lymphatic system as quantified in rat mesenteric lymph node and spleen isolated T cells and human lymphocytes.66
The immunomodulatory effects of CBD have also been observed in Type 1 diabetes mellitus. Type 1 diabetes mellitus is an autoimmune disease leading to T lymphocyte-mediated destruction of insulin-producing pancreatic β cell.67 Administration of CBD (5 mg/kg/day ip) has shown to significantly prevent the onset of autoimmune diabetes in non-obese diabetes (NOD) prone mice in comparison to mice that did not receive CBD.68,69 These mice displayed significant reduction in Th1 inflammatory cytokine production of IFN- γ and TNF- α, and an increase in Th2 cytokine production of IL-4 and IL-10, suggesting that CBD may exert an immunomodulatory mechanism resulting in a Th1 to Th2 immune response shift.70 Similarly, CBD (5 mg/kg/day) was shown to significantly reduce pro-inflammatory cytokine IFN-γ and TNF-α in the plasma of NOD mice treated with CBD.69 These mice also exhibited a decrease in Th1 cytokine production and increase in the production of Th2-associated cytokines.69 These results suggest that CBD may have an immunomodulatory role, shifting immune responses from Th1 to Th2.69 Expression of other initiators and activators of JAK/STAT including IL-6, TNF-α, and IL-12 were also reduced in the CBD-treated splenocytes derived from these non-obese diabetes-prone (NOD) female mice.
CBD has been shown to reduce proinflammatory signaling by modulation of IFNβ/STAT pathway71 such that CBD (10 μM) reduced IL-1β, IL-6, and IFNβ expression in LPS activated microglial cells. IL-1β activates myeloid differentiation factor 88 (MyD88)-adaptor protein-dependent pathway that leads to activation of NF-κB-dependent transcription. The NF-κB pathway is primarily responsible for regulating the expression of many pro-inflammatory genes including cytokines TNF α, IL-1, and IL-6. IFNβ, on the other hand, activates chemokines such as CXCL10, CCL5, and CCL2 by binding to IFN receptor and inducing phosphorylation of JAK leading to STAT pathway activation. CBD also suppresses NF-κB mediated transcription, in turn enhancing the anti-inflammatory effects. This process is mediated via STAT3 by increasing anti-inflammatory STAT3 phosphorylation while reducing pro-inflammatory STAT1 phosphorylation.71 Collectively, these studies further illuminate the pharmacodynamics of CBD and underline the immunomodulatory properties of CBD by down-regulation of JAK/STAT signaling cascade, thus supporting the development of CBD as a novel immune modulation therapeutic.
Nucleotide-Binding Oligomerization Domain-Like Receptor (NLR) Signaling Pathway
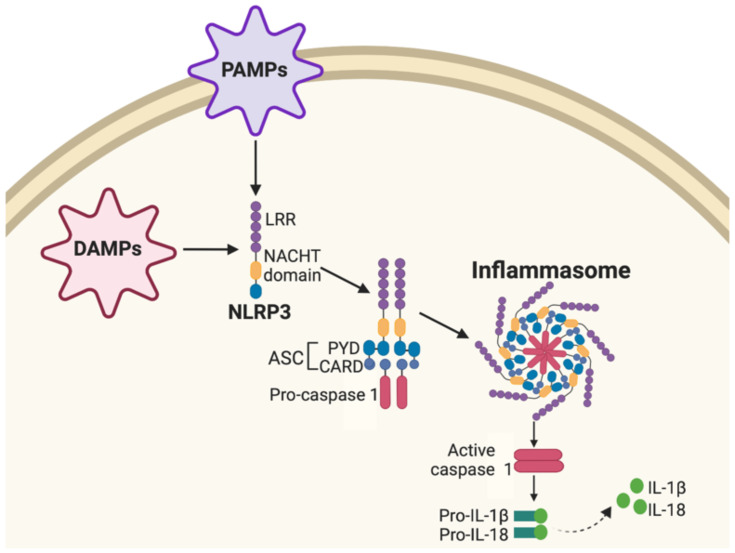
Nucleotide-binding oligomerization domain-like receptors (NLRs) serve as cellular receptors that detect and orchestrate innate immune system protection in response to cellular stress and insult, such as pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs).72,73 When dangerous cellular stimuli or stress is detected, the NLR Family Pyrin Domain Containing 3 gene (NLRP3) consisting of a central nucleotide-binding and oligomerization (NACHT) domain and leucine-rich repeat (LRR) forms an inflammasome complex with an adaptor protein apoptosis-associated speck-like protein (ASC). The ASC consists of an N-terminal pyrin domain (PYD) and C-terminal caspase-recruitment domain (CARD) and cysteine protease caspase 1 (Figure 5).74 This activated NLRP3 inflammasome complex leads to activation of caspase 1, in turn, regulating pro-inflammatory interleukin-1β (IL-1β) and interleukin-18 (IL-18) cytokine production that play significant roles in NF-κB, MAPK and IFN pro-inflammatory pathways.75
Figure 5.
Nucleotide-binding oligomerization domain-like receptor (NLR) signaling pathway. Pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) activate NLR Family Pyrin Domain Containing 3 (NLRP3) consisting of a central nucleotide-binding and oligomerization (NACHT) domain and leucine-rich repeat (LRR). NLRP3 forms an inflammasome complex with adaptor protein apoptosis-associated speck-like protein (ASC) consisting of a protein pyrin domain (PYD) caspase-recruitment domain (CARD) and cysteine protease caspase 1. Activated NLRP3 inflammasome complex activates caspase 1, leading to regulation of pro-inflammatory interleukin-1β (IL-1β) and interleukin-18 (IL-18) cytokine production.
CBD and NLR
An alternative pharmacological mechanism by which CBD induces immunomodulatory effects is by inhibiting activation of the NLR signaling cascade. Next-generation sequencing and gene ontology data reveal that inflammasome activation is inhibited in mesenchymal stem cells treated with CBD (5 μM) in comparison to stem cells not treated with CBD.76 In this study, CBD prevented NLRP3-inflammasome pathway activation by suppressing the expression of key genes belonging to NLRP3-inflammasome pathway including NLRP3 and caspase 1 and inhibited downstream production of IL-18 in Human Gingival Mesenchymal Stem Cells (hGMSCs).76 Down-regulation of NLRP3 genes in CBD-treated hGMSCs was also confirmed using immunocytochemical and protein extraction. Gene expression profiling also indicated CBD-induced down-regulation of encoding genes belonging, but not limited to the NLRP3-inflammasome pathway, including pro-inflammatory cytokines (IL6ST, IL-1β), Interleukin receptors or subunits (IL1R1, IL11RA, IL13RA), Toll-like receptor adaptor myeloid differentiation 88 (MYD88), Interferon Gamma Receptors (IFNGR1 and IFNGR2), Mitogen-Activated Protein Kinases (MAPK1, MAPK12, and MAPK14), transcription factors (STAT3 and STAT6), NF-κB complex (NFKB2, NFKB3/RELA), and the Matrix Metallopeptidase 3 (MMP3).76
To further understand the immunosuppressive effects and modulation of NLRP3 inflammasome pathway, CBD has also been compared to known NLRP3 inflammasome inhibitors, oridonin and MCC950, for their respective inhibition of NLRP3-inflammasome activity.77 Before evaluating anti-inflammasome activity of CBD, in vitro toxicity studies were conducted to ensure cellular viability of human monocytes treated with CBD. No significant cytotoxicity was observed among cells treated with CBD (0.1, 1, 10 and 100 μM), suggesting CBD as a safe and non-toxic potential phyto-therapeutic. LPS was used to significantly increase the production of pro-inflammatory cytokine IL-1β in monocytes, and CBD (0.1, 1, and 10 μM) successfully attenuated this IL-1β production.77 As the NLRP3 inflammasome complex is responsible for IL-1β production, specific CBD-induced modulation of NLRP3 was evaluated by comparing IL-1β concentration in LPS-stimulated monocytes treated with CBD, oridonin, and MCC950. CBD and these NLRP3 inhibitors exhibited comparable IL-1β cytokine inhibitory effects, thus stressing the immunomodulatory effects of CBD by inhibition of the NLRP3 inflammasome pathway, hence, further emphasizing CBD as a prospective immunomodulatory agent.
Adenosine Signaling
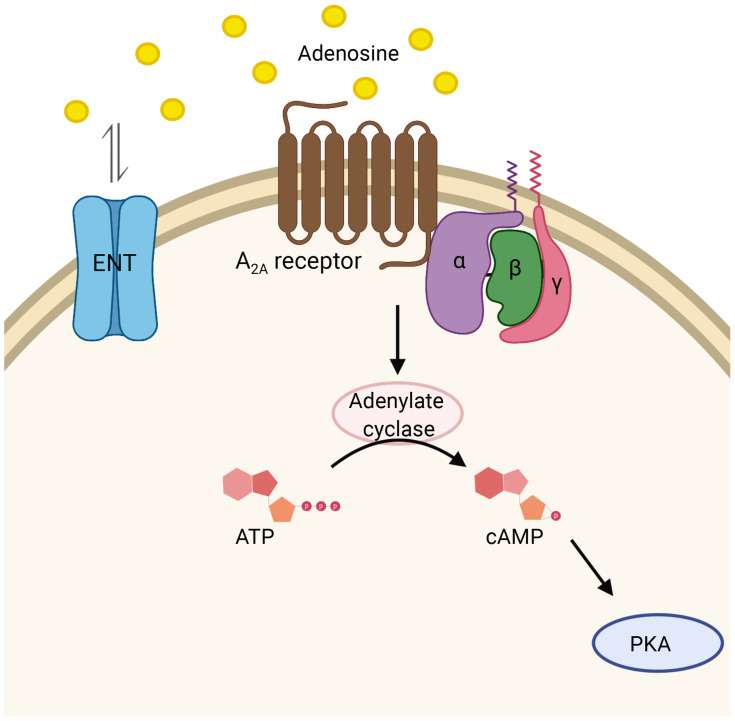
Adenosine is a purine nucleoside that protects against excessive cellular damage during stress and inflammation.78,79 The protective effects of adenosine are mediated by G protein-coupled A2A receptor, which attenuates inflammation via activation of cAMP-mediated pathway, and ultimately leading to inhibition of T cell differentiation, downregulation of neutrophil superoxide production, and inhibition of proinflammatory cytokines expression including TNF-α (Figure 6).80,81 However, rapid intracellular uptake of adenosine, facilitated by the equilibrative nucleoside transporter (ENT), prevents adenosine interaction with their respective immune cell adenosine receptors, and, consequently, blocking these protective effects.82,83 One method for hindering adenosine reuptake and promoting immune modulation is to prevent intracellular transport of adenosine by use of an ENT inhibitor.84
Figure 6.
Adenosine signaling. Equilibrative nucleoside transporter proteins (ENT) facilitate diffusion of adenosine across the cell membrane. Adenosine activates G protein-coupled adenosine receptor (A2A), leading to cAMP formation mediated by adenylate cyclase activity and subsequent activation of protein kinase A (PKA).
CBD and Adenosine Signaling
CBD is a competitive ENT inhibitor (Ki < 250 nM) resulting in subsequent anti-inflammatory and immunosuppressive effects by inhibiting adenosine uptake and enhancing adenosine signaling.85 In vitro data highlight properties of CBD similar to adenosine receptor agonists such as enhancing endogenous adenosine signaling and inhibiting adenosine uptake in murine microglia and macrophages pretreated with CBD.86 Moreover, in vivo treatment with CBD (1 mg/kg) decreased TNF-α as measured in the serum of LPS-treated mice.86 However, this effect was eliminated in an A2A receptor knockout mouse model and annulled in the presence A2A adenosine receptor antagonist.86 In a separate study, adenosine uptake inhibition was compared in rat retinal microglial cells treated with CBD and the ENT inhibitor S-(4-nitrobenzyl)-6-thioinosine (NBMPR) to understand if the ENT isinvolved in the pharmacodynamic role of CBD 87 In this study, CBD (0.5 μM) alone was shown to inhibit adenosine uptake in retinal microglial cells and treatment with NBMPR alone inhibited adenosine uptake in a dose-dependent manner. However, adenosine uptake inhibition was not enhanced in CBD-treated cells that were also treated with NBMPR, which lead to the suggestion that the immunosuppressive effects are mediated by competitive binding to ENT.87 Other studies have reported that TNF-α in LPS-treated mice is reduced in the presence of adenosine uptake inhibitors.88 As a result, endogenous adenosine is readily available to bind to the A2A receptor. To study if CBD inhibits TNF-α by A2A receptor mediation, rats were treated with CBD (1 mg/kg) and A2A receptor antagonist ZM241385 (10 mg/kg). In these experiments, CBD-treated rats showed significant reduction of TNF-α, while treatment with the ZM241385 alone or treatment with CBD and ZM241385 did not inhibit TNF-α concentration, suggesting that CBD inhibits adenosine uptake and reduces TNF-α production by A2A receptor modulation.87
The immunosuppressive effects of CBD by inhibition of adenosine reuptake and regulation of A2A receptor have also been evaluated in a murine model of Multiple Sclerosis. For that, mice were infected with Theiler’s Murine Encephalomyelitis Virus (TMEV) RNA to induce the deleterious inflammatory effects, such as encephalomyelitis, and then treated with CBD.89 Treatment with CBD (5 mg/kg ip) inhibited VCAM-1 production, a mediator of lymphocyte adhesion and leukocyte infiltration produced in response to inflammation. CBD was also found to reduce CCL2 and CCL5 chemokine expression as well as pro-inflammatory cytokines TNF-α and IL-1β.89 CCL2 and CCL5 are associated with macrophage, T cell, and leukocyte recruitment during inflammation.90 These pharmacological effects of CBD were also observed to be dependent on A2A receptor mediation in vivo. ZM241385 was administered to TMEV-induced mice and then treated with CBD shortly after. CBD attenuated VCAM-1 expression and immune cell infiltration. However, these effects were not observed in mice receiving ZM241385 alone, suggesting that the A2A receptor is involved in the mechanism of action of CBD.89 These studies further solidify the immunosuppressive effects of CBD and link the modulatory properties of CBD to inhibition of adenosine uptake and its enhancement of adenosine signaling.
Conclusions
Mechanistic understanding of fundamental pharmacological pathways and receptors followed by a survey and analytical review of promising work involving CBD in the context of the aforementioned receptors and signaling cascades validate CBD as a prospective immune modulatory therapeutic agent. CBD exerts immunomodulatory effects in CB1 and CB2 receptor knockout mice, suggesting that the significant reduction of immune response by CBD is independent of CB1 and CB2 receptors. However, receptor antagonists of CB1 and CB2 receptors have shown to reverse the immune modulatory effects of CBD, indicating that CBD is also an inverse agonist of CB1 and CB2 receptors. Co-administration of CBD with a TRPV1 antagonist reversed CBD inhibition of immune activation, further indicating the involvement of TRPV1 in the immunomodulatory effects of CBD. Moreover, CBD also down-regulates JAK/STAT signaling by inhibiting immune regulatory cytokines, such as TNF-α and IFN-γ, involved in the modulation of JAK/STAT. CBD also prevents NLRP3-inflammasome pathway activation as well as suppresses gene expression of downstream proteins of the NLRP3-inflammasome pathway. These immunosuppressive effects result in decreased production of pro-inflammatory cytokines, including IL-1β and IL-18. CBD has also been shown to inhibit cellular adenosine uptake, resulting in enhanced adenosine signaling and protection against tissue injury during inflammation and immune response. Adenosine receptor, A2A, inhibition with an antagonist or in A2A receptor knockout mouse, was shown to reverse these immune modulatory effects of CBD. Ultimately, thefindings emphasize the immune-suppressive properties of CBD and its potential use as an attractive therapeutic strategy for immunomodulation.
However, to enhance CBD as a promising therapeutic option, additional mechanistic studies should be conducted in the context of other autoimmune disorders, using various immune system models and stimuli, to generate new knowledge of the immune modulatory effects of CBD in vitro and in vivo. In addition, long-term studies are needed to determine CBD’s therapeutic efficacy in chronic immune diseases. Safety and toxicity should be evaluated in these long-term studies to identify any limitations associated with using CBD as a recurring immune modulator. Furthermore, as CBD is a highly hydrophobic molecule and subject to extensive first pass metabolism, its dosages are relatively high. Therefore, there are limitations on how varying dosage affects exhibited immunomodulatory properties. Conclusively, further studies should be conducted to attain a comprehensive understanding of CBD pharmacokinetics and dosages required for therapeutic immune modulation with the ultimate purpose of enhancing CBD as a prospective immune modulatory therapeutic.
Acknowledgments
This work was supported by the National Institutes of Health (R01GM114321, R01GM127706, R01MH104656, R01MH110415, HL126559, DA039576, DA040537, and DA044579) and the National Science Foundation (CHE-1506740, CBET-1841419). SD thanks the Miller School of Medicine of the University of Miami for the Lucille P. Markey Chair in Biochemistry and Molecular Biology.
Disclosure
Nadia Peyravian, Sapna Deo, Sylvia Daunert, and Joaquin J Jimenez report a patent pending: 62/985,529. The authors report no other potential conflicts of interest in this work.
References
- 1.VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin Proc. 2019;94(9):1840–1851. doi: 10.1016/j.mayocp.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 2.Berman P, Futoran K, Lewitus GM, et al. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci Rep. 2018;8(1):14280. doi: 10.1038/s41598-018-32651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafaye G, Karila L, Blecha L, Benyamina A. Cannabis, cannabinoids, and health. Dialogues Clin Neurosci. 2017;19(3):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abilio VC. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9:482. doi: 10.3389/fphar.2018.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAllister SD, Soroceanu L, Desprez PY. The antitumor activity of plant-derived non-psychoactive cannabinoids. J Neuroimmune Pharmacol. 2015;10(2):255–267. doi: 10.1007/s11481-015-9608-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schonhofen P, de Medeiros LM, Bristot IJ, et al. Cannabidiol Exposure During Neuronal Differentiation Sensitizes Cells Against Redox-Active Neurotoxins. Mol Neurobiol. 2015;52(1):26–37. doi: 10.1007/s12035-014-8843-1 [DOI] [PubMed] [Google Scholar]
- 7.Katchan V, David P, Shoenfeld Y. Cannabinoids and autoimmune diseases: A systematic review. Autoimmun Rev. 2016;15(6):513–528. doi: 10.1016/j.autrev.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150. doi: 10.1016/j.pharmthera.2017.02.041 [DOI] [PubMed] [Google Scholar]
- 9.Devinsky O, Cross JH, Wright S. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N Engl J Med. 2017;377(7):699–700. [DOI] [PubMed] [Google Scholar]
- 10.Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled Phase 3 trial. Lancet. 2018;391(10125):1085–1096. doi: 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- 11.Kozela E, Lev N, Kaushansky N, et al. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol. 2011;163(7):1507–1519. doi: 10.1111/j.1476-5381.2011.01379.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: signaling and Function in the Central Nervous System. Int J Mol Sci. 2018;19(3). doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turcotte C, Blanchet MR, Laviolette M, Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci. 2016;73(23):4449–4470. doi: 10.1007/s00018-016-2300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res. 2011;51(1):26–38. doi: 10.1007/s12026-011-8210-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busquets-Garcia A, Bains J, Marsicano G. CB1 Receptor Signaling in the Brain: extracting Specificity from Ubiquity. Neuropsychopharmacology. 2018;43(1):4–20. doi: 10.1038/npp.2017.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65(18):2913–2923. doi: 10.1007/s00018-008-8263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saroz Y, Kho DT, Glass M, Graham ES, Grimsey NL. Cannabinoid Receptor 2 (CB2) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes. ACS Pharmacol Transl Sci. 2019;2(6):414–428. doi: 10.1021/acsptsci.9b00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haushalter KJ, Casteel DE, Raffeiner A, Stefan E, Patel HH, Taylor SS. Phosphorylation of protein kinase A (PKA) regulatory subunit RIalpha by protein kinase G (PKG) primes PKA for catalytic activity in cells. J Biol Chem. 2018;293(12):4411–4421. doi: 10.1074/jbc.M117.809988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harb Perspect Biol. 2012;4(12):a011148–a011148. doi: 10.1101/cshperspect.a011148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobovisek L, Hojnik M, Ferk P. Overlapping molecular pathways between cannabinoid receptors type 1 and 2 and estrogens/androgens on the periphery and their involvement in the pathogenesis of common diseases (Review). Int J Mol Med. 2016;38(6):1642–1651. doi: 10.3892/ijmm.2016.2779 [DOI] [PubMed] [Google Scholar]
- 21.Dalton GD, Howlett AC. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br J Pharmacol. 2012;165(8):2497–2511. doi: 10.1111/j.1476-5381.2011.01455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102(4):1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x [DOI] [PubMed] [Google Scholar]
- 23.Sanchez MG, Ruiz-Llorente L, Sanchez AM, Diaz-Laviada I. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal. 2003;15(9):851–859. doi: 10.1016/s0898-6568(03)00036-6 [DOI] [PubMed] [Google Scholar]
- 24.Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78(6):549–563. doi: 10.1016/j.lfs.2005.05.055 [DOI] [PubMed] [Google Scholar]
- 25.Khan MI, Sobocinska AA, Czarnecka AM, Krol M, Botta B, Szczylik C. The therapeutic aspects of the endocannabinoid system (ECS) for Cancer and their development: from nature to laboratory. Curr Pharm Des. 2016;22(12):1756–1766. doi: 10.2174/1381612822666151211094901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlebnik NE, Cheer JF. Beyond the CB1 receptor: is cannabidiol the answer for disorders of motivation? Annu Rev Neurosci. 2016;39:1–17. doi: 10.1146/annurev-neuro-070815-014038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouron A. Phyto and endocannabinoids exert complex actions on calcium and zinc signaling in mouse cortical neurons. Biochem Pharmacol. 2018;152:244–251. doi: 10.1016/j.bcp.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 29.Juknat A, Rimmerman N, Levy R, Vogel Z, Kozela E. Cannabidiol affects the expression of genes involved in zinc homeostasis in BV-2 microglial cells. Neurochem Int. 2012;61(6):923–930. doi: 10.1016/j.neuint.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Kaplan BL, Springs AE, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol. 2008;76(6):726–737. doi: 10.1016/j.bcp.2008.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Filippis D, Iuvone T, d’amico A, et al. Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol Motil. 2008;20(8):919–927. doi: 10.1111/j.1365-2982.2008.01114.x [DOI] [PubMed] [Google Scholar]
- 32.Pazos MR, Mohammed N, Lafuente H, et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology. 2013;71:282–291. doi: 10.1016/j.neuropharm.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 33.Assas BM, Miyan JA, Pennock JL. Cross-talk between neural and immune receptors provides a potential mechanism of homeostatic regulation in the gut mucosa. Mucosal Immunol. 2014;7(6):1283–1289. doi: 10.1038/mi.2014.80 [DOI] [PubMed] [Google Scholar]
- 34.Huang WX, Yu F, Sanchez RM, et al. TRPV1 promotes repetitive febrile seizures by pro-inflammatory cytokines in immature brain. Brain Behav Immun. 2015;48:68–77. doi: 10.1016/j.bbi.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 35.Kong WL, Peng YY, Peng BW. Modulation of neuroinflammation: role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain Behav Immun. 2017;64:354–366. doi: 10.1016/j.bbi.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 36.Elokely K, Velisetty P, Delemotte L, et al. Understanding TRPV1 activation by ligands: insights from the binding modes of capsaicin and resiniferatoxin. Proc Natl Acad Sci U S A. 2016;113(2):E137–45. doi: 10.1073/pnas.1517288113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang XX, Liu GY, Lei H, Li ZL, Feng QP, Huang W. Activation of transient receptor potential vanilloid 1 protects the heart against apoptosis in ischemia/reperfusion injury through upregulating the PI3K/Akt signaling pathway. Int J Mol Med. 2018;41(3):1724–1730. doi: 10.3892/ijmm.2017.3338 [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Yang Y, Yang H, et al. NF-kappaB feedback control of JNK1 activation modulates TRPV1-induced increases in IL-6 and IL-8 release by human corneal epithelial cells. Mol Vis. 2011;17:3137–3146. [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XL, Wang X, Shao L, et al. TRPV1 mediates astrocyte activation and interleukin-1beta release induced by hypoxic ischemia (HI). J Neuroinflammation. 2019;16(1):114. doi: 10.1186/s12974-019-1487-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du Q, Liao Q, Chen C, Yang X, Xie R, Xu J. The role of transient receptor potential vanilloid 1 in common diseases of the digestive tract and the cardiovascular and respiratory system. Front Physiol. 2019;10:1064. doi: 10.3389/fphys.2019.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowin T, Straub RH. Cannabinoid-based drugs targeting CB1 and TRPV1, the sympathetic nervous system, and arthritis. Arthritis Res Ther. 2015;17:226. doi: 10.1186/s13075-015-0743-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gouin O, L’Herondelle K, Lebonvallet N, et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8(9):644–661. doi: 10.1007/s13238-017-0395-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6(4):e18281. doi: 10.1371/journal.pone.0018281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19(7):433–447doi: 10.1038/s41577-019-0147-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143(2):247–250. doi: 10.1038/sj.bjp.0705920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassan S, Eldeeb K, Millns PJ, Bennett AJ, Alexander SP, Kendall DA. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br J Pharmacol. 2014;171(9):2426–2439. doi: 10.1111/bph.12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fonseca BM, Correia-da-Silva G, Teixeira NA. Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis. J Physiol Biochem. 2018;74(2):261–272.;. [DOI] [PubMed] [Google Scholar]
- 48.Harrison DA. The Jak/STAT pathway. Cold Spring Harb Perspect Biol. 2012;4(3):a011205–a011205. doi: 10.1101/cshperspect.a011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106(42):17841–17846. doi: 10.1073/pnas.0905006106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldmann TA, Chen J. Disorders of the JAK/STAT Pathway in T Cell Lymphoma Pathogenesis: implications for Immunotherapy. Annu Rev Immunol. 2017;35:533–550. doi: 10.1146/annurev-immunol-110416-120628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bousoik E, Montazeri Aliabadi H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front Oncol. 2018;8:287. doi: 10.3389/fonc.2018.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462(1):1–13. doi: 10.1042/BJ20140712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194(1):21–27. doi: 10.4049/jimmunol.1401867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455 [DOI] [PubMed] [Google Scholar]
- 55.Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11(1):18–26. doi: 10.1124/mi.11.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–746. doi: 10.1038/nrc3818 [DOI] [PubMed] [Google Scholar]
- 57.Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845(2):136–154. doi: 10.1016/j.bbcan.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 58.Chiricosta L, Silvestro S, Pizzicannella J, et al. Transcriptomic analysis of stem cells treated with moringin or cannabidiol: analogies and differences in inflammation pathways. Int J Mol Sci. 2019;20(23):6039. doi: 10.3390/ijms20236039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murakami M, Kamimura D, Hirano T. Pleiotropy and Specificity: insights from the Interleukin 6 Family of Cytokines. Immunity. 2019;50(4):812–831. doi: 10.1016/j.immuni.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 60.Kaplan BL, Rockwell CE, Kaminski NE. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther. 2003;306(3):1077–1085. doi: 10.1124/jpet.103.051961 [DOI] [PubMed] [Google Scholar]
- 61.Verma R, Balakrishnan L, Sharma K, et al. A network map of Interleukin-10 signaling pathway. J Cell Commun Signal. 2016;10(1):61–67. doi: 10.1007/s12079-015-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava MD, Srivastava BI, Brouhard B. Delta9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology. 1998;40(3):179–185. doi: 10.1016/S0162-3109(98)00041-1 [DOI] [PubMed] [Google Scholar]
- 63.Miscia S, Marchisio M, Grilli A, et al. Tumor necrosis factor alpha (TNF-alpha) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 2002;13(1):13–18. [PubMed] [Google Scholar]
- 64.Horvath CM. The Jak-STAT pathway stimulated by interferon gamma. Sci STKE. 2004;2004(260):tr8. doi: 10.1126/stke.2602004tr8 [DOI] [PubMed] [Google Scholar]
- 65.Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97(17):9561–9566. doi: 10.1073/pnas.160105897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zgair A, Lee JB, Wong JCM, et al. Oral administration of cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci Rep. 2017;7(1):14542. doi: 10.1038/s41598-017-15026-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burrack AL, Martinov T, Fife BT. T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol (Lausanne). 2017;8:343. doi: 10.3389/fendo.2017.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiss L, Zeira M, Reich S, Slavin S, Raz I, Mechoulam R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54(1):244–249. doi: 10.1016/j.neuropharm.2007.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiss L, Zeira M, Reich S, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39(2):143–151. doi: 10.1080/08916930500356674 [DOI] [PubMed] [Google Scholar]
- 70.Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol–recent advances. Chem Biodivers. 2007;4(8):1678–1692. doi: 10.1002/cbdv.200790147 [DOI] [PubMed] [Google Scholar]
- 71.Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem. 2010;285(3):1616–1626. doi: 10.1074/jbc.M109.069294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–215. doi: 10.1038/nri2725 [DOI] [PubMed] [Google Scholar]
- 73.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 74.Ruland J. Inflammasome: putting the pieces together. Cell. 2014;156(6):1127–1129. doi: 10.1016/j.cell.2014.02.038 [DOI] [PubMed] [Google Scholar]
- 75.Kolly L, Busso N, Palmer G, Talabot-Ayer D, Chobaz V, So A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010;129(2):178–185. doi: 10.1111/j.1365-2567.2009.03174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Libro R, Scionti D, Diomede F, et al. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front Physiol. 2016;7:559. doi: 10.3389/fphys.2016.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu C, Ma H, Slitt AL, Seeram NP. Inhibitory effect of cannabidiol on the activation of nlrp3 inflammasome is associated with its modulation of the p2x7 receptor in human monocytes. J Nat Prod. 2020. doi: 10.1021/acs.jnatprod.0c00138 [DOI] [PubMed] [Google Scholar]
- 78.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25(1):33–39. doi: 10.1016/j.it.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 79.Noji T, Karasawa A, Kusaka H. Adenosine uptake inhibitors. Eur J Pharmacol. 2004;495(1):1–16. doi: 10.1016/j.ejphar.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 80.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98(3):1591–1625. doi: 10.1152/physrev.00049.2017 [DOI] [PubMed] [Google Scholar]
- 81.Cronstein BN, Sitkovsky M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13(1):41–51. doi: 10.1038/nrrheum.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247–264. doi: 10.1038/nrd1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pastor-Anglada M, Perez-Torras S. Who is who in adenosine transport. Front Pharmacol. 2018;9:627. doi: 10.3389/fphar.2018.00627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets–what are the challenges? Nat Rev Drug Discov. 2013;12(4):265–286. doi: 10.1038/nrd3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ibeas Bih C, Chen T, Nunn AV, et al. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics. 2015;12(4):699–730. doi: 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103(20):7895–7900. doi: 10.1073/pnas.0511232103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liou GI, Auchampach JA, Hillard CJ, et al. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci. 2008;49(12):5526–5531. doi: 10.1167/iovs.08-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noji T, Takayama M, Mizutani M, et al. KF24345, an adenosine uptake inhibitor, suppresses lipopolysaccharide-induced tumor necrosis factor-alpha production and leukopenia via endogenous adenosine in mice. J Pharmacol Exp Ther. 2002;300(1):200–205. doi: 10.1124/jpet.300.1.200 [DOI] [PubMed] [Google Scholar]
- 89.Mecha M, Feliu A, Inigo PM, et al. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141–150. doi: 10.1016/j.nbd.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 90.Owen JL, Criscitiello MF, Libreros S, et al. Expression of the inflammatory chemokines CCL2, CCL5 and CXCL2 and the receptors CCR1-3 and CXCR2 in T lymphocytes from mammary tumor-bearing mice. Cell Immunol. 2011;270(2):172–182. doi: 10.1016/j.cellimm.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]