Key Points
Question
Is there an association between Notch homolog 2 N-terminal-like C (NOTCH2NLC) GGC repeats and Parkinson disease (PD)?
Findings
A genetic screening of NOTCH2NLC GGC repeat expansion was carried out in a cohort of 1000 patients with sporadic PD and 1076 healthy control participants. A total of 13 patients with PD were identified as carrying NOTCH2NLC GGC repeat expansions greater than 40 units, and no repeat expansions were detected in healthy control participants (P < .001).
Meaning
Per this analysis, individuals with sporadic PD who carried pathogenic NOTCH2NLC GGC repeat expansions (>79 repeats) can present with typical PD, requiring low levodopa dosages, with no other clinical or imaging features of neuronal intranuclear inclusion body disease even after several years of follow-up.
This case-control study investigates if NOTCH2NLC GGC expansions are associated with Parkinson disease in a cohort of affected individuals and healthy control participants.
Abstract
Importance
The presence of Notch homolog 2 N-terminal-like C (NOTCH2NLC) repeat expansions are associated with neuronal intranuclear inclusion body disease (NIID), with varied neurological signs, including neuropathy, ataxia, parkinsonism, and tremor. To date, genetic screening of NOTCH2NLC GGC repeats in a cohort with typical Parkinson disease (PD) appears not to have been reported.
Objective
To investigate if NOTCH2NLC GGC expansions are present in a cohort of patients with PD and controls.
Design, Setting, and Participants
This case-control study was conducted in 2 tertiary movement disorder centers in Singapore. Participants were recruited and followed up from January 2005 to January 2020. The presence of NOTCH2NLC GGC expansion repeats was screened using polymerase chain reaction tests, and representative samples were verified with long-read genome sequencing.
Main Outcomes and Measures
Qualitative and quantitative comparisons between participants with sporadic PD, healthy control participants, and individuals with NIID.
Results
A total of 2076 participants, including 1000 with sporadic PD (600 men [60.0%]; mean age at onset, 62.6 [7.7] years) and 1076 healthy controls (581 men [54.0%]; mean age at study recruitment, 54.9 [9.4] years) were recruited. A total of 13 patients with PD and no healthy control participants were identified as carrying NOTCH2NLC GGC repeat expansions of more than 40 units; the frequency of more than 40 repeat expansions was higher in participants with PD than controls (P < .001). None of the patients with PD were carriers of known PD-associated genes. Ten patients with PD carried a GGC expansion of between 41 and 64 repeats (1% of patients with sporadic PD; mean [SD], 49.4 [9.2] repeats). The other 3 patients carried GGC repeats of 79 or more units, 2 with 122 and 79 repeats, respectively, exhibited typical parkinsonism and were responsive to small dosages of levodopa over many years, with no clinical or imaging features of NIID. The other patient with PD, who had 130 repeats, only developed cognitive impairment before death. Within the GGC expansions, there was no GGA interruptions (mean [SD] GGA percentage in the 3 patients with PD vs patients with NIID, 0% vs 12% [9%]), and the frequency of AGC interruptions was 3 times higher in these patients with PD than patients with NIID (mean [SD], 25% [12%] vs 8% [8%]).
Conclusions and Relevance
This study demonstrated that individuals with sporadic PD who carried pathogenic NOTCH2NLC GGC repeat expansions can present with typical parkinsonism, requiring only low dosages of levodopa, without displaying other clinical or imaging features of NIID even after several years of follow-up. None of the patients with PD had GGA interruptions within their GGC expansions, and the frequency of AGC interruptions was much higher than that of patients with NIID. The functional significance of a higher moderate repeat expansion in patients with PD compared with healthy controls needs to be further investigated.
Introduction
The presence of Notch homolog 2 N-terminal-like C (NOTCH2NLC) GGC expansion causes neuronal intranuclear inclusion body disease (NIID), a neurodegenerative condition characterized by eosinophilic intranuclear inclusions in neuronal and glial cells.1,2,3 Large GGC repeat numbers, ranging from 66 to 517 units, have been reported in patients affected by NIID, while control participants had fewer than 40 repeats (5-39).1,2,3,4 Thus far, to our knowledge, there have been no studies reporting intermediate-length (40-60) NOTCH2NLC GGC expansion in any human neurological disease. Patients with NIID have overlapping clinical features of dementia, ataxia, parkinsonism, and peripheral neuropathy.4,5,6 While parkinsonism is part of the spectrum of the clinical phenotype and may be a predominant sign in some patients with NIID and a family history, it is unclear if NOTCH2NLC GGC expansions are associated with typical Parkinson disease (PD)7 with good levodopa response and without other neurological signs. To date, to our knowledge, NOTCH2NLC GGC repeat expansions have not been reported in patients with PD, even though participants with parkinsonism plus other signs have been reported.4,5 To address these gaps in knowledge, we screened a large PD case-control cohort for GGC repeat expansions.
Methods
Patients with PD were recruited over a period of January 2005 to January 2020 in 2 tertiary movement disorder clinics in Singapore and diagnosed according to UK Brain Bank criteria for PD. Exclusion criteria included those with PD plus syndromes and those with atypical parkinsonism with a poor levodopa response. Biochemical tests and neuroimaging were performed to exclude secondary causes of parkinsonism. Healthy control participants without any neurological diseases and signs were also recruited. Ethical approval was obtained from the Singapore Health Services Centralised Institutional Review Board, and all patients provided written informed consent. The detailed protocol of repeat-primed amplification refractory mutation system polymerase chain reaction (repeat-primed PCR), fluorescence amplification refractory mutation system PCR, and long-read sequencing is provided in the eAppendix in the Supplement. Details of long-read sequencing, such as depth of sequencing, number of reads, and read length, are provided in the eTable in the Supplement. Statistical analysis was performed using SPSS version 23 (IBM). A P ≤ .05 was considered statistically significant.
Results
A total of 2076 participants, including 1000 with sporadic PD (600 men [60.0%]; mean [SD] age at onset, 62.6 [7.7] years) and 1076 healthy control participants (581 men [54.0%]; mean age at study recruitment, 52.9 [9.4] years) were recruited (a success rate of >90%). Approximately 90% of participants were ethnically Chinese, and the remainder were of mixed Chinese or other Asian ethnicities. All patients and control participants were screened for NOTCH2NCL GGC repeats using repeat-primed PCR, after which 10 patients (3 with positive expansions and 7 with 41-64 repeats) were sent for confirmation using long-read sequencing. We identified 13 patients with PD carrying NOTCH2NLC GGC repeat expansions of more than 40 units from the 1000 individuals with sporadic PD (summary in the Table), while all 1076 healthy control participants had fewer than 40 repeats, which was compatible with previous literature.1,2,3,4 Of the 13 patients with PD, 10 carried a GGC expansion of between 41 and 64 repeats (1% of those with sporadic PD; mean [SD], 49.4 [9.2] repeats). The frequency of more than 40 repeat expansions in individuals with PD was higher than in control participants (P < .001). None of the patients with PD were carriers of known PD-associated genes.
Table. Clinical Features of Patients With Parkinson Disease and Notch Homolog 2 N-Terminal-Like C (NOTCH2NLC) Repeat Expansions Greater Than 40a.
| Patient No. | Tremor-dominant PD | Age, decade | Age at onset, decade | Imaging results | Dementia | Peripheral neuropathy | NOTCH2NLC repeat size | No. (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GGC | GGA | AGC | Others | ||||||||
| 1 | Yes | 60s | 50s | MRI: normal | No | No | 122 | 102 (79.7) | 0 | 20 (15.6) | 6 (4.7) |
| 2 | Yes | 80s | 70s | MRI: periventricular ischemia, mild atrophy | No | No | 79 | 66 (83.5) | 0 | 13 (16.5) | 0 |
| 3 | No | 80s | 60s | CT: mild cerebral atrophy | Developed cognitive impairment in late 80s | No | 130 | 75 (57.7) | 0 | 55 (42.3) | 0 |
| 4 | Yes | 80s | 70s | MRI: subperiventricular ischemia | No | No | 64 | 61 (95.3) | 2 (1.6) | 1 (3.1) | 0 |
| 5 | Yes | 80s | 70s | No neuroimaging done | No | No | 62 | 62 (100) | 0 | 0 | 0 |
| 6 | Yes | 60s | 50s | No neuroimaging done | No | No | 60 | 59 (98.3) | 1 (1.7) | 0 | 0 |
| 7 | No | 60s | 50s | MRI: normal | No | No | 49 | 47 (95.9) | 2 (4.1) | 0 | 0 |
| 8 | Yes | 80s | 30s | CT: lacunar infarct in external capsule | No | No | 47 | NA | NA | NA | NA |
| 9 | No | 70s | 60s | CT: subcortical and periventricular ischemia | No | Yes (associated with diabetes) | 41 | 40 (97.5) | 1 (2.5) | 0 | 0 |
| 10 | No | 70s | 60s | MRI: periventricular ischemia | No | No | 47 | 45 (95.7) | 2 (4.3) | 0 | 0 |
| 11 | No | 60s | 60s | No neuroimaging done | No | No | 42 | NA | NA | NA | NA |
| 12 | No | 70s | 60s | CT: normal | No | No | 41 | 39 (92.9) | 2 (2.4) | 1 (4.8) | 0 |
| 13 | Yes | 70s | 70s | CT: chronic microvascular ischemia | No | No | 41 | NA | NA | NA | NA |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; NA, not available; PD, Parkinson disease.
All patients were ethnically Chinese and had no family history of Parkinson disease. They did not show any sign of ataxia or other complications, such as motor fluctuations or dyskinesias.
Two patients with PD with GGC repeat expansions of 122 and 79 repeats (0.2% of those with PD; Figure 1 and Figure 2) exhibited a typical PD phenotype, received low doses of levodopa for several years with good response, and had no other clinical or imaging features of NIID. The other patient with PD had 130 repeats and only developed cognitive impairment shortly before death. Within the GGC expansions, there was no GGA interruption (mean [SD] GGA percentage in the 3 patients with PD vs patients with NIID, 0% vs 12% [9%]), and the frequency of AGC interruptions was higher in these 3 patients with PD compared with in patients with NIID (mean [SD], 25% [12%] vs 8% [8%]).6
Figure 1. An Integrative Genomics Viewer Snapshot, Showing Long-Read Sequencing Reads Overlapping the Notch Homolog 2 N-Terminal-Like C (NOTCH2NLC) 5′ Region for Patient 2 and a Negative Control Sample.
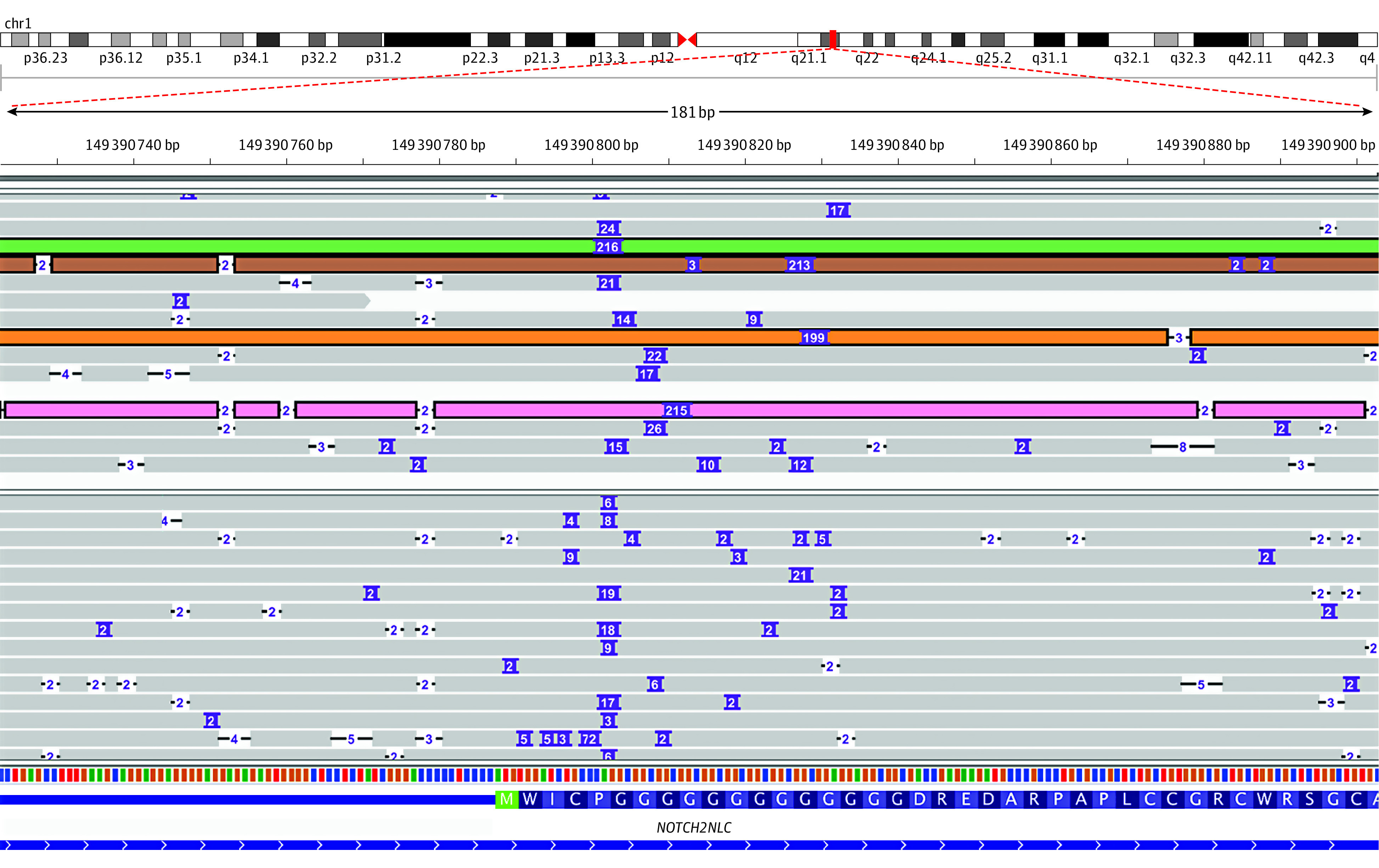
Purple boxes denote insertions, with the number within indicating insertion size. Reads harboring long (>141–base pair) insertions are highlighted.
Figure 2. Representative Electropherogram of the Repeat-Primed Polymerase Chain Reaction Showing Abnormal Notch Homolog 2 N-Terminal-Like C (NOTCH2NLC) GGC Repeat Expansions.
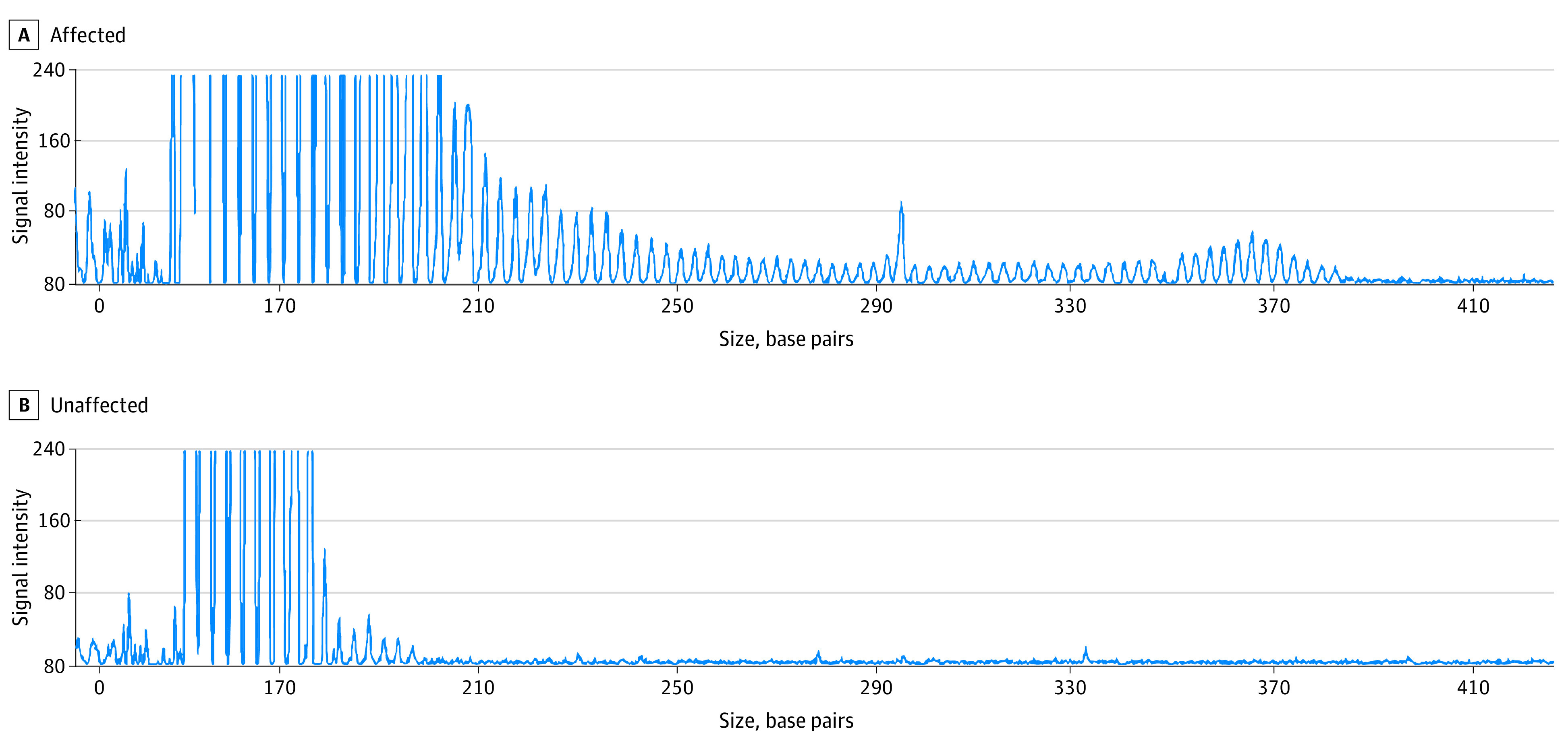
A, Sawtooth tail pattern of the repeat expansion in patient 2, with 79 repeats. B, Sixteen repeats in an unaffected individual.
Patient 1 was older than 60 years and had presented with rest tremor approximately 1 decade earlier, which was associated with mild bradykinesia and rigidity consistent with tremor-dominant PD. The patient had no cognitive dysfunction, ataxia, or peripheral neuropathy over a 9-year period. A magnetic resonance image of the brain did not have remarkable results. Genetic testing revealed a GGC expansion of 122 repeats. The patient’s parkinsonism progressed, and the patient responded well to low doses of levodopa and was functionally independent, with no motor fluctuation or levodopa complications at the last follow-up.
Patient 2 was in their late 80s and had presented about 15 years earlier with rest tremor, rigidity, and bradykinesia, with no cognitive dysfunction or other neurological signs. This individual was diagnosed with tremor-dominant PD and responded to small doses of levodopa (150 mg/d). A magnetic resonance image of the brain showed mild atrophy and periventricular ischemia, and repeated imaging a few years later did not show progression of atrophy. The patient remained stable and received follow-up for several years before dying of causes not associated with PD.
Patient 3 presented in their late 60s with rigidity and bradykinesia but no ataxia, memory loss, or neuropathy. The patient had no family history of PD and responded well to levodopa (150-300 mg) for several years with no drug complications. The patient also had multiple medical problems, including cardiac and gastrointestinal diseases. The patient developed cognitive impairment more than 10 years after PD diagnosis, and this was attributed to PD. A computed tomography brain scan showed mild cerebral atrophy with no other lesions. The patient died of other medical conditions in their late 80s.
Discussion
To our knowledge, this is the first study reporting 2 patients with PD with GGC repeat expansions of 122 and 79 repeats. The subgroup of patients with PD showed significantly higher repeat expansions compared with control participants, but the pathogenicity of the 40-to-64-repeat range still needs to be further investigated, because clinical NIID cases were reported to have more than 65 repeats. It is possible that expansions of 41 to 64 repeats constitute a genetic risk for PD or represent a different association. Whether such intermediate repeat range represents an endophenotype with the potential for subsequent clinical manifestations remains speculative. In addition to GGC repeats, there are 2 forms of repeated interruptions, GGA and AGC, within the repeat expansions in patients with PD and NIID. It is interesting to highlight that all 3 patients with PD and long GGC expansions did not carry any GGA interruption within the GGC expansion, compared with a reported 12% frequency in NIID cases.6 In addition, the frequency (25%) of AGC interruptions was also more than 3 times higher than that seen in NIID. This has been demonstrated in other repeat-expansion diseases, such as spinocerebellar ataxia type 1, in which CAT trinucleotide interruptions can reduce the development of slipped strand structures, which may be protective against the formation of abnormal expansions,8 whereas for expansions without interruptions in spinocerebellar ataxia type 10, a negative correlation between age at onset and size of expansion has been reported.9 In spinocerebellar ataxia 2, smaller CAG repeat sizes have been reported in patients (which was more common among individuals of Chinese and certain other races/ethnicities) with a pure parkinsonian phenotype compared with an ataxia phenotype, with some having interruptions within CAG repeat expansions.10
Limitations
It is possible that the absence of GGA interruptions in the 3 patients with PD in this study may have a role in either affecting the stability of the expansion or influence phenotypic expression through unknown factors. Studies in cohorts of other ethnicities may be useful to corroborate our finding.
Conclusions
Our study suggests that, while the condition is uncommon, those who carry NOTCH2NLC GGC repeat expansions can present with typical sporadic PD with no other clinical or imaging features of NIID, even after several years of follow-up. The higher frequency of GGC expansions of 41 to 64 repeats in patients with PD compared with controls, and the absence of GGA interruptions and higher AGC interruptions in patients with PD and expanded repeats compared with patients with NIID needs to be evaluated with further longitudinal clinical and functional studies.
eAppendix. Supplementary Methods.
eReferences.
eTable. Details on LRS sequencing on patients with long and intermediate repeat expansions.
References
- 1.Ishiura H, Shibata S, Yoshimura J, et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat Genet. 2019;51(8):1222-1232. doi: 10.1038/s41588-019-0458-z [DOI] [PubMed] [Google Scholar]
- 2.Sone J, Mitsuhashi S, Fujita A, et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51(8):1215-1221. doi: 10.1038/s41588-019-0459-y [DOI] [PubMed] [Google Scholar]
- 3.Deng J, Gu M, Miao Y, et al. Long-read sequencing identified repeat expansions in the 5'UTR of the NOTCH2NLC gene from Chinese patients with neuronal intranuclear inclusion disease. J Med Genet. 2019;56(11):758-764. doi: 10.1136/jmedgenet-2019-106268 [DOI] [PubMed] [Google Scholar]
- 4.Tian Y, Wang JL, Huang W, et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet. 2019;105(1):166-176. doi: 10.1016/j.ajhg.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okubo M, Doi H, Fukai R, et al. GGC repeat expansion of NOTCH2NLC in adult patients with leukoencephalopathy. Ann Neurol. 2019;86(6):962-968. doi: 10.1002/ana.25586 [DOI] [PubMed] [Google Scholar]
- 6.Sun QY, Xu Q, Tian Y, et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain. 2020;143(1):222-233. doi: 10.1093/brain/awz372 [DOI] [PubMed] [Google Scholar]
- 7.Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71(4):499-504. doi: 10.1001/jamaneurol.2013.6233 [DOI] [PubMed] [Google Scholar]
- 8.Kraus-Perrotta C, Lagalwar S. Expansion, mosaicism and interruption: mechanisms of the CAG repeat mutation in spinocerebellar ataxia type 1. Cerebellum Ataxias. 2016;3:20. doi: 10.1186/s40673-016-0058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland KN, Liu J, Landrian I, et al. Paradoxical effects of repeat interruptions on spinocerebellar ataxia type 10 expansions and repeat instability. Eur J Hum Genet. 2013;21(11):1272-1276. doi: 10.1038/ejhg.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu CS, Wu Chou YH, Kuo PC, Chang HC, Weng YH. The parkinsonian phenotype of spinocerebellar ataxia type 2. Arch Neurol. 2004;61(1):35-38. doi: 10.1001/archneur.61.1.35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplementary Methods.
eReferences.
eTable. Details on LRS sequencing on patients with long and intermediate repeat expansions.


