Abstract
The treatment of leg telangiectasias could be done with liquid sclerotherapy or Nd:YAG laser. We evaluated randomly, in a simple blind, the efficacy of the treatment with 0,5% polidocanol (POL-0,5), 1% polidocanol (POL-1) and Nd:YAG laser (LAS) on 132 patient (264 limbs) with telangiectasia of the lower limbs with less than 2mm diameter (C1EAP). The main objective was to evaluate the efficacy of the sclerotherapy (chemical compared with Nd:YAG-LAS). Secondary objectives were: possible major complications (deep thrombosis, severe burns, ischemic complications, etc.), the percentage of the local complications, the cosmetic aspect-evaluated by both the patient and the doctor and the grade of discomfort of the patient during and after the procedure. Comparing the treatment with Nd:YAG laser (LAS), polidecanol-0.5% (POL-0.5), polidecanol 1% (POL-1), it was noticed that telangiectasias smaller than 1mm led to good and very good results in all the cases treated with Nd:YAG laser and the same outcome was obtained in one third of the subjects treated with POL-0.5, vs. 47.81% of patients treated with POL-1. When telangiectasias were larger than 1mm diameter, good and very good results occurred in 86.36% of patients treated with LAS and 100% of the cases treated with POL-0.5 and POL-1. In conclusion, we consider that leg telangiectasias can be treated with good results using Nd:YAG laser or sclerotherapy with polidocanol, Nd:YAG laser being reccomended for telangiectasia under than 1 mm diameter while sclerotherapy in larger vessels.
Keywords: Sclerotherapy, Nd:Yag laser, telangiectasia, polidocanol
Introduction
Chronic venous disease (CVD) of the lower limbs is a condition that affects up to 30% of the population [1]; current studies [2] revealing that a proactive diagnostic system can detect this condition in almost 2/3 of the population.
During the time, the definition of this disease has changed [3,4] in order to allow both the staging and the standardization of the applied treatment.
Currently, CEAP classification is mostly used by the specialists although there are numerous improvement suggestions.
Practically, the chronic venous disease of the lower limbs is actually considered as a general expression of the disease regardless of its stages of evolution (C1-C6)-this term targets both the morphological and functional modifications, the prolonged evolution comprising the signs and symptoms and underlying the need for a diagnosis and a treatment [5].
On the other hand, the chronic venous insufficiency of the lower limbs is seen as a venous disease in advanced stage (C3=C6)-the term fits the functional anomalies of the venous system [6].
The treatment of the chronic venous disease of the lower limbs depends on, as we previously said, the staging, existing effective methods for it such as liquid sclerotherapy and Nd:YAG laser for the telangiectatic vessels.
Sclerotherapy of the varicose veins of the lower limbs is a technique that involves the chemical alteration of the venous wall (endothelium but also some surrounding structures) due to the injection of a specific chemical solution [7,8].
The solution produces the sclerosis of the blood vessel, phenomenon that succeeds after the destruction of the endothelium.
Despite the fact the purpose of this procedure is not thrombosis, a certain percentage of the sclerotic vessels are affected by this process. The resulted thrombus can recanalize later.
The sclerotic vein enters a remodelation process which, in time, over years, will lead to its resorption.
The laser targets the skin by transferring the light energy into thermic energy that will heat some cutaneous structures named chromophores.
These absorb differently the light according to the wavelengths.
The Nd:YAG with 1064-nm neodymium yttrium-aluminum-garnet laser, acts mainly on the hemoglobin which it heats abruptly leading to the destruction of the vascular wall.
The effect is almost immediate, the damage of the endothelium and the surrounding tissues being followed by the vascular sclerosis and later by the remodelation, same like in the sclerotherapy process.
In this study we evaluated randomly, simple blind, the efficacy and the compliance of the treatment (evaluated by the degree of discomfort of the patients) of the patients with chronic venous disease of the lower limbs C1EAP that underwent sclerotherapy with 0,5%, 1% polidocanol and Nd:YAG laser.
Material and Methods
We randomised during October 2017 and March 2018, 132 patient (264 limbs) with telangiectasia of the lower limbs with less than 2mm diameter (C1EAP) for the treatment of the venectasias with 0,5% and 1% polidocanol and Nd:YAG laser.
The study was performed in the Medical Center Dr. Ianosi in Craiova, Romania and each patient signed the informed consent.
Also the study was approved by the ethical committee of the Medical Center according to (no. 411/24.07.2017) the World Medical Association Declaration of Helsinki.
To exclude most of the biases due to the demographic data of the study groups, we performed a different procedure on each lower limb of the patient.
Therefore, were randomly 3 groups of 88 lower limbs: first group treated with sclerotherapy with 0,5% polidocanol (POL-0,5-Aetoxysklerol-Kreussler, Germany), second group with 1% polidocanol (POL-1,) and the third with laser (LAS-StarLux 500; Palomar Technologies, Carlsbad, CA,. USA) platform having a 3mm-wide spot, a pulse length of 30 msec, and the fluency of 290-350 J/cm2.
The treatment was performed by the same doctor and consisted of the injection of 0,5% or 1% liquid polidocanol or laser therapy.
For each group there were two identical sessions at 60 days interval, and no patient underwent a combine treatment.
O photo of the area that was treated was taken before every session in order to compare the results obtained after 60 days and 120 days by another doctor that was not aware of the type of the treatment that was done (randomized, simple-blind).
This physician evaluated the decreasing of the telangiectatic vessels, the patient satisfaction and the posible complications using a scale with 5 degrees: 0 (failure-no change), 1 (limited/modest-1-25% cleared), 2 (moderate-25-50% cleared), 3 (good-51-75% cleared), and 4 (very good-75-100% cleared).
The inclusion criteria were patients over 18 years with less than 2mm diameter telangiectasia of the lower limbs (C1AEpAS1PN) as only complaint and who agreed to participate in this trial therefore signing the informed consent form.
Exclusions criteria were the following:
-patients with symptomatic telangiectasia (C1S);
-patients having clear-cut visible superficial venous reflux on Doppler ultrasound imaging at the level of the lower limbs;
-patients having established deep venous thrombosis or with post thrombotic syndrome (ES);
-pregnant patients, or during breastfeeding;
-patients with cancers, significant health problems or taking chronic medication for different pathology.
The main objective was to evaluate the efficacy of the sclerotherapy with polidocanol 0.5% (POL-0.5) and polidocanol 1% (POL-1) versus Nd:YAG laser (LAS).
Secondary objectives were:
-possible major complications (deep thrombosis, severe burns, ischemic complications, etc.)
-the level of the local complications;
We used a Microsoft Excel (Microsoft Corporation, USA) sheet to include all data.
These included transversal and longitudinal data statistical analysis. In these studies, we expressed proportions as percentages and numerical variables as mean and/or standard deviation.
To compare a homogeneous group of patients we used treatment effects statistical analysis in order to prove causality hypothesis.
The longitudinal data analysis was time-to-event analysis, calculation of incidence rates and statistical modeling.
The conditional distribution of the outcome variable given the random effects is assumed to be multinomial with success probability determined by the logistic cumulative distribution function.
In comparison with the biological systems that have unpredictable results, the model can predict a better chance of success of each type of treatment compared with usual regression models applied transversely ignoring the time factor.
The failure variable for survival analysis was represented by the good and very good results and the time to event measured in full months from the start of the treatment.
The hazard ratios (recognized as risk ratio in terms of the transverse analysis) can be useful to measure the favorable outcomes rates in time.
In these conditions all the patients can make informed decisions taking into account the time needed and the final results of the treatment.
Results
Initially, there were evaluated a total of 132 patients but only 124 patients (248 lower limbs) finished the study, meaning they underwent the both scheduled meetings (after a period of 60 days and also after 120 days). 248 lower limbs were distributed as follow:
-83 lower limbs treated only with POL-0,5 (first group);
-82 lower limbs treated only with POL-1 (second group);
-83 lower limbs were treated only with ND:YAG laser (third group).
Between all 83 patients from first group POL-0.5 there were 33(39.76%) very good results and 22 (26.50%) good results.
Very good results were encountered in 35 (42.68%) of 82 the patients from group POL-1 (second group) and good results in 23 (28.04%) patients. (Table 1a and 1b)
Table 1a.
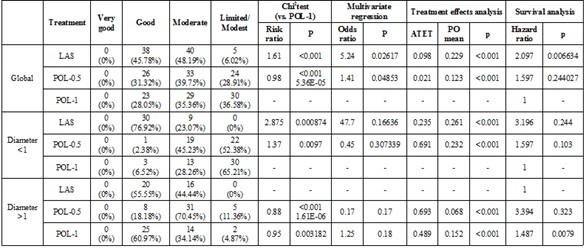
Statistical data for the treatment of telangiectasias-60 days
Table 1b.
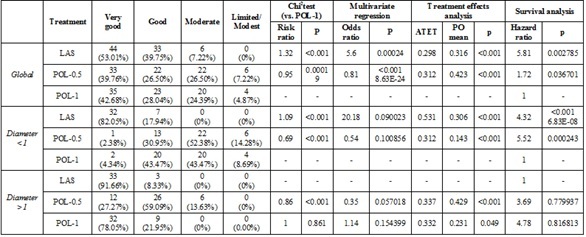
Statistical data for the treatment of telangiectasias-120 days
Looking for ND: YAG LASER patients (third group), we obtained very good results in 44 (53.01%) patients and good results in 33 (39.75%) patients.
Considering ND:YAG LASER vs. POL-1, it was noticed that for telangiectasias less than 1mm the good/very good results occurred in 100% of patients treated with laser, vs 47.81% of patients treated with POL-1 (Risk Ratio (RR)=1.09, p less than 0.0001).
For telangiectasias larger than 1mm diameter, the good/very good results were noticed in 86.36% of patients treated with ND:YAG LASER, versus 100% of patients treated using POL-1 (RR=0.86, p less than 0.0001).
Comparing POL-0.5 versus POL-1, it was noticed that for under 1mm diameter telangiectasias the good/very good results occurred in 33.33% of patients treated with POL-0.5, versus 47.81% of patients treated using POL-1 (Risk Ratio (RR)=0.69, p<0.0001).
For telangiectasias larger than 1mm, good and very good results occurred in all the patients treated with POL-0.5 and also 100% of patients treated with POL-1 (RR=1, p=0.861). (Table 1a and 1b)
The results of the treatment effect analysis showed that for telangiectasias under 1mm diameter, ND:YAG LASER treatment had a better potential outcome (PO) mean than POL-0.5 treatment (0.531 versus 0.312), whereas in the telangiectasias over 1mm diameter, Pol-0.5 treatment had a higher PO mean than the ND:YAG LASER treatment (0.337 versus 0.332). (Table 1a and 1b)
Multivariate analysis compares the outcome of applying all three types of treatments to the possibility of getting a good result. In telangiectasias smaller than one mm diameter, ND:YAG LASER treatment led to an OR of 20.18 (p=0.09) and POL-0.5 treatment led to an OR of 0.54 (p=0.1) versus POL-1 cure.
In over 1mm diameter telangiectasias, ND:YAG LASER treatment had an OR of 0.35 (p=0.05); the POL-0.5 treatment of 1.14 (p=0.154) compared to POL-1 cure. (Table 1b).
Following, the longitudinal data analysis of the outcome underlined that in the case of smaller telangiectasias, the good/very good results of ND:YAG LASER showed up from the first 60 days in 30 patients (76.92%), and after 120 days in 39 patients (100%), while for the POL-0.5 treatment, good and very good results occurred from the first 60 days in 1 patient (2.44%), and after 120 days in 14 patients (33.33%). POL-1 treatment achieved good results in 3 patients (6.97%) in the first 60 days and 22 patients after 120 days (47.82%). (Table 1a and 1b)
In larger telangiectasias, the good/very good results of POL-0.5 cure showed up from the first 60 days for 8 patients (18.18%), and after 120 days in 38 patients (86.36%).
In the same time, using the POL-1 cure, good/very good results showed up from the first 60 days in 25 patients (60.97%), and after 120 days in 41 patients (100%).
ND: YAG LASER treatment achieved good results in 20 patients (55.55%) in the first 60 days and 36 patients (100%) after 120 days. (Table 2).
Table 2.
The good and very good results in the treatment of telangiectasias
|
|
|
60 days |
120 days |
|
Diameter <1 |
LAS |
30 (76.92%) |
39 (100%) |
|
POL-0.5 |
1 (2.44%) |
14 (33.33%) |
|
|
POL-1 |
3 (6.97%) |
22 (47.82%) |
|
|
Diameter >1 |
LAS |
20 (55.55%) |
36 (100%) |
|
POL-0.5 |
8 (18.18%) |
38 (86.36%) |
|
|
POL-1 |
25 (60.97%) |
41 (100%) |
Using the hazard regression model, a hazard ratio of 4.32 (p<0.001) for laser treatment was an outcome and 5.52 (p=0.000243) for POL-0.5 treatment.
In the case of under one mm telangiectasias, sclerotherapy could be achieved with any of these two concentrations, the results being similar.
Using the hazard regression model, a hazard ratio of 3.69 (p=0.77) for POL-0.5 treatment was an outcome and 4.78 (p=0.81) for POL-1 treatment, vs. ND:YAG LASER treatment.
It can be concluded that for the telangiectasias larger than 1mm diameter we observed a slight superiority of POL-0.5 and POL-1 vs. LAS in terms of achieving good results treatment; the superiority of the POL-0.5 above the POL-1 could not be demonstrated. (Figure 1).
Figure 1.
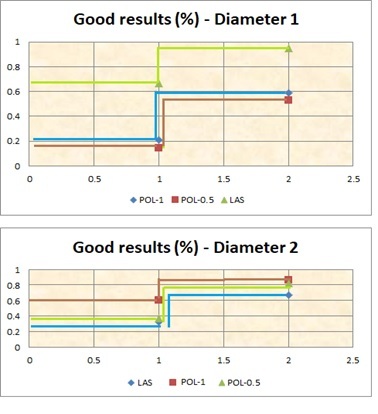
The diagram for the hazard function reflecting the good effect of the 3 cures in the two stages of disease, Diameter <1 (Diameter 1); Diameter >1 (Diameter 2)
In this study, for telangiectasias smaller than one mm, the ND:YAG LASER treatment was the best comparing with sclerotherapy: (for POL-1 p=0.02617 and for POL-0.5 p=0.04853) (Figures 2, 3, 4).
Figure 2.
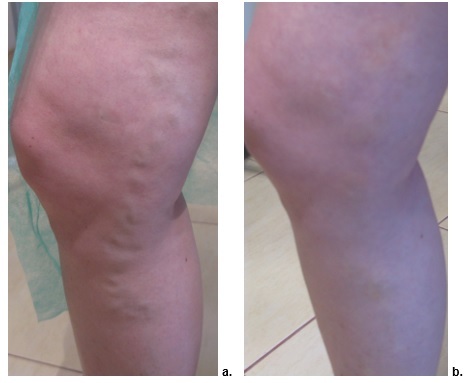
Sclerotherapy with POL-1. a. Day 0, b. Day 60
Figure 3.
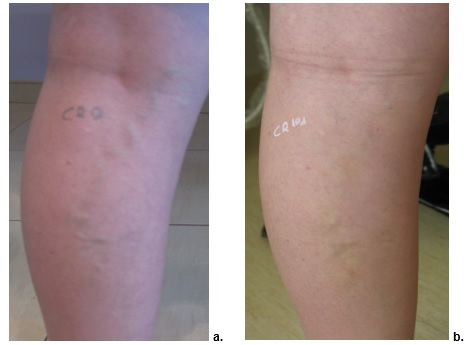
Sclerotherapy with POL-0,5. a. Day 0, b. Day 60
Figure 4.
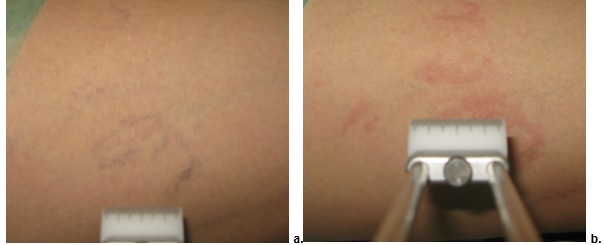
ND:YG LASER treatment. a. Day 0, b. Day 60
At the end of the study, from all 248 limbs enrolled, 190 had obtained good/very good outcome, 48 obtained a modest result and 10 had unsatisfactory result.
In our study, no major complications such as ischemic complications, deep thrombosis or severe burns occurred.
The minor side effects included cutaneous burns appeared in 14 limbs (7 limbs treated with LAS, 3 limbs treated with POL-0,5 and also in 4 limbs treated with POL-1) and hyper pigmentations in 3 limbs (2 limbs from LAS group and 1 limb from POL-1 group), but without any significant statistical correlation.
Discussion
Nowadays, there are many methods to evaluate the venous disease.
Venous Clinical Severity Score was invented to backup the CEAP classification and it offers a vast quantification of the venous disease severity and even more it takes into account the impact of this condition on the affected individuals.
This scale evaluates the symptoms and the severity of the varicose vein, edema, cutaneous pigmentation, inflammations, indurations, ulcers and the efficacy of the compressive therapy.
Venous Segmental Disease Score takes into account the anatomical and pathogenic mechanisms of the venous disease while the Venous Disability Score evaluates the impact of the venous disease on the daily activities, using a 4 stages scale (0-3 where 0 represents asymptomatic) and shows the severity of this condition.
The usage of the former or the later in the evaluation of the severity of the chronic venous disease of the lower limbs varies according to the objectives of the study: clinical appearance, the anatomical and pathogenic involved mechanisms, the impact on the quality of the patients’ life, etc.
In general, the venous symptoms of the lower limbs and their correlation with the chronic venous disease remain a controversial subject [9].
Nowadays, the treatment of the chronic venous disease is mostly a minimally invasive method that must ensure two major advantages: firstly an acceptable cosmetic aspect with disappearance of the pain as main symptom of the disease and secondly to be accordingly to the guidelines of the healthcare insurance of each country [3].
To be noted that there are major differences between the health insurances of different countries, some supporting only the therapy for chronic venous disease associated with cutaneous signs while others with varicose veins!
Our study aimed solely less than 2mm diameter lower limbs telangiectasia so the monitoring was based on the efficacy of the applied treatment (laser or sclerotherapy) on those vessels.
The indications of the sclerotherapy for reticular and telangiectatic vessels is absolute (IA grade) according to the international flebological guidelines [3,4].
If foam sclerotherapy is more efficient for larger vessels, the liquid sclerotherapy is the first line of treatment for smaller veins [10].
Repeated sessions may be required if neoclusion or early recanalization of the treated veins appears [11].
Although there are many sclerotic substances used in current practice, 2 or 3 are the most frequently utilized. Among those, polidocanol (Lauromacrogol 400) is the mostly used due to its high efficacy and low risk of local side effects (ulcerations, burns of the surrounding tissues, etc).
Chemically, this substance is a non-ionic detergent which produces the thermal injury of the venous intimae by reacting with the membrane lipoproteins [12].
The reconstruction, that lasts 6-7 weeks, will lead to the venous fibrosis.
Practically, this will resorb into the subcutaneous lax tissue but it may take up to 1 year, the duration being direct proportional with the diameter of the vessels and according to some studies with the concentration of the administered substance [13].
In our study the treatment was aimed at relatively small vessels. In this scenario, the results are according to this purpose so it was noted almost simultaneously the esthetic and the functional aspects, by closing the vessel.
Those are the consequences of the sclerosis process and the vasospasm due to the chemical or thermal agent [14,15].
The literature mentions numerous studies that show the efficacy of different concentrations of polidocanol for the cure of the varicose veins of the lower limbs.
Our study is among the few that compares the result of the sclerotherapy with various concentrations of polidocanol to ND:YAG laser therapy.
Even more, according to our knowledge there is no other similar study published in Romania until now.
Sclerotherapy is a minimally invasive procedure that can be done repeatedly but not exceeding 2mg/kg/day.
In our study we used 2 different concentration of polidocanol, 0,5% and 1%, both being recommended for the treatment of the telangiectasia of the lower limbs and we monitored the comparative efficiency in inducing sclerosis of the treated vessels.
The laser is a pure light regarding the wavelength.
In contrast with intense pulse light, which is also used to treat cutaneous vascular lesions, laser has only one wavelength (IPL has many wavelengths) that has a more or less powerful action on different skin structures according to the its spectrum.
An adequate wavelength for treating vascular lesions is in the green area or infrared.
A laser device that emits such a wavelength will transfer the light energy to the target vein and transform it into thermal energy and it will cause overheat.
It will lead to the destruction of the vascular wall being followed by the vascular sclerosis and later by the remodelation, same like in the sclerotherapy process [16].
There are indications for laser treatment of the telangiectasia of the lower limbs as well as there are also studies that showed a better outcome of this method when the targeted area previously underwent a surgical procedure where there is a higher risk for hyper pigmentation or needle phobia [17].
Last but not least, it must be mentioned that the association of sclerotherapy with laser can have more benefits for patients with telangiectasia of the lower limbs [18,19].
Generally, the complications after treating small vessels are very low as long as it is done respecting the guidelines of sclerotherapy (the injection is done strictly intravascular) and of laser (using adequate spot diameter, fluency and widthness of the spotum and every area should be treated only once).
In our study the hyperpigmentation appeared only in 3 limbs and regarding the other complications (ulcerations, erythema, burns, oedema, pain) they were mild and temporary.
The literature describes a variety of complications but with extremely low incidence. Usually, they occur whenever there are not taken into account the particularities of the patient, so there are some recommendations such as:
-postponing the laser treatment with extremely tanned patients to avoid the excessive absorption of light by the melanin granulations [17];
-avoid the injection or use a reduced laser fluency around bone structures [17];
-be aware of sensitive areas like popliteal fossa [10];
-not exceed 0,1-0,2ml of sclerotic substance in one area and avoid to treat twice the same area [21,22].
If ulcerations do appear the treatment is the classic, performing gentle debridement and bandages that stimulate the granulation and prevent the infections.
In case of burns it is enough to apply emollients and cold bandages, same treatment being used also for edema.
Conclusions
Telangiectasias and reticular veins of the lower limbs can be successfully treated with Nd:YAG laser or sclerotherapy with polidocanol.
Nd:YAG laser is recommended for the treatment of small telangiectasia with less than 1mm diameter while sclerotherapy with polidocanol is more efficient as the diameter of the vessels increases.
Acknowledgments
Cristina Violeta Tutunaru and Madalina Xenia Calbureanu-Popescu have an equal contribution with the first author.
Conflict of interests
None to declare.
References
- 1.Van der Velden SK, Shadid NH, Nelemans PJ, Sommer A. How specific are venous symptoms for diagnosis of chronic venous disease. Phlebology. 2014;9(9):580–586. doi: 10.1177/0268355513515859. [DOI] [PubMed] [Google Scholar]
- 2.Kahle B, Leng K. Efficacy of sclerotherapy in varicose veins-prospective, blinded, placebo-controlled study. Dermatol Surg. 2004;30(5):723–728. doi: 10.1111/j.1524-4725.2004.30207.x. [DOI] [PubMed] [Google Scholar]
- 3.Rabe E, Breu FX, Cavezzi A, Coleridge Smith P, Frullini A, Gillet JL, Guex JJ, Hamel-Desnos C, Kern P, Partsch B, Ramelet AA, Tessari L, Pannier F. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(6):338–354. doi: 10.1177/0268355513483280. [DOI] [PubMed] [Google Scholar]
- 4.Parsons ME. Sclerotherapy basics. Dermatol Clin. 2004;22(4):501–508. doi: 10.1016/j.det.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Kern P, Ramelet AA, Wutschert R, Bounameaux H, Hayoz D. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30(3):367–372. doi: 10.1111/j.1524-4725.2004.30102.x. [DOI] [PubMed] [Google Scholar]
- 6.Gianesini S, Obi A, Onida S, Baccellieri D, Bissacco D, Borsuk D, Campisi C, Campisi CC, Cavezzi A, Chi YW, Chunga J, Corda D, Crippa A, Davies A, De Maeseneer M, Diaz J, Ferreira J, Gasparis A, Intriago E, Jawien A, Jindal R, Kabnick L, Latorre A, Lee BB, Liew NC, Lurie F, Meissner M, Menegatti E, Molteni M, Morrison N, Mosti G, Narayanan S, Pannier F, Parsi K, Partsch H, Rabe E, Raffetto J, Raymond-Martimbeau P, Rockson S, Rosukhovski D, Santiago FR, Schul A, Schul M, Shaydakov E, Sibilla MG, Tessari L, Tomaselli F, Urbanek T, van Rijn MJ, Wakefield T, Wittens C, Zamboni P, Bottini O. Global guidelines trends and controversies in lower limb venous and lymphatic disease: Global guidelines trends and controversies in lower limb venous and lymphatic disease: Narrative literature revision and experts' opinions following the vWINter international meeting in Phlebology, Lymphology & Aesthetics. Phlebology. 2019;34(suppl 1):S4–S66. doi: 10.1177/0268355519870690. [DOI] [PubMed] [Google Scholar]
- 7.Hamel-Desnos C, Ouvry P, Benigni IP, Boitelle G, Schadeck M, Desnos P, Allaert FA. Comparison of 1% and 3% polidocanol foam in the ultrasound guided sclerotherapy of great saphenous vein: a randomized double-blind trial with two years follow-up. Eur J Vasc Endovasc Surg. 2007;34(6):723–729. doi: 10.1016/j.ejvs.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Breu FX, Guggenbichler S. European consensus meeting on foam sclerotherapy. Dermatol Surg. 2004;30(5):709–717. doi: 10.1111/j.1524-4725.2004.30209.x. [DOI] [PubMed] [Google Scholar]
- 9.Perrin C, Eklof B, VAN Rij A, Labropoulos N, Vasquez M, Nicolaides A, Blattler W, Bouhassira D, Bouskela E, Carpentier P, Darvall K, DE Maeseneer M, Flour M, Guex JJ, Hamel-Desnos C, Kakkos S, Launois R, Lugli M, Maleti O, Mansilha A, NEGLéN P, Rabe E, Shaydakov E. Venous symptoms: the SYM Vein Consensus statment development under the auspices of the European Venous Forum. Int Angiology. 2016;35(4):374–398. [PubMed] [Google Scholar]
- 10.Ianosi NG, Calbureanu-Popescu MX, Dragusin L, Ianosi SL, Tutunaru CV, Neagoe CD. Treatment of Lower Limbs Telangiectasias with Nd:Yag Laser-Prospective Study on 446 Patients. Curr Health Sci J. 2018;44(3):235–242. doi: 10.12865/CHSJ.44.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CH, Chiu CS, Yung CH. Ultrasound-guided foam sclerotherapy for treating incompetent great saphenous veins-results of five years analysis and morphologic evolvement study. Dermatol Surg. 2012;38(6):851–857. doi: 10.1111/j.1524-4725.2012.02408.x. [DOI] [PubMed] [Google Scholar]
- 12.Uncu H. Sclerotherapy: a study comparing Polidocanol in foam and liquid form. Phlebology. 2010;25:44–49. doi: 10.1258/phleb.2009.008064. [DOI] [PubMed] [Google Scholar]
- 13.Rabe E, Schliephake D, Otto J, Breu FX, Panier F. Sclerotherapy of teleangiectases and reticular veins: a double-blind, randomized, comparative clinical trial of polidocanol, sodium tetradecylsulphate and isotonic saline (EASI study) Phlebology. 2010;25(3):124–131. doi: 10.1258/phleb.2009.009043. [DOI] [PubMed] [Google Scholar]
- 14.Guex JJ, Allaert FA, Gillet JL, Chleir F. Immediate and midterm complications of sclerotherapy: report of a prospective multicenter registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31(2):123–128. doi: 10.1111/j.1524-4725.2005.31030. [DOI] [PubMed] [Google Scholar]
- 15.Sadick NS. Predisposing factors of varicose and telangiectatic leg veins. J Dermatol Surg Oncol. 1992;18(10):883–886. doi: 10.1111/j.1524-4725.1992.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 16.Bauersachs R, Gerlach HE, Heinken A, Hoffmann U, Langer F, Noppeney T, Pittrow D, Klotsche J, Rabe E. Rationale, design, and methodology of the observational INSIGHTS-SVT study on the current state of care and outcomes of patients with superficial vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2017;5(4):553–560. doi: 10.1016/j.jvsv.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Ianosi G, Ianosi S, Calbureanu-Popescu MX, Tutunaru CV, Calina D, Neagoe D. Comparative study in leg telangiectasias treatment with Nd:YAG laser and sclerotherapy. Exp Ther Med. 2019;17(2):1106–1112. doi: 10.3892/etm.2018.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabe E, Berboth G, Pannier F. Epidemiology of chronic venous diseases. Wien Med Wochenschr. 2016;166(9-10):260–263. doi: 10.1007/s10354-016-0465-y. [DOI] [PubMed] [Google Scholar]
- 19.Rabe E, Carpentier P, Maggioli A. Understanding lower leg volume measurements used in clinical studies focused on venous leg edema. Int Angiol. 2018;37(6):437–443. doi: 10.23736/S0392-9590.18.04057-9. [DOI] [PubMed] [Google Scholar]
- 20.Kern P, Ramelet AA, Wutschert R, Hayoz D. Compression after sclerotherapy for telangiectasias and reticular leg veins: a randomized controlled study. J Vasc Surg. 2007;45(6):1212–1216. doi: 10.1016/j.jvs.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 21.Gillet JL, Desnos CH, Lausecker M, Daniel C, Guex JJ, Allaert FA. Sclerotherapy is a safe method of treatment of chronic venous disorders in older patients: A prospective and comparative study of consecutive patients. Phlebology. 2017;32(4):234–240. doi: 10.1177/0268355516642659. [DOI] [PubMed] [Google Scholar]
- 22.Munia MA, Wolosker N, Guarnieri-Munia C, Chao WS, Puech-Leao P. Comparison of laser versus sclerotherapy in the treatment of lower extremity telangiectasias: a prospective study. Dermatol Surg. 2012;38(4):635–639. doi: 10.1111/j.1524-4725.2011.02226.x. [DOI] [PubMed] [Google Scholar]


