Abstract
Background.
Radical hysterectomy and complete pelvic lymphadenectomies are the most commonly performed procedures for women with early stage cervical cancer. Sentinel lymph node mapping (SLN) could be an alternative to routine pelvic lymphadenectomy (PLN), aiming to diagnose accurately nodal extension and decrease lymphatic morbidity.
Primary objectives.
To compare three year disease free survival (DFS) and health related quality of life (HRQoL) after SLN or SLN + PLN in early cervical cancer.
Study hypothesis.
We hypothesize that DFS is non-inferior and HRQoL superior after SLN compared to SLN + PLN.
Trial design.
International, randomized, multicenter, single blind trial. The study will be run in trained teams to SLN biopsy, belonging to clinical research cooperative groups or recognized as expert in this field. Patients with an optimal mapping (MSKCC criteria) and a negative frozen section will be randomized 1:1 to SLN biopsy only or SLN biopsy + PLN.
Inclusion, exclusion criteria.
Patients with early stages (Ia1 with lymphovascular invasion to IIa1). Histological types are limited to squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma.
Primary endpoint.
Main endpoint will be co-primary endpoint, associating 3-year DFS and quality of life (QLQ-C30 and QLQ-CX24).
Sample size.
950 patients have to be randomized.
Estimated dates for completing accrual and presenting results.
Study started on Q2 2018, last accrual is scheduled on Q2 2021 and last follow-up in Q2 2026.
Trial registration.
ClinicalTrials.gov Identifier: NCT03386734.
Introduction.
Sentinel lymph node mapping is gaining interest among the gynecologic oncology community. This technique was introduced nearly 20 years ago, as a potential alternative to lymphadenectomy in the nodal staging of early cervical cancer (1). The concept is to perform a targeted biopsy on a small number of nodes deemed to be at the greatest risk of harboring metastases due to the specific drainage of the organ involved with malignancy. This approach has been validated in several solid tumors, such as breast cancer, melanoma and vulvar cancer in the field of gynecologic oncology.
There is strong rationale to justify this technique in early cervical cancer. The cervix is easily accessible with straightforward dye injection. Systematic pelvic lymphadenectomy does increase surgical time and morbidity. Overall, the low rate of lymph node metastasis makes it logical to perform a targeted biopsy to limit the morbidity in patients who ultimately have no nodal metastases. These rationales are in line with the current trend in the treatment of early cervical cancer to decrease radicality with more conservative surgery, fertility preservation, and minimally invasive approaches.
Nearly 150 publications report on sentinel lymph node mapping in early cervical cancer with majority being retrospective analyses but there are 5 prospective series and 7 meta-analyses. The diagnostic accuracy of sentinel lymph node mapping is critical. The main goal is to minimize the false negative rate (i.e. missing a metastatic node) as nodal status is an important determinant of treatment decisions and outcome. Bilateral pelvic detection of sentinel lymph node is of utmost importance in minimizing the false negative rates and false negative predictive values (2,3). The Memorial Sloan Kettering Cancer Center team developed a sentinel lymph node mapping algorithm known as “MSKCC criteria” and has published that this results in a significantly lower false negative rate as compared to merely removing colored nodes (4). In a meta-analysis by Tax and colleagues false negative predictive value was 0.08% when this Memorial Sloan Kettering Cancer Center algorithm was applied (5).
We now realize that sentinel lymph node mapping results in precision surgery and provides enhanced information. The technique identifies sentinel lymph node in “unexpected” nodal basins in approximately 18% of patients that are not addressed in routine standard lymphadenectomies (6). In fact, we observe the same anatomical distribution of SLN than that of solitary metastatic nodes described after historical extensive dissection (7). Anatomical distribution of SLN is also concordant with the anatomical descriptions of main and accessory pathway of the lymphatic drainage of the uterus. Ultrastaging with serial sectioning and immune-histochemistry (IHC) detects low volume disease such micrometastases or isolated tumors cells that would not have otherwise been detected with routine pathologic nodal processing (8,9). This increases the rate of metastatic patients and the sensitivity of the technique, making sentinel lymph node the most sensitive technique for the diagnosis of nodal involvement. One criticism is that sentinel lymph node are ultrastaged and not the non-sentinel nodes. Mathevet P et al. ultrastaged sentinel lymph node and non- sentinel lymph node and confirmed that the false negative rate was the same. The same author also demonstrated a decreased post-operative morbidity and a better quality of life after sentinel lymph node vs lymphadenectomy through a randomized trial (SENTICOL II trial)(10).
The GCIG organized a brainstorming session in Melbourne in 2014, to develop future trial concepts in cervical cancer (11). The committee stated that a validation study was necessary on sentinel lymph node, launching the SENTICOL III trial. One of the requirements was to use an innovative methodology. At that time we proposed to use a co-primary endpoint, associating survival and quality of life. We also proposed a design with a comparison of prospective cohorts, coming from “sentinel lymph node” or “dissection” centers. However, several criticisms rose against this methodology and a randomized design was finally decided.
Methods
Trial design.
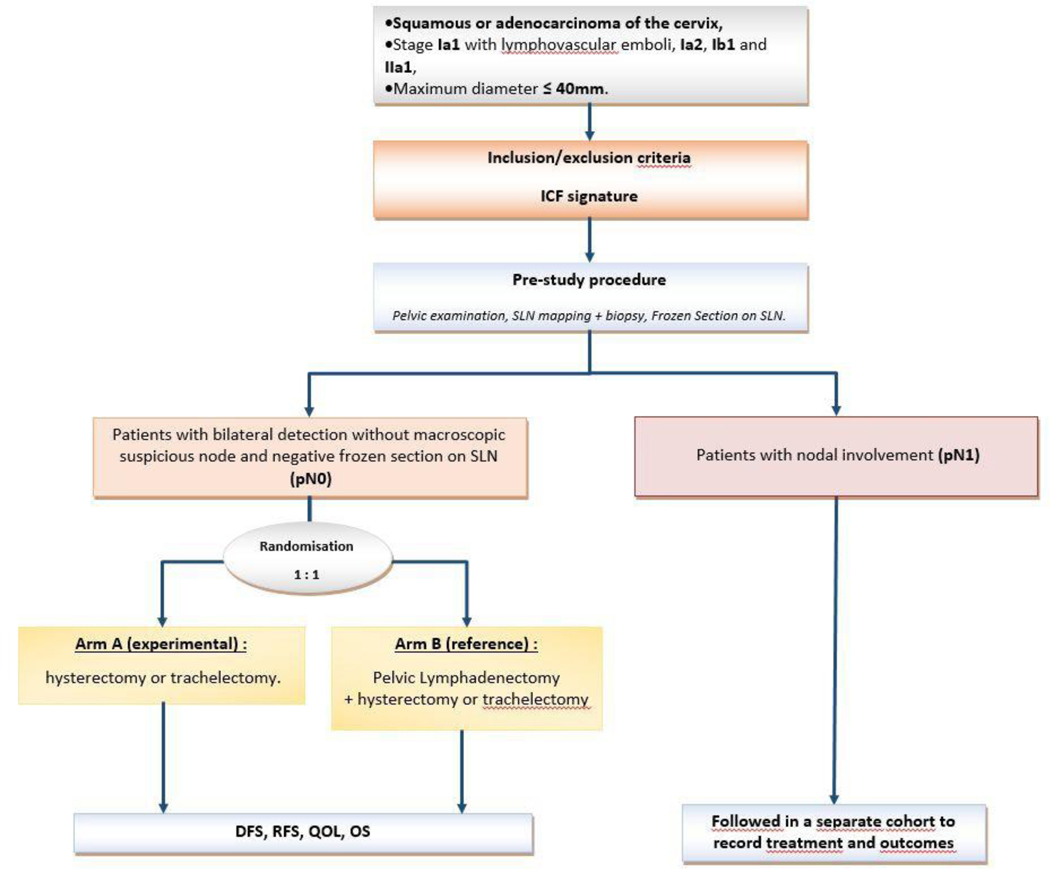
SENTICOL III is an international, randomized, multicenter, single blind trial. It will compare sentinel lymph node biopsy alone versus SLN biopsy + pelvic lymphadenectomy in terms of disease free survival and health-related quality of life in patients with negative sentinel lymph node (Figure 1).
Figure. 1.
Trial schema. DFS, disease-free survival; ICG, indocyanine green; OS, overall survival; QOL, quality of life; RFS, recurrence-free survival; SLN, sentinel lymph node.
Patients will undergo a sentinel lymph node biopsy using a strict methodology. For those in which a radiotracer will be used, the injection will be performed the day before surgery. Long and short protocols are allowed. Pre-operative imaging is required for those who undergo radiotracer mapping with a SPECT-CT being the recommended option but lymphoscintigram also acceptable. Laparoscopic, robotic or open access will be obtained and adhesiolysis will be performed if necessary. The cervical injection can be performed either before or after accessing the abdomen. At least two deep injections (3 and 9 o’clock) are needed (supplementary injections are allowed). Detection can be performed with isotope alone, isotope + blue dye, isotope + ICG or ICG alone. Blue dye alone is not allowed.
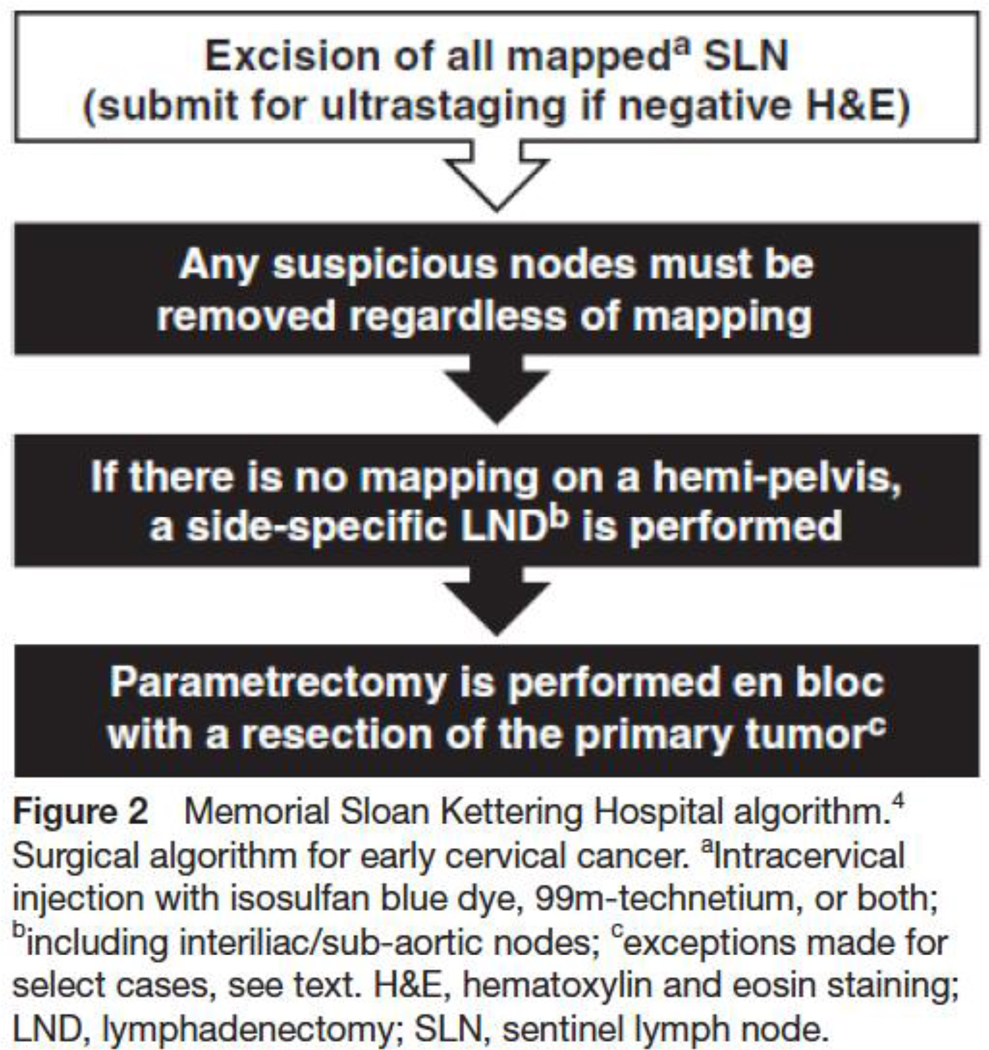
The Memorial Sloan Kettering Cancer Center algorithm will be followed (Figure 2)(4). All peritoneal surfaces inspected and any suspicious lesions biopsied. If there is extra-cervical spread then sentinel lymph node mapping is unnecessary and procedure will be terminated as patient will require other therapy. The retroperitoneal spaces must be systematically opened and examined. Any suspicious nodes are to be extracted regardless of mapping. The Memorial Sloan Kettering Cancer Center criteria must be fulfilled, especially the bilateral detection of sentinel lymph node. Sentinel lymph node will be systematically analyzed by frozen section: sentinel lymph node should be cut in half along their long axis, one level in one of the two parts, staining with Hematoxylin Eosin Safran. Patients with negative sentinel lymph node on frozen will then be randomized 1:1 between the experimental arm (sentinel lymph node alone) or the standard arm (sentinel lymph node + pelvic lymph node dissection)(Figure 1). Patients with a unilateral detection or a non-optimal mapping will undergo a side-specific lymphadenectomy. Patients with positive frozen section will not be randomized and included in the pN1 cohort. In these patients, a full nodal dissection with paraaortic staging may be considered but treatment decisions will be left to the individual treating surgeons. Additionally, completion radical hysterectomy, radicality, fertility sparing surgery will be performed according to the tumor and patients characteristics. However operative and postoperative treatment should comply with national or international guidelines. Open and minimally invasive accesses are allowed for the realization of the hysterectomy. Teams using minimally invasive surgery (MIS) should be properly trained (according to data provided by the center). Results of the recently published LACC randomized trial should be discussed with patients (12). Patients will be stratified according to use of open of MIS access.
Figure 2.
Memorial Sloan Kettering Hospital algorithm (4)
SLNs will undergo permanent pathologic ultrastaging with serial sectioning each 200 microns and staining with Hematoxylin Eosin Safran. Negative sentinel lymph node with Hematoxylin Eosin Safran, will undergo immune-histo-chemistry with anti-cytokeratin AE1-AE3. Non sentinel lymph node will be sectioned once and examined after staining with Hematoxylin Eosin Safran. Standard definition of isolated tumor cells, micrometastases and macro metastases will be used (13).
Patients will be followed at 30 days postoperative and then every 3 months during the first year, every 4 months during the second year and then every 6 months to complete 5 years of follow -up.
These visits will focus on morbidity related to the treatment, especially lower-limb and lymphatic morbidity, survival and health-related quality of life (EORTC QLQ-C30). No systematic imaging is required for screening of recurrence.
This trial has been funded by a grant of Institut National du Cancer (INCa)(PHRC ) for the French part and the international coordination. Each participating group will have to find its own funding.
Setting.
This study will be run in France with the collaboration of the INCA labeled French Cooperative Group ARCAGY-GINECO. This large randomized study will also be widely run in Europe through the ENGOT network (European Network of Gynecological Oncology Trial) and in non-European countries thanks to GCIG (Gynecological Cancer Intergroup) and CCRN (Cervix Cancer Research Network) accredited centers. 300 centers will participate.
Participants.
We will include patients with early cervical cancer of squamous, adenosquamous or adenocarcinoma histology. Stages Ia1 with lymphovascular space invasion to stage IIa1 will be included. Tumor size must be <40mm on clinical examination and magnetic resonance imaging. No suspicious node should be seen on magnetic resonance imaging (RECIST 1.1). Patients must be ≥18 years of age, have an Eastern Cooperative Oncology Group performance status of 0 to 2, have signed the informed consent and accept to comply with follow-up. For French patients, being affiliated to, or a beneficiary of, a social security category is mandatory. Exclusion criteria include pregnancy, previous pelvic or abdominal cancer, previous chemotherapy or external beam radiation therapy for the cervical cancer (brachytherapy is allowed). Participation cannot be proposed in case of proven allergy to blue dye, isotope or ICG.
Outcomes.
The primary objective is a co-primary objective associating disease free survival and health-related quality of life. The hypothesis is that disease free survival is non-inferior and health-related quality of life better after SLN compared to sentinel lymph node + pelvic lymph node dissection. Secondary objectives include outcome of pN1 patients through a specific cohort (taking into account the size of the metastasis), evaluation of mapping with indocyanine green, surgical mortality and morbidity, other dimensions of health-related quality of life, positive and negative predictive values of sentinellymph node, overall survival, recurrence free survival, cost analysis in France and lymphatic and lower limb complications.
The primary endpoint will be a co-primary outcome:
-
-
the disease free survival defined as the time interval between randomization and physical or radiographic evidence of recurrence (local/distant) or second cancer or death (all causes) whichever occur first,
-
-
and health-related quality of life assessed with European Organisation for Research and Treatment QLQ-C30 and QLQ-CX24 with 3 targeted dimensions: pain, global health score and physical functioning scores at 3 years.
Secondary endpoints will be:
-
-
Disease free survival of patients with isolated tumor cells, micrometastases and macrometastases in sentinel lymph node
-
-
Detection rate and diagnostic accuracy of IndoCyanine Green
-
-
Surgical morbidity assessed by the NCI-CTCAE v4.03 and the Clavien Dindo classification and mortality (per and 30-day post-operative)
-
-
Other dimensions of health-related quality of life of both QLQ-C30 and QLQ-CX24
-
-
True positive patients, true and false negative patients in patients with sentinel lymph node biopsy + pelvic lymph node dissection
-
-
Overall survival defined as time interval between randomization and death (all causes); alive patients will be censored at the last date of news
-
-
Recurrence-free interval defined as the time interval from randomization to physical or radiographic evidence of recurrence (local/distant) or death (all causes) whichever occur first
-
-
Cost of the procedure only for France,
-
-
Lymphatic and lower limb complications, LEL Screening questionnaire (only for France)
Quality assurance is an important issue in this trial. Several criteria concerning surgery and pathology will be surveyed: training of the surgeon, selection of patients, surgical training and quality, morbidity, false negative rate and false negative predictive values in the standard arm, training of the pathologist, etc. Centers will be selected according to their ability to comply with the protocol and quality criteria. Centers belong to GINECO, European Network of Gynecological Oncology Trial, Gynecologic Cancer InterGroup or are part of the Cancer Cervix Research Network.
Tumor specimen (cervical tumors, sentinel lymph nodes and non-sentinel lymph nodes), as well as blood samples will be stored in the majority of centers. A translational program is being developed and should improve the understanding and treatment of patients with early cervical cancer.
Sample size.
We aim to demonstrate the non-inferiority of sentinel lymph node alone vs sentinel lymph node+pelvic lymph node dissection for disease free survival using a gatekeeping procedure to control the type I error rate. Using a 5% non-inferiority margin and a standard 3 years disease free survival of 85%, 900 randomized patients are required to observe the required (unilateral alpha error of 5%, a statistical power of 80% and a 5 year follow-up). An interim analysis will be planned when at least 132 events will be observed to reject H0 or H1 using O-Brien Fleming and alpha spending function. Stopping guidelines for efficacy will be −2.532 on Z scale and −0.346 for futility For health-related quality of life, 3 dimensions of the European Organisation for Research and Treatment QLQ-C30 and QLQ-CX24 will be targeted: global health status, pain and physical functioning. At 3 years, to demonstrate a superiority of at least one of the 3 targeted dimensions without significant deterioration in at least one with a minimal clinically important difference in mean score of at least 5 points (standard deviation of 20), and a bilateral alpha type one error of 0.016 (Bonferroni adjustment for 3 dimensions) it would be required to have 815 patients with available health-related quality of life scores at 3 years to reach a 85% statistical power. Taking into account a 5% of lost to follow-up, 950 patients are needed. Accrual will last for 3 years (14).
Randomization and blinding.
Patients will be randomized in a 1:1 ratio using an Interactive Web Response System (IWRS) in a 1:1 ratio one of the two treatment arms: sentinel lymph node biopsy alone versus sentinel lymph node biopsy + pelvic lymph node dissection by minimization technique and stratified by center and stage of the disease using the 2018 Federation Internationale de Gynécologie Obstétrique classification. A double blinded study is not feasible since the details of the operation cannot be masked to the surgeon and treating physician. Thus, the treatment will be blinded to the patients only.
Statistical methods.
Efficacy analyses will be conducted on intent-to-treat population including all randomized patients whatever treatment received and eligibility criteria. For non-inferiority analysis of disease free survival, results will be also reported on per protocol analysis. For health-related quality of life analysis, a modified intent to treat population will be considered, including all randomized patients with at least the baseline health-related quality of life score available. Safety analyses will be conducted on all randomized patients and with at least one post-baseline safety assessment. This population will be considered for safety data and treatment exposure data.
All tests will be performed two-sided at a significance level of 5% (95% Confidence interval), with the exception of the tests for the primary endpoint of Non inferiority using one sided at a statistical significance level of 5% (90% CI). Indeed, for health-related quality of life analyses, all tests will be performed two-sided at a significance level of 1.6%. For the DFS, the nominal significance levels for the interim and final analyses of DFS will be derived from the alpha spending function with O’ Brien Fleming boundaries which are dependent on the information fraction in the intention to treat population.
Confidence intervals will be calculated, these will be 2-sided with a confidence level of 95% with the exception of the estimates for the primary endpoint using a confidence level of 100% - nominal alpha and quality of life using a confidence interval of 100–1.6, i.e. 98.4%.
Clinical and demographic at baseline will be described by treatment arm using rules form. The statistical parameters mean, median, standard deviation, interquartile range and range will be presented for continuous baseline variables. For categorical baseline variables, number and percentages will be calculated.
Kaplan Meier estimation method will be used to estimate disease free survival as well as other time to event endpoints. For the primary objective of non-inferiority in disease free survival, the 2 years disease free survival rate will be computed with its 90% confidence interval. Follow-up will be estimated using the reverse Kaplan-Meier method, and will be described using the median with its 95%CI. For primary health-related quality of life analysis, a t-test will be used to compare health- related quality of life level at three years according to treatment arm for each targeting dimension (global health status, pain and physical functioning). The statistical level for significance will be 0.016. The normal distribution of the scores will be checked. In case of non- normally distribution, a non- parametric Mann-Whitney test will be done. Overall survival will be estimated using the Kaplan- Meier method, and will be described using the median with its 95% confidence interval. Univariate Cox proportional hazards model will be used to estimate hazard ratios with 95% Confidence interval.
Multivariate Cox analyses will be done in respect of the Peduzzi rule of one variable per ten events. An univariate selection procedure will serve to identify eligible explanatory variables with univariate Cox p-value according to the Wald test lower than 0.10 as potential prognostic value. For each Cox analysis, the proportionality assumption of the risk will be graphically checked.
For safety analyses, the report will take into account all adverse events observed during and after the acts performed or methods used. Categorical data will be summarized in contingency tables displaying frequencies and percentages. Continuous data will be presented using median, minimum and maximum values. The safety data of the different strategy will be compared using the Kruskal- Wallis test or Fisher exact test or Chi square tests. Particular interest will be given to rates of grade 3– 4 toxicities according to the NCI-CTCAE v4.
Discussion.
Few oncologic surgical techniques have been validated by a complete pathway including proof of concept, diagnostic accuracy study, safety study and then survival and QoL by a randomized trial. SENTICOL III is an ambitious study, requiring a large number of patients and an international collaboration. This kind of study is clearly a challenge for the community, necessitating sharing ideas and energy to conduct a large trial despite budget and administrative constraints. Participation of the French cooperative group GINECO, the European Network of Gynecological Oncological Trial, the Cervix Cancer Research Network, the Gynecological Cancer InterGroup, as well as referent centers around the world will offer a greater chance of successfully completing this ambitious trial.
SENTICOL III presents several particularities. First, we decided to use the co-primary endpoints of survival and quality of life. This choice was guided by the aim to assess non-inferior survival in a randomized fashion as well as quality of life which is also equally important. It would have been difficult to make a choice between these two fundamental parameters. Women with early cervical cancer and negative sentinel lymph node have an excellent prognosis with long survivals, thus making quality of life preservation quite important. Second, quality criteria assessment will be largely developed in this trial. This aspect is logically more considered in surgical studies. Respect of minimal rules and algorithms, verification of the surgical and pathological skills, etc. are mandatory to guaranty the validity of the results. It would not be acceptable that results, their interpretation and implementation could be impaired by technical limitations of errors. This aspect is not simple since surgery and pathology have several hand-craft aspects, and emphasizes the need for a minimal standardization of techniques. Third, SENTICOL III is a large, international randomized trial. This design is clearly the most difficult and the most challenging with significant financial implications as well as having to manage various research rules and requirements across multiple countries.
However, this is necessary to be able to achieve successful enrollment and provide robust results and subsequently implement safely the sentinel lymph node technique in the armamentarium of ECC management.
Secondary objectives are important also. Treatment and survival of patients with minimal nodal disease is a major challenge for the coming years. These patients have been traditionally categorized as high risk and managed as well. However, data coming from the SENTICOL I trial indicate that patients without and patients with one metastatic node share a similar prognosis (15). We clearly need modern data on this specific subset of patients, who could benefit a more personalized treatment approach. Similarly, this study will evaluate the accuracy of ICG in a routine situation. ICG is more and more commonly used despite not being approved in a majority of countries for SLN detection. A prospective trial recently presented shows that ICG detects ≥1 and bilateral SLN more often than blue dye alone (FILM study) (16). However, a large prospective validation will be welcome. Finally, SENTICOL III is also a unique opportunity to record prospective data on this particular population of early cervical cancer. We will get data on a large number of patients with early cervical cancer, which have not been investigated for many years. Several ancillary studies will be possible. It is at least a unique opportunity to develop translational research on this disease. Early cervical cancer has been less investigated than other gynecological tumors. Studies on human papilloma virus, patients immunity, tumor biology are now necessary to progress in the treatment of pN1 patients, but also for pN0 patients with recurrence or poor prognosis.
The SENTICOL III trial has received a large grant in France to cover for the per case funding of French patients and for the international coordination. It has been approved by the Ethics Committee and Competent Authority in France late 2017 and accrual has just started. Other groups and CCRN centers should start soon, when administrative and ethical approval will be obtained.
References
- 1).Medl M, Peters-Engl C, Schütz P, Vesely M, Sevelda P. First report of lymphatic mapping with isosulfan blue dye and sentinel node biopsy in cervical cancer. Anticancer Res. 2000; 20:1133–4. [PubMed] [Google Scholar]
- 2).Hauspy J, Beiner M, Harley I, Ehrlich L, Rasty G, Covens A. Sentinel lymph nodes in early stage cervical cancer. Gynecol Oncol 2007; 105: 285–290. [DOI] [PubMed] [Google Scholar]
- 3).Lecuru F, Mathevet P, Querleu D, Leblanc E, Morice P, Darai E et al. Bilateral Negative Sentinel Nodes Accurately Predict Absence of Lymph Node Metastasis in Early Cervical Cancer: Results of The SENTICOL Study. J Clin Oncol 2011; 29: 1686–1691. [DOI] [PubMed] [Google Scholar]
- 4).Cormier B, Diaz JP, Shih K, Sampson RM, Sonoda Y, Park KJ, et al. Establishing a sentinel lymph node mapping algorithm for the treatment of early cervical cancer. Gynecol Oncol 2011; 122: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Tax C, Rovers MM, de Graaf C, Zusterzeel PL, Bekkers RL. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol Oncol. 2015. December;139(3):559–67. [DOI] [PubMed] [Google Scholar]
- 6).Bats AS, Mathevet P, Buenerd A, Orliaguet I, Mery E, Zerdoud S, et al. The sentinel node technique detects unexpected drainage pathways and allows nodal ultrastaging in early cervical cancer: insights from the multicentre prospective SENTICOL study. Ann Surg Oncol 2013; 20: 413–422. [DOI] [PubMed] [Google Scholar]
- 7).Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R et al. Incidence and Distribution Pattern of Pelvic and Paraaortic Lymph Node Metastasis in Patients with Stages IB, IIA, and IIB Cervical Carcinoma Treated with Radical Hysterectomy. Cancer 1999; 85: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 8).Horn LC, Hentschel B, Fischer U, Peter D, Bilek K. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of the cervix uteri using step sectioning: Frequency, topographic distribution and prognostic impact. Gynecol Oncol 2008; 111: 276–281. [DOI] [PubMed] [Google Scholar]
- 9).Gortzak-Uzan L, Jimenez W, Nofech-Mozes S, Ismiil N, Khalifa MA, Dubé V et al. Sentinel lymph node biopsy vs. pelvic lymphadenectomy in early stage cervical cancer: is it time to change the gold standard? Gynecol Oncol 2010; 116: 28–32. [DOI] [PubMed] [Google Scholar]
- 10).Mathevet P, Lecuru F and SENTICOL II group. Sentinel lymph-node biopsy alone decreases the morbidity of the surgical treatment of early cervical cancer: results from the prospective randomized study Senticol2. Poster. ASCO meeting 2016. [Google Scholar]
- 11).Sagae S1, Monk BJ, Pujade-Lauraine E, Gaffney DK, Narayan K, Ryu SY, et al. Advances and Concepts in Cervical Cancer Studys: A Road Map for the Future. Int J Gynecol Cancer. 2016. January;26(1):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, Isla D, Tamura M, Zhu T, Robledo KP, Gebski V, Asher R, Behan V, Nicklin JL, Coleman RL, Obermair A. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N Engl J Med. 2018. November 15;379(20):1895–1904. [DOI] [PubMed] [Google Scholar]
- 13).Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999; 86:2668–73. [PubMed] [Google Scholar]
- 14).Bonnetain F et al. Statistical Challenges in the Analysis of Health-Related Quality of Life in Cancer Clinical Trials. J Clin Oncol. 2016. June 1;34(16):1953–6. [DOI] [PubMed] [Google Scholar]
- 15).Guani B, Dorez M, Magaud L, Buenerd A, Lecuru F, Boutitie F, Mathevet P. Recurrence risk after sentinel lymph node biopsy in patients with early cervical cancer: three years follow-up results of a prospective study (Senticol)submitted for publication.
- 16).Frumovitz M, Plante M, Lee PS, Sandadi S, Lilja JF, Escobar PF, Gien LT, Urbauer DL, Abu-Rustum NR. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018. October;19(10):1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]