Abstract
Objectives:
Neoadjuvant chemotherapy (NAC) is an important method for breast cancer treatment. By monitoring its pathological response, the selection of clinical treatment strategies can be guided. In this study, the meta-analysis was used to compare the accuracy of contrast-enhanced MRI (CE-MRI) and contrast-enhanced spectral mammography (CESM) in detecting the pathological response of NAC.
Methods:
Literatures associated to CE-MRI and CESM in the evaluation of pathological response of NAC were searched from PubMed, Cochrane Library, web of science, and EMBASE databases. The Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool was used to assess the quality of studies. Pooled sensitivity, specificity, and the area under the SROC curve were calculated to evaluate the diagnostic accuracy of CE-MRI and CESM in monitoring the pathological response of NAC.
Results:
There were 24 studies involved, 18 of which only underwent CE-MRI examination, three of which only underwent CESM examination, and three of which underwent both CE-MRI and CESM examination. The pooled sensitivity and specificity of CE-MRI were 0.77 (95%CI, 0.67–0.84) and 0.82 (95%CI, 0.73–0.89), respectively. The pooled sensitivity and specificity of CESM were 0.83 (95%CI, 0.66–0.93) and 0.82 (95%CI, 0.68–0.91), respectively. The AUCs of SROC curve for CE-MRI and CESM were 0.86 and 0.89, respectively.
Conclusions:
Compared to CE-MRI, CESM has equal specificity, greater sensitivity and excellent performance, which may have a brighter prospect in evaluating the pathological response of breast cancer to NAC.
Advances in knowledge:
CESM showed equal specificity, greater sensitivity, and excellent performance than CE-MRI.
Introduction
Breast cancer is the most common malignant tumor in females, and its mortality rate ranks the first among female malignant tumors.1 Neoadjuvant chemotherapy (NAC), as the standard treatment for patients with locally advanced breast cancer, has unique advantages in the treatment of breast cancer.2 It can improve the success rate of breast-conserving surgery and reduce the proportion of mastectomy by reducing the rate of tumor progression and the size of preoperative tumor.3 Previous studies have shown that the pathological response of breast cancer patients to NAC is closely related to the long-term prognosis of breast cancer. The disease-free survival and overall survival were improved in patients with pathological complete response (pCR). However, around 10–35% of patients have chemotherapy resistance.4 For patients who were not responding to NAC, surgery might be delayed due to excessive chemotherapy. Thus, accurate and timely evaluation of the pathological response of breast cancer patients to NAC is extremely important. It can not only predict the scope of surgical resection and avoid additional postoperative treatment, but also provide guidance for the selection of treatment schemes for non-responders.5 Pathological examination is the gold standard for evaluating tumor response. However, it can only be applied in the postoperative specimens, and patients often miss the best time to adjust the treatment plan. Therefore, it is necessary to find a reliable and noninvasive imaging method to evaluate the pathological response of NAC in early stage.6
Currently, common imaging techniques used to evaluate the pathological response of NAC include mammography, ultrasound, positron emission tomography (PET/CT), and contrast-enhanced magnetic resonance imaging (CE-MRI). Many studies have focused on finding the best imaging mode to evaluate the efficacy of NAC on breast cancer, but there is no final conclusion presented.7,8 In the last 10 years, contrast-enhanced magnetic resonance imaging (CE-MRI) has been increasingly used to early predict the pathological response of breast cancer to NAC. And some studies have shown that it is superior to mammography and ultrasonography in evaluating the efficacy of NAC. A meta-analysis about evaluating the efficacy of NAC by Gu YL et al indicated that the pooled sensitivity and specificity of CE-MRI were 0.64 (95% CI range = 0.56–0.70) and 0.92 (95% CI range = 0.89–0.94), respectively. The AUC for CE-MRI was 0.88 (95%CI range = 0.85–0.91). CE-MRI can not only evaluate the change of tumor size, but also evaluate the change of morphological characteristics on tumor. However, because of its high cost, lengthy imaging time and contraindications in some patients, it is still controversial on using it routinely.9,10 Contrast-enhanced spectral mammography (CESM) is a novel breast imaging technique which has developed rapidly in recent years. It is an effective tool for the accurate diagnosis of breast cancer and has a vital value in evaluating the tumor neovascularity. The principle of this technique is to combine contrast enhancement with digital mammography. After i.v. injection of nonionic iodinated contrast agent, different enhancement modes can be obtained by subtracting the dual exposures of low and high x-ray energies, thus determining the characteristics of the lesion.11 some studies have shown that CESM is valuable in monitoring the pathological response of NAC, even comparable to CE-MRI.12 However, there has been no meta-analysis about CESM to evaluate the pathological response of breast cancer to NAC. The purpose of this study is to systematically review relevant literatures and compare the diagnostic value of CESM and CE-MRI in evaluating the pathological response of breast cancer to NAC.
Methods and materials
Literature search
Literatures on CE-MRI and CESM in the evaluation of the pathological response of breast cancer to NAC were searched on PubMed, Cochrane Library, web of science, and EMBASE databases. And gray literature was excluded. The search strategy was based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist and the recommendations of the Cochrane collaboration. The search terms included “contrast-enhanced magnetic resonance imaging” or “CE-MRI,” “contrast-enhanced spectrum mammography” or “mammography” or “contrast enhancement spectrum mammogram,” “breast neoplasms” or “breast neoplastic” or “breast carcinoma” or “breast cancer” or “locally advanced breast cancer,” “NAC” or “primary chemotherapy” or “preoperative” or “pre-surgery,” “sensitivity,” “specificity.” The search was performed in March 2020, without a start date limit. The search strategy used in Pubmed was as follows: (“Breast Neoplasms” [MeSH Term] OR “breast neoplastic” [All Fields] OR “breast carcinoma” [All Fields] OR “breast cancer” [All Fields] OR “locally advanced breast cancer” [All Fields]) AND (“contrast-enhanced magnetic resonance imaging” [All Fields] OR “CE-MRI” [All Fields] OR “contrast-enhanced spectrum mammography” [All Fields] OR “mammography” [All Fields] OR “contrast enhancement spectrum mammogram” [All Fields]) AND (“NAC” [All Fields] OR “primary chemotherapy” [All Fields] OR “preoperative” [All Fields] OR “pre-surgery” [All Fields]) AND (“sensitivity” [All Fields]) AND (“specificity” [All Fields]). In order to avoid missing eligible studies, the references of the enrolled studies were searched.
Study selection
The study selection was independently performed by two researchers, in two different phases. First, the two researchers eliminated irrelevant literature by reviewing the title and abstract of the literature, and all discrepancies or omissions were resolved by the third researcher. Then, articles were eligible for full-text review if (1) the subjects were patients who were diagnosed with breast cancer and had received NAC before operation; (2) all patients should have undergone CE-MRI and/or CESM to assess the pathological response of breast cancer to NAC before operation; (3) pathological examination should serve as the gold standard; (4) Sufficient original data can be directly or indirectly extracted, including the number of true positive (TP), true negative (TN), false positive (FP), and false negative (FP) findings; (5) studies that with different additional surgery or other adjunctive treatment were acceptable. We excluded studies with incomplete data, duplicated articles, case reports, animal and cell studies, comments, and other non-related studies.
Data extraction
Two reviewers extracted data from each included study and reached an agreement with the third reviewer when they encountered differences. To better describe the underlying information of the included literature, a table was used to extract the following information: (1) number of patients, patient age, the definition of pathological responder, the percentage of responders, tumor classification, and grading; (2) first author, year of publication, study design (prospective or retrospective); (3) equipment and magnet field strength, the dose of the contrast agent; (4) the number of TP, TN, FP, and FN were obtained from the pathological results of the CE-MRI and CESM.
Methodological quality assessment
Quality assessment of diagnosis accuracy study form (QUADAS-2) was used in the assessment of methodological quality of each included study. The quality evaluation tool includes four domains: patient selection, index test, reference standard, and flow and timing.13 The QUADAS list consisted of 14 items, 11 of which were used according to the Cochrane collaborative diagnostic test system evaluation methods group. These 11 items were tested to assess the risk of bias, reliability, and quality of reporting for each study. For each item, judgments of “yes” (low degree of bias or good applicability), “no” (high degree of bias or poor applicability), and “unclear” (lack of relevant information or uncertainty of bias) were made. Finally, Review Manager v.5.3 software was used to draw the risk of bias’ figure.
Statistical analysis
Meta-DiSc 1.4 and Stata v.15.0 statistical software were used for data analysis. All the included studies were analyzed using a 2×2 contingency table. Using these tables, the sensitivity, specificity, area under the curve (AUC), diagnostic odds ratios (DOR), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and the confidence intervals (CIs) were calculated. AUC was the final comparison indicator, which is close to one for a perfect test and close to 0.5 for a poor test. The threshold effect is one of the main causes of heterogeneity. We used Spearman correlation coefficients to determine if there was a threshold effect. If p > 0.05, there would be no threshold effect observed among the studies. We used the chi-square test and I2 statistics to assess heterogeneity between different studies. If I2 >50%, the random effects model would be used for outcome estimation, and it adopted the method of D-L (DerSimonian-Laird) to reduce heterogeneity by increasing the weight of the studies with small sample sizes; otherwise, the fixed effects model was used and adopted the method of Mantel-Haenszel. In addition, meta-regression analyses and sensitivity analyses were used to further assess possible explanations for the heterogeneity. Publication bias was analyzed using Deeks’ funnel plot. If the Deeks’ funnel plot was symmetrical, there would be no publication bias in the included study.
Results
Study selection
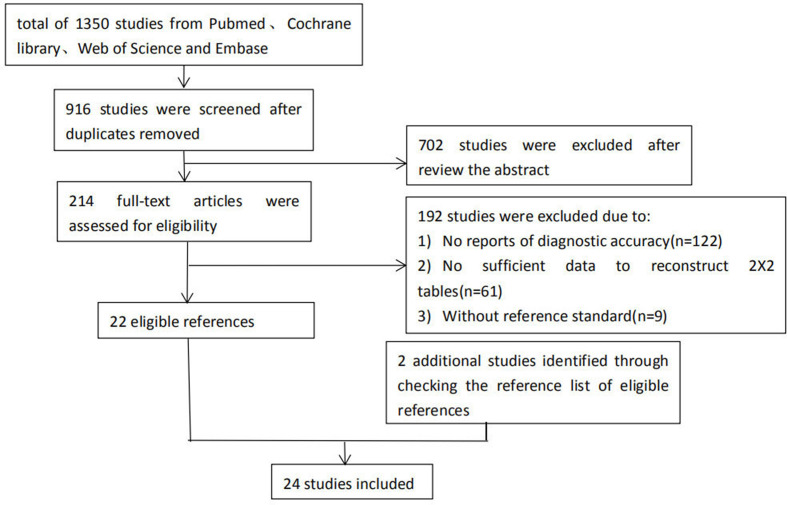
A total of 1350 studies were obtained after searching the databases of Cochrane, PubMed, EMBASE, and Web of Science. 434 duplicate studies were excluded. By reading the titles and abstracts, 214 studies were left after excluding irrelevant articles. After in-depth reading, 192 studies were excluded for the following reasons: (1) no reports of diagnostic accuracy (n = 122); (2) unable to extract complete data (n = 61); and (3) the gold standard was not pathology (n = 9). By searching the references list, two studies that met all inclusion criteria were included. This meta-analysis eventually included 2414–37 studies with a total of 2528 patients. The flowchart of literature search is shown in Figure 1.
Figure 1.
Flowchart of literature search
Characteristics of included studies
Age of the patients included in the study ranged from 20 to 80. Eighteen studies only evaluated the value of CE-MRI in monitoring the pathological response of breast cancer to NAC,14–31 three studies only evaluated the value of CESM in monitoring the pathological response of breast cancer to NAC,32,33,36 while other three studies evaluated the value of CE-MRI and CESM in monitoring the pathological response of breast cancer to NAC.34,35,37 Of the 24 included studies, seven were retrospective14,16,21,23,24,29,36 and 17 were prospective.15,17–20,22,25–28,30–35,37 The definition of pCR was not consistent in the included studies. Fourteen studies defined pCR as the complete disappearance of invasive carcinoma and ductal carcinoma in situ (DCIS),14,15,17–19,24,26,30,32–37 Ten studies defined pCR as the absence of invasive cancer.16,20–23,25,27–29,31 Histological subtypes of the tumor mainly included invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC). The characteristics of the included study are shown in Table 1.
Table 1.
Characteristics of included studies
| StudY | Year | No.of cases | Age(mean range) | Initial Tumor Size | Histologic SubtYpe |
Equipment (MRI,C-ESM)and Magnet Strength (T) | Duration of the patients (year. month) | pCR rate (%) |
Definition of pCR | Contrast Material (MRI,CESM,d-ose) |
CE-MRIor CESM used |
The research type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Los Santos14 | 2013 | 746 | 49 (20–86) | T1—4 | IDC,ILC | GE,3/1.5T | 2002.01–2011.02 | 23.99 | No residual | NR | CE-MRI | retrospective |
| Chen16 | 2008 | 51 | 49.5 (31–77) | T2—4 | NR | Philips,1.5T | NR | 54.9 | No invasive | 0.1 cc/kg | CE-MRI | retrospective |
| Nicoletto17 | 2008 | 26 | 47 (30–57) | T2—4 | IDC,ILC | NR | 2001.03–2003.06 | 23.08 | No residual | NR | CE-MRI | prospective |
| Choi, J.H18 | 2010 | 29 | 45.1 (24–63) | T2—4 | IDC,ILC | NR | 2004.12–2008.03 | 24.17 | No residual | NR | CE-MRI | prospective |
| Dose—Schwarz19 | 2010 | 46 | 50 (30–66) | T2—4 | NR | NR | NR | 10.87 | No residual | NR | CE-MRI | prospective |
| Fangberget20 | 2011 | 22 | 50.7 (37–72) | T2—4 | IDC,ILC | Siemens,1.5T | 2007.04–2008.10 | 36.36 | No invasive | 0.1 mmol/kg | CE-MRI | prospective |
| Park21 | 2011 | 32 | 45 (28–67) | NR | IDC | Siemens,1.5T | 2006.08–2008.05 | 25.00 | No invasive | 0.1 mmol/kg | CE-MRI | retrospective |
| Hayashi, Y22 | 2013 | 264 | 51 (23–71) | T1—4 | IDC | Philips,1.5T | 2003.02–2008.06 | 37.12 | No invasive | NR | CE-MRI | prospective |
| Ko, E.S23 | 2013 | 166 | 44 (23–72) | T2—4 | IDC,ILC | Philips,3T | 2007.04–2010.12 | 24.09 | No invasive | 0.1 mmol/kg | CE-MRI | retrospective |
| Williams24 | 2013 | 87 | 50 (25–83) | T2—4 | IDC,ILC | Fairfield,1.5T | 2004.01–2009.11 | 25.29 | No residual | 0.1 mmol/kg | CE-MRI | retrospective |
| Choi, BB25 | 2015 | 98 | 50 (29–81) | NR | IDC,ILC | Philips,3/1.5T | 2006–2011 | 17.35 | No invasive | 0.2 mmol/kg | CE-MRI | prospective |
| Diguisto26 | 2015 | 102 | 48.5 | NR | IDC,ILC | Siemens,1.5T | 2008.01–2011.12 | 29.41 | No residual | NR | CE-MRI | prospective |
| Lee, M.C27 | 2015 | 39 | 46.9 (24–64) | T1—4 | NR | NR | 2008–2012 | 28.21 | No invasive | NR | CE-MRI | prospective |
| Vriens28 | 2016 | 149 | NR | NR | NR | >1.5T | 2006.02–2009.04 | 16.78 | No invasive | NR | CE-MRI | prospective |
| Fukuda29 | 2016 | 265 | 49.9 (25–78) | T2—4 | NR | GE,1.5T | 2005.01–2007.12 | 7.17 | No invasive | 0.2 mmol/kg | CE-MRI | retrospective |
| Weber,JJ31 | 2017 | 129 | 50.8(27–80) | T1—4 | NR | Milwaukee,3/1.5T | 2014.06–2015.08 | 31 | No residual | 0.1 mmol/kg | CE-MRI | prospective |
| ElSaid32 | 2017 | 21 | 50 (30–77) | T3—4 | IDC,ILC,other | GE Healthcare | NR | 28.5 | No residual | 1.5 ml/kg | CESM | prospective |
| Moustafa33 | 2019 | 42 | NR | NR | IDC,ILC,other | GE Seno graphe | NR | 92.85 | No residual | NR | CESM | prospective |
| Iotti34 | 2017 | 46 | 54 (33–72) | T2—4 | IDC,ILC,other | GE,1.5T,GE Healthcare | 2012.10–2014.12 | 17 | No residual | NR | CE-MRI/CESM | prospective |
| Patel37 | 2018 | 65 | 52.7 (30–76) | T1—4 | IDC,ILC,other | GE,3.0T,GE Healthcare | NR | 30.76 | No residual | NR,1.5 ml/kg | CE-MRI/CESM | prospective |
| Barra35 | 2018 | 33 | 45(22-76) | NR | IDC,ILC,other | GE,1.5T,GE Healthcare | 2015.08–2017.12 | 24.2 | No residual | 0.1 mmol/kg,NR | CE-MRI/CESM | prospective |
| Barraa36 | 2017 | 8 | 46.41(22-76) | NR | NR | GE Healthcare | 2011–2013 | 75 | No residual | 1–2 mL/kg | CESM | retrospective |
| Trecate15 | 1999 | 30 | NR | T4/LABC | NR | Siemens,1.5T | 1995.11–1998.04 | 13.33 | No residual | 0.1 mmol/kg | CE-MRI | prospective |
| BhattacharYYa30 | 2008 | 32 | 42.7 (24–60) | T2—3 | NR | GE,1.5T | NR | 15.63 | No invasive | 16 mmol/kg | CE-MRI | prospective |
NR, not reported; pCR, pathological complete response.
Quality assessment
a study of the same author but different publication year;
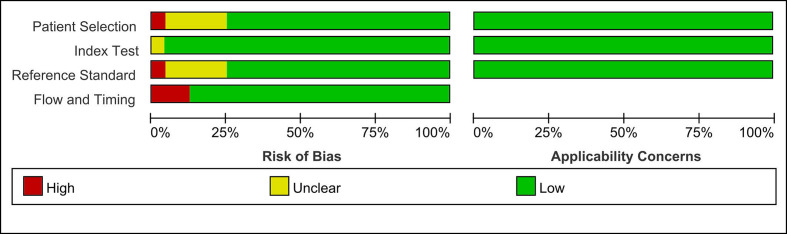
The results of QUADAS-2 quality assessment showed that the overall quality of the included studies was high. The detailed information of risk of bias of each study is shown in Figure 2. The risk of bias in five studies was considered to be high. In terms of patient selection domain, one of the studies included only patients diagnosed with inflammatory breast cancer. In terms of reference standard domain, one of the studies did not have detailed indexes to explain the final results. In terms of flow and timing domain, not all patients receive the same reference standard in the other three studies. Besides, all included studies had low concerns regarding applicability for this three domain (patient selection, index test, index test). Finally, no studies were excluded on the basis of quality assessment.
Figure 2.
Quality analysis of included studies based on QUADAS-2 criteria
Accuracy of CE-MRI and CESM
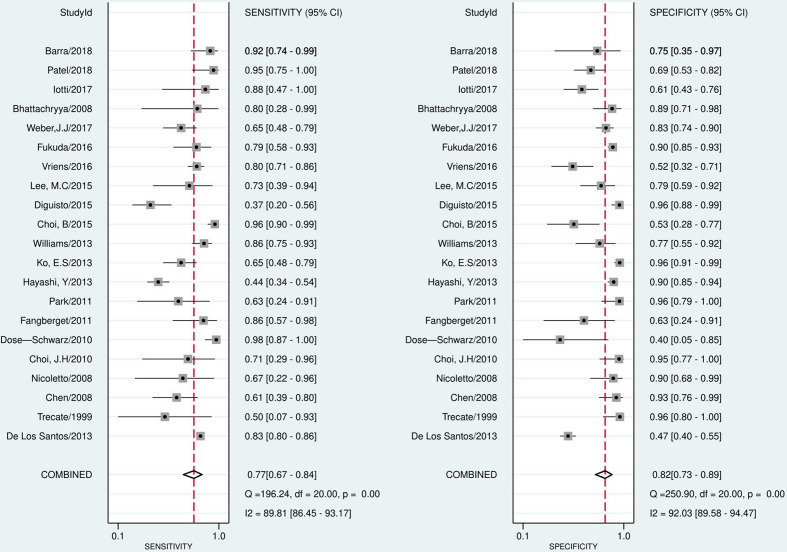
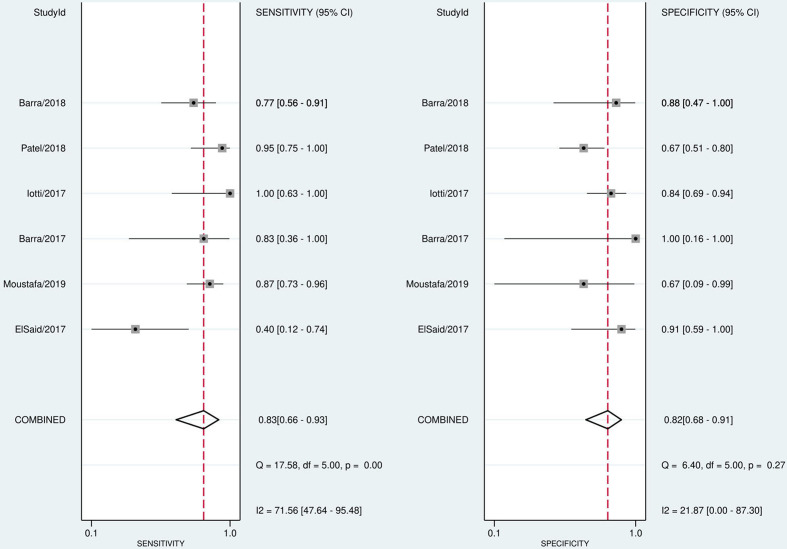
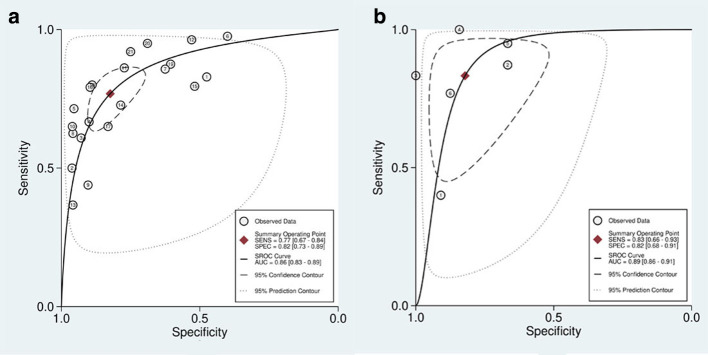
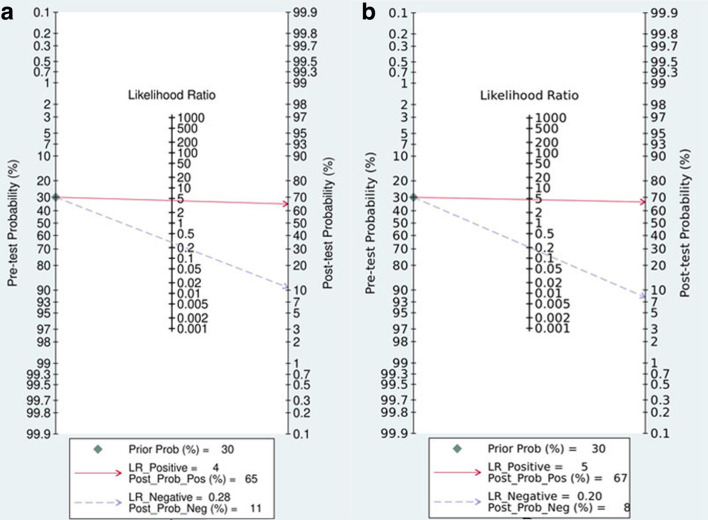
The pooled sensitivity, specificity, PLR, NLR, and DOR of the pathological response of breast cancer to NAC assessed by CE-MRI were 0.77 (95% CI, 0.67–0.84), 0.82 (95% CI, 0.73–0.89), 4.35 (95% CI, 3.00–6.33), 0.28 (95%CI, 0.20–0.39), and 15.48 (95% CI, 9.97–24.03), respectively (Figure 3). The pooled sensitivity, specificity, PLR, NLR, and DOR of the pathological response of breast cancer to NAC assessed by CESM were 0.83 (95% CI, 0.66–0.93), 0.82 (95% CI, 0.68–0.91), 4.66 (95% CI, 2.59–8.41), 0.20 (95% CI, 0.10–0.43), and 22.91 (95% CI, 8.66–60.62), respectively (Figure 4). Compared with MRI, CESM has the same specificity and higher sensitivity. The SROC curves of CE-MRI and CESM are shown in Figure 5. The AUC of CESM was higher than that of CE-MRI (0.89 vs 0.86). The likelihood ratios and post-test probabilities of CE-MRI and CESM studies were moderate (Figure 6). Use of a CE-MRI test would raise the post-test probability to 68 from 30% with a PLR of 5 when the pretest was positive and would reduce the post-test probability to 11% with an NLR of 0.35 when the pretest was negative. Use of a CESM test would raise the post-test probability to 69 from 30% with a PLR of 5 when the pretest was positive and would reduce the post-test probability to 8% with an NLR of 0.2 when the pretest was negative. These results indicate that CE-MRI and CESM were helpful for increasing the accuracy of evaluating the pathological response of breast cancer to NAC.
Figure 3.
Forest plot of CE-MRI sensitivity and specificity to predict pCR
Figure 4.
Forest plot of CESM sensitivity and specificity to predict pCR
Figure 5.
sROC to predict pathological response of breast cancer to NACT by CE-MRI (A) and CESM (B)
Figure 6.
Fagan plot of the CE-MRI (A) and CESM (B test for evaluation of the pathological response of breast cancer to NACT
Heterogeneity test
The statistical results showed that the heterogeneity of CE-MRI was high in terms of both sensitivity (I2 = 89.81%) and specificity (I2 = 92.03%). There was heterogeneity of CESM only in sensitivity (I2 = 71.56%). The spearman correlation coefficients and p values of CE-MRI and CESM were 0.840 (p = 0.000, p < 0.05) and 0.853 (p = 0.031, p < 0.05), respectively. These results indicated a threshold effect both in CE-MRI group and CESM group. CE-MRI group used the meta-regression analyses to evaluate the non-threshold effect. The results showed that the definition of pathological responder, magnetic field strength, study design, and number of patients included in the study had no significant effect on heterogeneity (Table 2). The CESM group did not conduct meta-regression analysis because it included only six studies and the sample size was too small. The sensitivity analysis of CESM group showed that the study of ElSaid et al had a significant impact on heterogeneity. The statistical analysis of the remaining five CESM studies after excluding the study of ElSaid et al showed that there was no obvious heterogeneity in sensitivity (I2 = 29.7%) and specificity (I2 = 26.4%) of the five CESM studies. The pooled sensitivity and specificity of the five CE-MRI studies to evaluate the pathological response of breast cancer to NACT were 0.87 (95% CI, 0.79–0.93), and 0.76 (95% CI, 0.66–0.84), respectively. The definition of pCR in this study was different from other studies. This study defined minimal residual disease as pCR (ypT < mic), while the other five studies defined pCR as the complete disappearance of invasive carcinoma and ductal carcinoma in situ (DCIS), which may be the main cause of heterogeneity.
Table 2.
Results of meta-regression analysis of 21 CE-MRI studies
| Parameter | Coefficient | SE | P-value |
|---|---|---|---|
| Definition of responder (pCR or near pCR) | −0.378 | 0.419 | 0.386 |
| Magnetic field (three or 1.5 T) | −0.045 | 0.462 | 0.925 |
| Study design (retrospective or prospective) | −0.051 | 0.418 | 0.905 |
| Number of patients included (≤100 or >100) | 0.081 | 0.436 | 0.856 |
Publication bias
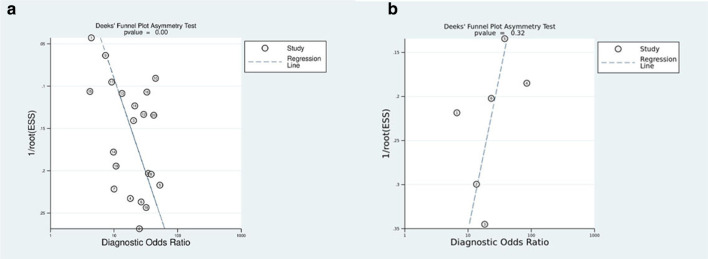
According to the results of the Deek’s test, the P-values for CE-MRI and CESM were 0.00 and 0.32, respectively. This showed that there was publication bias in 21 MRI studies and no publication bias in six CESM studies (Figure7).
Figure 7.
Deeks funnel plot of CE-MRI (A and CESM (B
Discussion
In the 1970s, NAC for breast cancer was introduced. As a preoperative treatment, it can reduce the stage of local advanced breast cancer and increase the feasibility of breast-conserving surgery.38 Early evaluation of the pathological response of breast cancer to NAC and differentiation of patients with complete pathological response (PCR) and patients without response can guide clinicians to develop a treatment plan suitable for each patient. Early assessment of the pathological response of NAC can guide the scope of surgical resection of patients with pCR and improve its clinical prognosis. Patients who have no response can find appropriate alternative treatment to avoid unnecessary toxicity.39
Currently, MRI is considered as an effective method to detect the efficacy of NAC in breast cancer, but its application is limited due to its long examination time, high cost, and intolerance of some patients. Some studies have shown that CESM has the potential as an alternative to breast MRI, and it performs breast MRI in detecting breast cancer and assessing the efficacy of treatment for breast cancer.40 A recent meta-analysis41 of 18 studies with 2859 patients was conducted to analyze the diagnostic performance of CESM for screening breast cancer. The results illustrated that the pooled sensitivity and specificity were 0.89 (95% CI, 0.88–0.91) and 0.84 (95%CI, 0.82–0.85), respectively, indicating the efficiency of CESM to diagnose breast cancer.
To our knowledge, there is currently no meta-analysis to evaluate the diagnostic performance of CESM for evaluating the pathological response of breast cancer to NAC. The purpose of this study is to use pathology as the gold standard and compare the diagnostic value of CE-MRI and CESM in evaluating the pathological response of breast cancer to NAC by meta-analysis. The study included 24 researches with a total of 2528 patients. The results of QUADAS-2 quality assessment showed that the overall quality of the included studies was high. The results of this study showed that the pooled sensitivity of 21 CE-MRI studies was 0.77 (95% CI, 0.67–0.84), the specificity was 0.82 (95% CI, 0.73–0.89), and the AUC was 0.86. The pooled sensitivity of 6 CESM studies was 0.83 (95% CI, 0.66–0.93), specificity was 0.82 (95% CI, 0.68–0.91), and the AUC was 0.89. These results indicated that CESM had the same specificity and higher sensitivity than CE-MRI, and CESM was more accurate in evaluating the pathological response of breast cancer to NAC.
In this meta-analysis, substantial heterogeneity was detected. Therefore, we performed a meta-regression analysis on 21 CE-MRI studies. The covariates were as follows: (1) the definition of pathological responder (pCP or near pCR); (2) number of patients included in the study (≤100 or>100); (3) magnetic field strength (1.5T or 3t); and (4) study design (prospective or retrospective). The results showed that the above covariates were not significant factors affecting heterogeneity. However, due to the limited sample size, we were unable to investigate all possible causes of heterogeneity. And more unmentioned differences in the study, such as tumor histological subtypes and stages, patient age, and NAC treatment options, may also be the cause of heterogeneity. In addition, we conducted sensitivity analysis on six CESM studies. After excluding ElSaid et al.’s study, the heterogeneity of sensitivity and specificity decreased significantly. We believe that this study has a significant impact on heterogeneity. We carefully reviewed Elsaid et al.’s study and found that the main difference between this study and other five CESM studies was the definition of pCR. In this study, the minimal residual disease was pCR (ypT < mic), and the other five studies suggested that the complete disappearance of invasive carcinoma and ductal carcinoma in situ was pCR, which may be the main cause of heterogeneity.
At present, there is no uniform standard for the definition of pCR. The Japanese Breast Cancer Association considers pCR to be no residual cancer cells, or necrotic or non-viable residual cancer cells, while the University of Texas MD Anderson Cancer Center (MD Anderson) believes that pCR requires not only the complete response of the primary lesion but also the disappearance of axillary lymph node metastasis. The most controversial question is whether to classify ductal carcinoma in situ (DCIS) as pCR. In our meta-analysis, 22 studies explained the definition of pCR. Ten of these 22 studies defined pCR as the absence of invasive cancer with or without DCIS, and 12 studies considered that pCR did not include DCIS. Marinovich et al42 think that the definition of pCR is the key factor affecting the accuracy of test, and it is very necessary to standardize the definition of pCR.
There were some limitations to our meta-analysis. First, 21 CE-MRI studies had high heterogeneity, and they had differences in the histological subtypes, tumor grades, and the definition of PCR. However, due to the limited sample size, we did not conduct a subgroup analysis of these 21 CE-MRI studies. Second, not all included studies were prospective, and the NAC treatment regimens were not uniform, which was one of the reasons for heterogeneity. Third, threshold effect was also a factor causing heterogeneity. Fourth, there were only six studies about the evaluation of pathological response of breast cancer to NAC assessed by CESM included in this study, which may not be enough to reflect the true performance of CESM. Therefore, more high-quality and large-scale prospective studies are needed to verify the diagnostic value of CESM in evaluating the pathological response of breast cancer to NAC. At last, there was publication bias in the 21 CE-MRI studies. One reason for publication bias may be that statistically significant studies are more likely to be published.
Conclusion
Our study showed that compared with CE-MRI, CESM has equal specificity, greater sensitivity, and excellent performance, in diagnosing the pathological response of breast cancer to NAC, and it had the advantages of being cheaper and more patient-tolerant. Therefore, we believe that CESM may have a brighter prospect in evaluating the pathological response of breast cancer to NAC in the future.
Contributor Information
Sudan Tang, Email: 1152646756@qq.com.
Chunhong Xiang, Email: 2451712639@qq.com.
Quan Yang, Email: flyingstar1120@163.com.
REFERENCES
- 1.Budny A, Starosławska E, Budny B, Wójcik R, Hys M, Kozłowski P, et al. . Epidemiology and diagnosis of breast cancer. Pol Merkur Lekarski 2019; 46: 195–204PMID. [PubMed] [Google Scholar]
- 2.Gao W, Guo N, Dong T, Wen G, Ning G, Ting D. Diffusion-Weighted imaging in monitoring the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis. World J Surg Oncol 2018; 16: 145. doi: 10.1186/s12957-018-1438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu Y-L, Pan S-M, Ren J, Yang Z-X, Jiang G-Q. Role of magnetic resonance imaging in detection of pathologic complete remission in breast cancer patients treated with neoadjuvant chemotherapy: a meta-analysis. Clin Breast Cancer 2017; 17: 245–55. doi: 10.1016/j.clbc.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 4.Colleoni M, Goldhirsch A. Neoadjuvant chemotherapy for breast cancer: any progress? Lancet Oncol 2014; 15: 131–2. doi: 10.1016/S1470-2045(13)70584-9 [DOI] [PubMed] [Google Scholar]
- 5.Tudorica A, Oh KY, Chui SY-C, Roy N, Troxell ML, Naik A, et al. . Early prediction and evaluation of breast cancer response to neoadjuvant chemotherapy using quantitative DCE-MRI. Transl Oncol 2016; 9: 8–17. doi: 10.1016/j.tranon.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Q, Huang J, Liang J, Ma M, Ye K, Shi C, et al. . The diagnostic performance of DCE-MRI in evaluating the pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Front Oncol 2020; 10: 93. doi: 10.3389/fonc.2020.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L-M, Hu J-N, Gu H-Y, Hua J, Chen J, Xu J-R, et al. . Can diffusion-weighted MR imaging and contrast-enhanced MR imaging precisely evaluate and predict pathological response to neoadjuvant chemotherapy in patients with breast cancer? Breast Cancer Res Treat 2012; 135: 17–28. doi: 10.1007/s10549-012-2033-5 [DOI] [PubMed] [Google Scholar]
- 8.Dialani V, Chadashvili T, Slanetz PJ. Role of imaging in neoadjuvant therapy for breast cancer. Ann Surg Oncol 2015; 22: 1416–24. doi: 10.1245/s10434-015-4403-9 [DOI] [PubMed] [Google Scholar]
- 9.Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. . Diagnostic accuracy of mammography, clinical examination, us, and MR imaging in preoperative assessment of breast cancer. Radiology 2004; 233: 830–49. doi: 10.1148/radiol.2333031484 [DOI] [PubMed] [Google Scholar]
- 10.Morrow M. Magnetic resonance imaging in the breast cancer patient: curb your enthusiasm. J Clin Oncol 2008; 26: 352–3. doi: 10.1200/JCO.2007.14.7314 [DOI] [PubMed] [Google Scholar]
- 11.Dromain C, Balleyguier C, Adler G, Garbay JR, Delaloge S. Contrast-Enhanced digital mammography. Eur J Radiol 2009; 69: 34–42. doi: 10.1016/j.ejrad.2008.07.035 [DOI] [PubMed] [Google Scholar]
- 12.Lobbes MBI, Lalji UC, Nelemans PJ, Houben I, Smidt ML, Heuts E, et al. . The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer 2015; 6: 144–50. doi: 10.7150/jca.10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–36. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 14.De Los Santos JF, Cantor A, Amos KD, Forero A, Golshan M, Horton JK, et al. . Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. translational breast cancer research Consortium trial 017. Cancer 2013; 119: 1776–83. doi: 10.1002/cncr.27995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trecate G, Ceglia E, Stabile F, Tesoro-Tess JD, Mariani G, Zambetti M, et al. . Locally advanced breast cancer treated with primary chemotherapy: comparison between magnetic resonance imaging and pathologic evaluation of residual disease. Tumori 1999; 85: 220–8PMID. doi: 10.1177/030089169908500402 [DOI] [PubMed] [Google Scholar]
- 16.Chen JH, Feig B, Feig B, Agrawal G, Yu H, Carpenter PM, et al. . Mri evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer 2008; 112: 17–26. doi: 10.1002/cncr.23130 [DOI] [PubMed] [Google Scholar]
- 17.Nicoletto MO, Nitti D, Pescarini L, Corbetti F, Mencarelli R, Cappetta A, et al. . Correlation between magnetic resonance imaging and histopathological tumor response after neoadjuvant chemotherapy in breast cancer. Tumori 2008; 94: 481–8PMID. doi: 10.1177/030089160809400407 [DOI] [PubMed] [Google Scholar]
- 18.Choi JH, Lim HI, Lee SK, Kim WW, Kim SM, Cho E, et al. . The role of PET CT to evaluate the response to neoadjuvant chemotherapy in advanced breast cancer: comparison with ultrasonography and magnetic resonance imaging. J Surg Oncol 2010; 102: 392–7. doi: 10.1002/jso.21424 [DOI] [PubMed] [Google Scholar]
- 19.Dose-Schwarz J, Tiling R, Avril-Sassen S, Mahner S, Lebeau A, Weber C, et al. . Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br J Cancer 2010; 102: 35–41. doi: 10.1038/sj.bjc.6605427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fangberget A, Nilsen LB, Hole KH, Holmen MM, Engebraaten O, Naume B, et al. . Neoadjuvant chemotherapy in breast cancer-response evaluation and prediction of response to treatment using dynamic contrast-enhanced and diffusion-weighted MR imaging. Eur Radiol 2011; 21: 1188–99. doi: 10.1007/s00330-010-2020-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JS, Moon WK, Lyou CY, Cho N, Kang KW, Chung J-K, et al. . The assessment of breast cancer response to neoadjuvant chemotherapy: comparison of magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography. Acta Radiol 2011; 52: 21–8. doi: 10.1258/ar.2010.100142 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi Y, Takei H, Nozu S, Tochigi Y, Ichikawa A, Kobayashi N, et al. . Analysis of complete response by MRI following neoadjuvant chemotherapy predicts pathological tumor responses differently for molecular subtypes of breast cancer. Oncol Lett 2013; 5: 83–9. doi: 10.3892/ol.2012.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko ES, Han B-K, Kim RB, Ko EY, Shin JH, Hahn SY, et al. . Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Ann Surg Oncol 2013; 20: 2562–8. doi: 10.1245/s10434-013-2925-6 [DOI] [PubMed] [Google Scholar]
- 24.Williams M, Eatrides J, Kim J, Talwar H, Esposito N, Szabunio M, et al. . Comparison of breast magnetic resonance imaging clinical tumor size with pathologic tumor size in patients status post-neoadjuvant chemotherapy. Am J Surg 2013; 206: 567–73. doi: 10.1016/j.amjsurg.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Choi BB, Kim SH. Effective factors to raise diagnostic performance of breast MRI for diagnosing pathologic complete response in breast cancer patients after neoadjuvant chemotherapy. Acta Radiol 2015; 56: 790–7. doi: 10.1177/0284185114538622 [DOI] [PubMed] [Google Scholar]
- 26.Diguisto C, Ouldamer L, Arbion F, Vildé A, Body G. Mri evaluation of residual breast cancer after neoadjuvant chemotherapy: influence of patient, tumor and chemotherapy characteristics on the correlation with pathological response. Anticancer Res 2015; 35: 581–5PMID. [PubMed] [Google Scholar]
- 27.Lee MC, Gonzalez SJ, Lin H, Zhao X, Kiluk JV, Laronga C, et al. . Prospective trial of breast MRI versus 2D and 3D ultrasound for evaluation of response to neoadjuvant chemotherapy. Ann Surg Oncol 2015; 22: 2888–94. doi: 10.1245/s10434-014-4357-3 [DOI] [PubMed] [Google Scholar]
- 28.Vriens BEPJ, de Vries B, Lobbes MBI, van Gastel SM, van den Berkmortel FWPJ, Smilde TJ, et al. . Ultrasound is at least as good as magnetic resonance imaging in predicting tumour size post-neoadjuvant chemotherapy in breast cancer. Eur J Cancer 2016; 52: 67–76. doi: 10.1016/j.ejca.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 29.Fukuda T, Horii R, Gomi N, Miyagi Y, Takahashi S, Ito Y, et al. . Accuracy of magnetic resonance imaging for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy: association with breast cancer subtype. Springerplus 2016; 5: 152. doi: 10.1186/s40064-016-1800-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharyya M, Ryan D, Carpenter R, Vinnicombe S, Gallagher CJ. Using MRI to plan breast-conserving surgery following neoadjuvant chemotherapy for early breast cancer. Br J Cancer 2008; 98: 289–93. doi: 10.1038/sj.bjc.6604171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber JJ, Jochelson MS, Eaton A, Zabor EC, Barrio AV, Gemignani ML, et al. . Mri and prediction of pathologic complete response in the breast and axilla after neoadjuvant chemotherapy for breast cancer. J Am Coll Surg 2017; 225: 740–6. doi: 10.1016/j.jamcollsurg.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ElSaid NAE, Mahmoud HGM, Salama A, Nabil M, ElDesouky ED, et al. . Role of contrast enhanced spectral mammography in predicting pathological response of locally advanced breast cancer post neo-adjuvant chemotherapy. The Egyptian Journal of Radiology and Nuclear Medicine 2017; 48: 519–27Available from. doi: 10.1016/j.ejrnm.2017.03.022 [DOI] [Google Scholar]
- 33.Moustafa AFI, Kamal RM, Gomaa MMM, Mostafa S, Mubarak R, El-Adawy M, et al. . Quantitative mathematical objective evaluation of contrast-enhanced spectral mammogram in the assessment of response to neoadjuvant chemotherapy and prediction of residual disease in breast cancer. Egyptian Journal of Radiology and Nuclear Medicine 2019; 50: 1–13. doi: 10.1186/s43055-019-0041-8 [DOI] [Google Scholar]
- 34.Iotti V, Ravaioli S, Vacondio R, Coriani C, Caffarri S, Sghedoni R, et al. . Contrast-Enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res 2017; 19: 106. doi: 10.1186/s13058-017-0899-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barra FR, Sobrinho AB, Barra RR, Magalhães MT, Aguiar LR, de Albuquerque GFL, et al. . Contrast-Enhanced mammography (CEM) for detecting residual disease after neoadjuvant chemotherapy: a comparison with breast magnetic resonance imaging (MRI. Biomed Res Int 2018; 2018: 1: 8531916–9. doi: 10.1155/2018/8531916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barra FR, de Souza FF, Camelo REFA, Ribeiro ACdeO, Farage L. Accuracy of contrast-enhanced spectral mammography for estimating residual tumor size after neoadjuvant chemotherapy in patients with breast cancer: a feasibility study. Radiol Bras 2017; 50: 224–30. doi: 10.1590/0100-3984.2016-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel BK, Hilal T, Covington M, Zhang N, Kosiorek HE, Lobbes M, et al. . Contrast-Enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following neoadjuvant systemic therapy. Ann Surg Oncol 2018; 25: 1350–6. doi: 10.1245/s10434-018-6413-x [DOI] [PubMed] [Google Scholar]
- 38.Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL, et al. . Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer 1980; 16: 351–6. doi: 10.1016/0014-2964(80)90352-7 [DOI] [PubMed] [Google Scholar]
- 39.Matsuda N, Kida K, Ohde S, Suzuki K, Yamauchi H, Nakamura S, et al. . Change in sonographic brightness can predict pathological response of triple-negative breast cancer to neoadjuvant chemotherapy. Breast Cancer 2018; 25: 43–9. doi: 10.1007/s12282-017-0782-z [DOI] [PubMed] [Google Scholar]
- 40.Fallenberg EM, Dromain C, Diekmann F, Renz DM, Amer H, Ingold-Heppner B, et al. . Contrast-Enhanced spectral mammography: does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res Treat 2014; 146: 371–81. doi: 10.1007/s10549-014-3023-6 [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Huang J-M, Zhang K, Xia L-J, Feng L, Yang P, et al. . Diagnostic value of contrast-enhanced spectral mammography for screening breast cancer: systematic review and meta-analysis. Clin Breast Cancer 2018; 18: e985–95. doi: 10.1016/j.clbc.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 42.Marinovich ML, Sardanelli F, Ciatto S, Mamounas E, Brennan M, Macaskill P, et al. . Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast 2012; 21: 669–77. doi: 10.1016/j.breast.2012.07.006 [DOI] [PubMed] [Google Scholar]