Abstract
The distinction of non-muscle-invasive bladder cancer and muscle-invasive bladder cancer is important for the selection of the optimal treatment. Multiparametric MRI (mp-MRI) has been an useful modality for the T staging of bladder cancer, and a systematic evaluation of mp-MRI is needed. The Vesical Imaging Reporting and Data System was designed to standardize the scanning and reporting criteria based on mp-MRI for clinical and research applications. This review briefly describes the method, interpretation, and timing of mp-MRI examinations in the clinical settings. Validation studies of Vesical Imaging Reporting and Data System and future perspectives are also considered.
Introduction
Bladder cancer is one of the most common diseases worldwide. There are approx. 430,000 incident cases of bladder cancer per year, with 165,000 deaths.1 The majority of bladder cancers are urothelial cell carcinomas, and these can be divided into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). The first choice of treatment for NMIBC has been transurethral resection of the bladder tumor (TUR-BT) or intravesical chemotherapy or immunotherapy, whereas the first choice of treatment for MIBC has been cystectomy or systemic chemotherapy. Therefore, the distinction of NMIBC and MIBC is one of the most important issues in the treatment of bladder cancer.
For the staging of bladder cancer, CT and MRI are the important modalities of choice. Although CT is the first choice for N (node) or M (metastasis) staging, the accuracy of T (tumor) staging, especially for the distinction of NMIBC and MIBC, is insufficient.2,3 Multiparametric-MRI (mp-MRI) has recently become one of the most important modalities of choice for the T staging of bladder cancer. Meta-analyses of mp-MRI were published in 2017–18,4,5 and the Vesical Imaging Reporting And Data System (VI-RADS) was released in 2018 to standardize the scanning and reporting criteria based on mp-MRI.6 VI-RADS score might fit in several settings, not only for initial staging to avoid second look biopsy, but also for the evaluation of response to chemotherapy to avoid unnecessary total cystectomy. It might contribute to reduce total lifetime medical cost of bladder cancers.7
.In this review, we briefly describe the method, interpretation and clinical timing of mp-MRI examinations, the staging method of mp-MRI using the VI-RADS, and clinical validation studies of mp-MRI based on the VI-RADS. We also discuss future perspectives regarding staging strategy using the VI-RADS with mp-MRI.
The method, interpretation, and timing of mp-MRI in clinical settings
The mp-MRI method
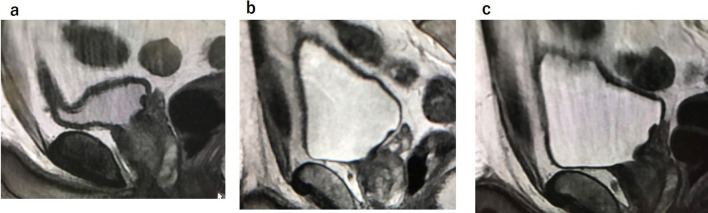
Adequate bladder distention is necessary for the optimal visualization of the bladder wall. To achieve sufficient distention, patients are asked to void 1–2 h before imaging or to drink 500–1000 ml of water 30 min before the examination.8 For the prevention of motion and susceptibility artifacts from bowel peristalsis, the administration of an intramuscular antispasmodic agent is recommended (Figure 1).9
Figure 1.
Vesical wall distension is very important. (a) The bladder wall is less distended. (b) Void and drink 500–1000 cc of water after urination 1 h prior to MRI. Antispasmodic agent is given. The bladder wall is well distended, and there is no motion artifact. (c) Over distended bladder wall without antispasmodic agent. Motion artifact occurs.
A mp-MRI examination for bladder cancer consists of a T2 weighted image (T2WI), a diffusion-weighted image (DWI), and a dynamic-contrast enhanced (DCE) image. To achieve high spatial resolution, 1.5 or 3.0 T MRI is recommended. Sensitivity and specificity were reported to be better with 3.0 T MRI as compared to 1.5 T MRI.4,5 T2WIs are usually obtained with two-dimensional (2D) fast spin echo (FSE) or three-dimensional (3D) spin echo acquisitions. On DWI, a spin echo EPI sequence combined with fat saturation is recommended. A high b-value (800–1000 s/mm2) is necessary for the visualization of bladder cancer. On DCE, a gadolinium-based contrast agent is administered with at a rate of 1.5–2.0 ml/s. The initial contrast image is obtained at 30 s after the injection of the contrast agent and is followed by the same sequences 4–6 times every 30 s for the visualization of inner layer enhancement (ILE) when the tumor is contrast-enhanced.10
The interpretation of mp-MRI findings
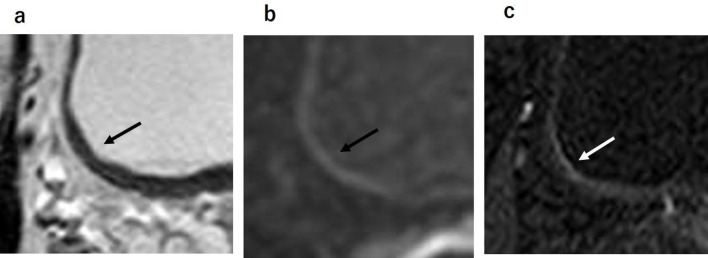
On T2WI, the urine, normal mucosa, and submucosa of the bladder wall is visualized as a high-signal intensity (SI) layer. The muscle layer of the bladder wall is visualized as a low-SI layer. As a result, in normal bladder wall the mucosa and submucosa are canceled out by urine (Figure 2a). On DWI, the urine, mucosa, and submucosa of the bladder wall are visualized as low-SI, and thus the mucosa and submucosa are canceled out by normal bladder wall.11 The muscle layer of the bladder wall is visualized as intermediate-SI on DWI (Figure 2b). In some cases, part of the muscle layer is affected by motion artifact from peristalsis the colon or small intestine. On DCE, the mucosa and submucosa are often enhanced early after the administration of the contrast agent, but the muscle layer maintains its hypo-SI and is later contrast-enhanced (Figure 2C).
Figure 2.
Normal bladder wall. (a, b) The urine, normal mucosa and submucosa of the bladder wall has high signal intensity on T2WI and has low signal intensity on DWI. Therefore, normal mucosa and submucosa are not visualized on T2WI and DWI. (c) On dynamic contrast enhanced image, the mucosa and submucosa are often enhanced early after contrast material administration. DWI, diffusion-weighted image; T2WI,T2weighted image.
Bladder cancer shows iso- to slightly higher SI compared to the SI of the muscle layer on T2WI. In patients with muscle-invasive tumors, the low SI of the muscle layer is often interrupted by the slightly high SI of the tumor. If the bladder cancer is iso-SI to the muscle layer, it is difficult to evaluate tumor invasion with T2WI alone. The SI values of bladder cancers are very high on DWI, but it is difficult to recognize anatomical structures on DWI. Comparisons of T2WI and DWI findings are thus necessary to identify the locations of tumors. For example, it was reported that the apparent diffusion coefficient (ADC) value of a bladder carcinoma was 1.18 × 10−3 mm2/s, which was significantly lower than that of urine (3.28 × 10−3 mm2/s) and normal bladder wall (2.27 × 10−3 mm2/s).12 A bladder cancer tumor is enhanced early after the administration of contrast material. Hayashi et al10 reported that intact ILE adjacent to a tumor indicated stage T1 or lower.
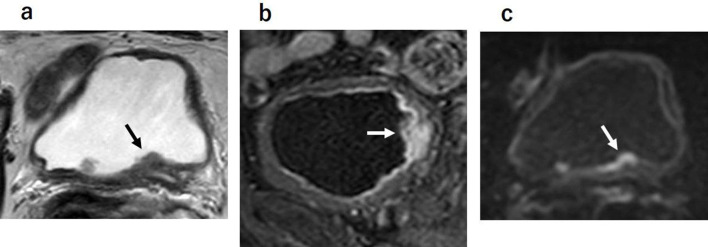
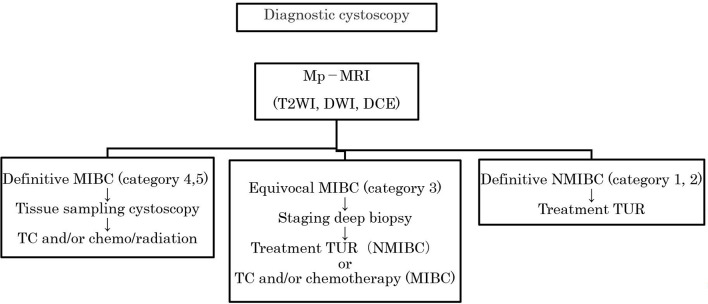
There have been several important reports on mp-MRI for the T staging of bladder cancer. Takeuchi et al13 reported that a high-SI bladder cancer with the presence of a low-SI submucosal stalk or thickened submucosa suggests bladder cancer at stage T1 or lower. The submucosal components have shown low signal intensity on DWI, representing a mixture of edematous submucosa, fibrous tissue, capillaries, and mild inflammatory cell infiltration that result in a tumor stalk or thickened submucosa (Figure 3). The SI of the tumor stalks observed in cases of stage Ta or T1 bladder cancer varies on T2WI,14 and it is thus sometimes difficult to make a T-stage-based diagnosis of bladder cancer with a stalk on T2WI alone.
Figure 3.
High SI bladder cancer over the low SI submucosal stalk or a thickened submucosa suggests bladder cancer with T1 or lower. Submucosal components showed low signal intensity on DWI. DWI, diffusion-weighted image; SI, signalintensity.
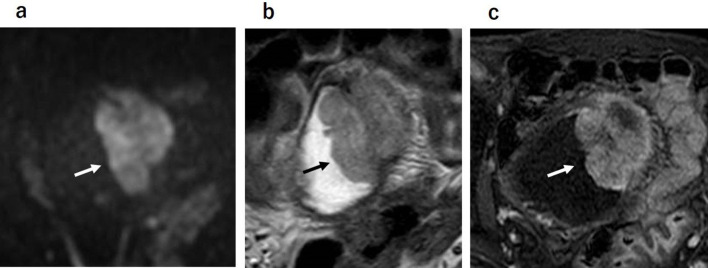
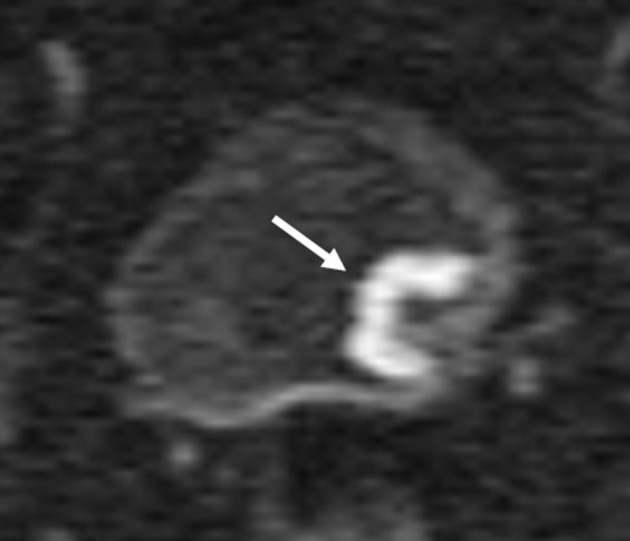
Wang et al15 reported that all of the <10.0 mm stage T1 or lower bladder cancer tumors without any stalks that they examined did not have ILE. In addition, all of the tumors without ILE had no stalks, of which 94.1% were stage T1 and 82.4% were <10.0 mm. Moreover, 20% of all of the tumors with discontinuous ILE and no stalk were NMIBC. According to that report, tumors with a dia. <10.0 mm would be NMIBC, and stalks are not usually seen in these tumors. On the other hand, tumors without ILE or with discontinuous ILE and no stalk would not be always MIBC (Figure 4).
Figure 4.
Bladder cancer of stage T1. Tumor without ILE or with discontinuous ILE and no stalk are not the finding of muscle invasion. ILE, inner layer enhancement.
Tekes et al16 reported that 81% of bladder tumors showed SI similar to that of muscle on T2WI, and over staging was the most common error when evaluating the T-stage on T2WI. They noted that a low-SI area similar to a tumor reflecting inflammatory change or fibrosis (which are sometimes seen around tumor invasion) could cause over staging. In contrast, the improvement of the spatial resolution of DCE would enable the detection of muscle or perivesical-fat invasion, which is considered to be perivesical-fat invasion rather than tumor invasion. These findings can cause false positive findings of muscle or perivesical-fat invasion of bladder cancer. On DWI, these inflammatory changes or fibrosis appear as low SI and thus distinguishable from tumor invasion (Figure 5).
Figure 5.
Bladder cancer of stage T1. (a, b) Inflammatory change or fibrosis, which are sometimes resemble tumor invasion, could cause over staging on T2 weighted image or dynamic-contrast enhanced image. (c) On DWI, these inflammatory changes or fibrosis appear as low signal intensity, thus enable distinct from tumor invasion on DWI. DWI, diffusion-weighted image
Although DWI is the most important sequence for the T-staging of bladder cancer. The images are often noisy with artefacts. They may obscure the tumor. This makes the diagnosis in all cases difficult. In these particular cases, an evaluation with T2WI and DCE is required instead of DWI (Figure 6).
Figure 6.
(a) (DWI sometimes causes motion artifact from colon or small intestine (arrow). (b, c) Evaluation with T2 weighted image and dynamic-contrast enhanced image is needed instead of DWI. DWI, diffusion-weighted image
The two recent meta-analyses of MRI for evaluations of bladder cancer4,5 revealed that the pooled sensitivity and specificity were 90–92% and 78–88%, and that 3.0 T MRI was better than 1.5 T MRI for differentiating NMIBC and MIBC. Sungmin et al4 mentioned that the sensitivity and specificity for distinguishing NMIBC and MIBC were highest with mp-MRI (94 and 95%). In the second meta-analysis,5 the sensitivity was slightly better with DCE (90%) than with DWI (86%), and the specificity was better with DWI (92%) than with DCE (64%). According to these meta-analyses,4,5 mp-MRI using DWI is the optimal protocol for the T-staging of bladder cancer.
The timing of the mp-MRI examination
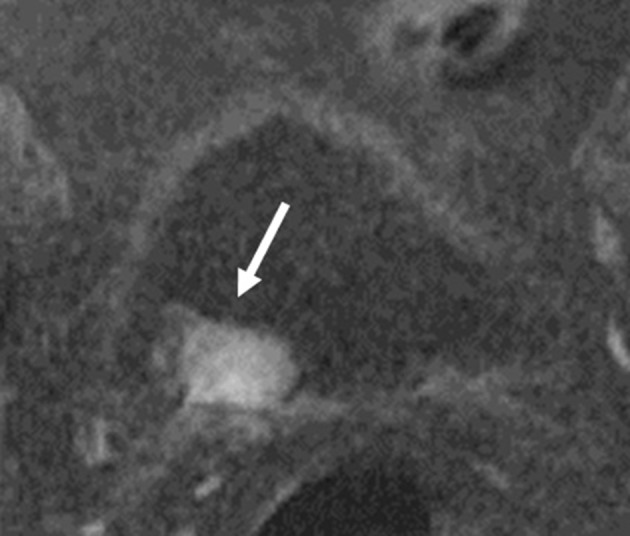
Previous TUR-BT and intravesical chemotherapy usually cause edema and inflammation in the bladder wall. In such cases, it may be difficult to distinguish the remaining tumor from benign treatment-related effects17 (Figure 7). As a result, the T-stage of bladder cancer may be overestimates on MRI or CT. Treatment-related effects can persist for up to 18–24 months.18 In contrast, Takes et al16 concluded that the time interval between MRI and TUR-BT (≤60 and ≥61 days) was not a statistically significant factor. However, we recommend mp-MRI before TUR-BT or intravesical chemotherapy, because the initial appearance (peduncle, ILE, surrounding inflammatory change) of tumors is lost after TUR-BT on mp-MRI, and thus the VI-RADS cannot be used for T-staging. If TUR-BT was performed in a patient before mp-MRI, an interval of ≥8 weeks would be recommended for correct T-staging.
Figure 7.
MRI obtained 2 weeks after TUR-BT. (a) Contrast enhanced CT. A tumor was detected on left-side wall. (b) There was thickness of left-side wall on T2 weighted image. Inflammatory change appeared due to TUR-BT. TUR-BT, transurethralresection of the bladder tumor
The mp-MRI staging method using the VI-RADS
Based on the reports of mp-MRI-based assessments of bladder cancer, Panebianco and Narumi et al6 released the VI-RADS for local bladder cancer staging in May 2018. The aim of the VI-RADS is to standardize the scanning and bladder reporting format based on mp-MRI for clinical and research applications. In the VI-RADS, an mp-MRI examination consists of a combination of T2WI, DWI, and DCE imaging sequences (Table 1). On T2WI, ≥2 planes of multiplanar (axial, coronal, and sagittal) images without fat suppression are usually obtained with 2D fast spin echo; 3D spin echo acquisitions may be used as an adjunct to 2D acquisitions. On DWI, axial and sagittal/coronal breathing-free spin echo EPI sequences combined with spectral fat saturation is recommended. The scanning protocol of the DWI should be similar to T2WI to permit localization. On DCE, although either a 2D or 3D T1 gradient echo (GRE) sequence with fat suppression may be used, 3D acquisition is preferred to obtain higher spatial resolution.19 If 3D images are acquired with isotropic voxels on T2WI or DCE, an arbitrary plane perpendicular to the tumor-bladder wall interface can be reformatted.
Table 1.
Examples of parameter setting for 3.0 T MRI
| T2WI | DWI | DCE | |
|---|---|---|---|
| Parameter setting at 3.0 T | |||
| TR (ms) | 4690 | 2500–5300 | 3.8 |
| TE (ms) | 119 | 61 | 1.2 |
| Flip angle (degree) | 90 | 90 | 15 |
| FOV (cm) | 23 | 32 | 27 |
| Matrix | 400 × 256–320 | 128 × 128 | 192 × 192 |
| Slice thickness (mm) | 3–4 | 3–4 | 1 |
| Slice gap (mm) | 0–0.4 | 0.3–0.4 | 0 |
| Number of excitations | 2–3 | 4–10 | 1 |
| b values (s/mm2) | 0–800 - 1000 |
DCE, dynamic contrast enhanced; DWI, diffusion-weighted image; FOV, filed of view; TE, echo time; TR, repetition time; T2 WI, T2 weighted image.
The VI-RADS classifies the probability of bladder cancer invasion to muscle by using a 5-point scoring system:
VI-RADS 1; muscle invasion is highly unlikely
VI-RADS 2; muscle invasion is unlikely.
VI-RADS 3; muscle invasion is equivocal.
VI-RADS 4; muscle invasion is likely.
VI-RADS 5; muscle invasion is very likely.
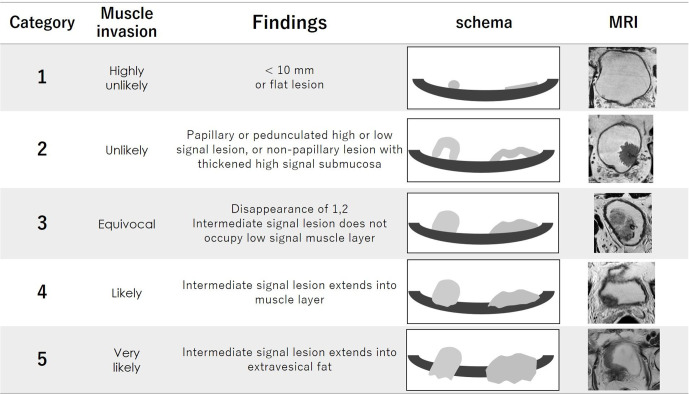
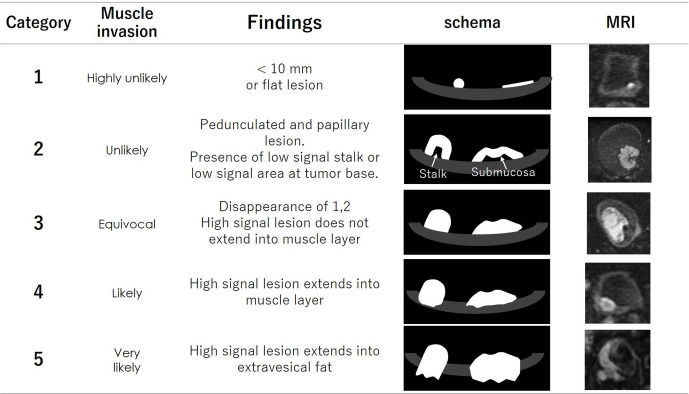
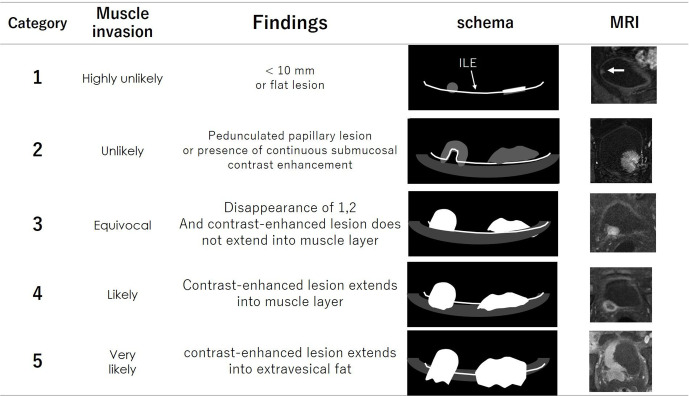
The VI-RADS score is defined using individual categories of T2WI, DWI and DCE. Tumors with a dia. of <10.0 mm and flat lesions were reported to be almost all non-muscle invasive,15 and these tumors were selected as Category 1. Category 2 is defined as tumors with a dia. ≥1 cm and a thickened inner layer. Category 3 is defined as the disappearance of Category 2 findings, but no clear disruption of a low-SI muscle layer. Category 4 is defined as the interruption of a low-SI line suggesting a muscle layer. Category 5 is defined as an extension of an intermediate SI tumor to extra vesical fat.
The VI-RADS scores 1 to 5 of each sequence are shown in Figures 8–10. The scoring is done based first on T2WI for the determination of morphology, because of the higher spatial resolution of T2WI in the evaluation of the integrity of the muscle layer. The most commonly used sequence for determining the VI-RADS score is DWI (first) and DCE (second).6 DWI is used for the final category when DWI is available. DCE is used for the final category when the DWI results are suboptimal due to artifacts or noises. If DCE is also suboptimal, T2WI is used for the final category.
Figure 8.
The category of T2WI. Category 1 to 5 are shown on schema and corresponding T2WI. Possibility of muscle invasion is higher with greater number of category. T2WI, T2 weighted image.
Figure 9.
The category of DWI. Category 1 to 5 are shown on schema and corresponding DWI. Possibility of muscle invasion is higher with greater number of category. DWI, diffusion-weighted image.
Figure 10.
The category of DCE image. Category 1 to 5 are shown on schema and corresponding DCE. Possibility of muscle invasion is higher with greater number of category. DCE dynamic-contrast enhanced.
Reporting of mp-MRI using VI-RADS
One issue regarding the VI-RADS has been its diagnostic performance and reproducibility. Several research groups recently evaluated the diagnostic performance of the VI-RADS20–25 (Table 2). Overall, the sensitivity and specificity of the VI-RADS for bladder cancer staging have been 82–92% and 44–91% respectively when the cut-off VI-RADS score ≥3 was used. The sensitivity and specificity of the VI-RADS for bladder cancer staging have been 76–91% and 76–93%, respectively when the cut-off VI-RADS score ≥4 was used. Interobserver agreements were good or excellent in all of those reports.
Table 2.
Sensitivity and specificity of VI-RADS in previous reports
| Number | Cut-off category | Sensitivity (%) | Specificity (%) | Interobserver agreement | |
|---|---|---|---|---|---|
| Ueno Y et al. | 74 |
>4 >3 |
76 88 |
93 77 |
0.85 |
| Barchetti G et al. | 75 |
>4 >3 |
82–91 77–82 |
85–89 89–94 |
0.73 |
| Wang H et al. | 340 | >3 | 87 | 97 | 0.92 |
| Kim SH et al. | 339 |
>4 >3 |
91 95 |
76 44 |
0.85 |
| Giudice FD et al. | 231 | >3 | 92 | 91 | 0.81 |
| Makboul M et al. | 50 | >4 | 78 | 88 | 0.87 |
VI-RADS, Vesical Imaging Reporting and Data System.
Category 3 or 4 was adopted for cut-off category ofmuscle invasion.
According to these results, the diagnostic performance of the VI-RADS is good. The sensitivity was slightly higher when the cut-off VI-RADS ≥3 was used, whereas the specificity was slightly higher when the cut-off VI-RADS ≥4 was used. The diagnostic ability of the VI-RADS for the T-staging of bladder cancer has been comparatively good, but there are still some false-positive or false-negative cases. Further multi-institutional and meta-analyses are needed for a more thorough validation of the VI-RADS.
Possible clinical algorithm using the VI-RADS in the future
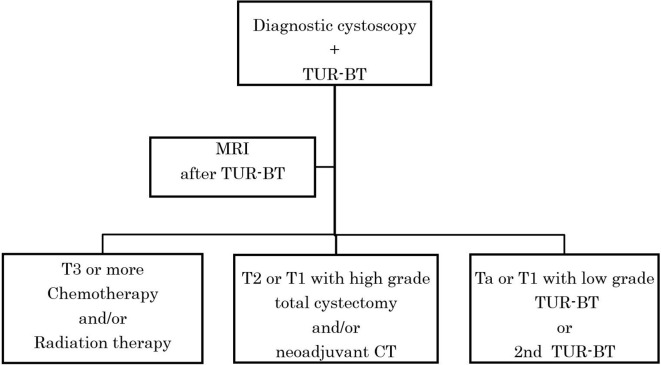
The current clinical algorithm used at many institutions as the gold standard of MIBC is TUR-staging first, followed by MRI staging (Figure 11). However, the TUR-staging is not perfect. It was reported that 48 and 46.7% of bladder cancers were under staged in the first- and second-look staging, respectively in a study in which total cystectomy was applied as the gold-standard.26 At present, MRI can be used as a first-line method for the stratification of diagnoses to distinguish apparent MIBC (VI-RADS score 4, 5) from apparent NMIBC (VI-RADS score 1, 2). A staging biopsy may be used for selective cases of equivocal MIBC (VI-RADS score 3) (Figure 12). In this way, we can reduce the total number of second look staging biopsy possibly resulting in reduction of total diagnostic cost. Future studies should justify this staging algorism by comparing TUR-staging biopsy and MRI staging applying total cystectomy as the gold-standard.
Figure 11.
Current Algorism for T staging of bladder cancer. Clinical algorism as the gold-standard of MIBC is TUR-staging first, followed by MRI staging. MIBC, muscle-invasivebladder cancer; TUR-BT, transurethral resection of the bladder tumor.
Figure 12.
Proposed New Algorism of VI-RADS with mp-MRI to stratify patients. Mp-MRI can be used as first line method for stratification of diagnosis to distinguish apparently MIBC (VI-RADS 4, 5) from apparently NMIBC (VI-RADS 1, 2). Staging biopsy may be used for selective cases of equivocal MIBC (VI-RADS 3). DWI, diffusion-weighted image; MIBC, muscle-invasivebladder cancer; mp-MRI, multiparametric-MRI; NMIBC, non-muscle-invasive bladdercancer; T2WI,T2weighted image; TUR-BT, transurethral resection of the bladdertumor.
Conclusion
Mp-MRI has been an important method for the T-staging of bladder cancer. We need to know the characteristics of each sequence of mp-MRI for bladder cancer. As VI-RADS scoring is an excellent tool, we proposed diagnostic strategy with mp-MRI before TUR-BT using VI-RADS scoring in patients with bladder cancer. Multi-institutional studies are awaited comparing TUR-BT staging and mp-MRI scoring using the VI-RADS.
Contributor Information
Hiroshi Juri, Email: rad103@poh.osaka-med.ac.jp.
Yoshifumi Narumi, Email: naru21747@gmail.com.
Valeria. Panebianco, Email: valeria.panebianco@uriroma1.it.
Keigo Osuga, Email: osuga@osaka-med.ac.jp.
REFERENCES
- 1.Cumberbatch MGK, Noon AP. Epidemiology, aetiology and screening of bladder cancer. Transl Androl Urol 2019; 8: 5–11. doi: 10.21037/tau.2018.09.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stojovska-Jovanovska E, Mitreska N, Stojovski M, Lazarova A, Stavridis S, Dodevski A. Computed tomography or magnetic resonance imaging – our experiences in determining preoperative TNM staging of bladder cancer. Pril 2013;: 3463–70. [PubMed] [Google Scholar]
- 3.Vargas HA, Akin O, Schöder H, Olgac S, Dalbagni G, Hricak H, et al. Prospective evaluation of MRI, ¹¹C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol 2012; 81: 4131–7. doi: 10.1016/j.ejrad.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 4.Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Diagnostic performance of MRI for prediction of muscle-invasiveness of bladder cancer: a systematic review and meta-analysis. Eur J Radiol 2017; 95: 46–55. doi: 10.1016/j.ejrad.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Kong Q, Liu Z, Wang J, Kang Z, Zhu Y. The diagnostic value of Mr imaging in differentiating T staging of bladder cancer: a meta-analysis. Radiology 2018; 286: 502–11. doi: 10.1148/radiol.2017171028 [DOI] [PubMed] [Google Scholar]
- 6.Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (vesical Imaging-Reporting and data system. Eur Urol 2018; 74: 294–306. doi: 10.1016/j.eururo.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Gonzalez JE, Reyes NR. Patterns of global health financing and potential future spending on health. The Lancet 2017; 389: 1955–6. doi: 10.1016/S0140-6736(17)31001-2 [DOI] [PubMed] [Google Scholar]
- 8.Panebianco V, Barchetti F, de Haas RJ, Pearson RA, Kennish SJ, Giannarini G, et al. Improving staging in bladder cancer: the increasing role of multiparametric magnetic resonance imaging. Eur Urol Focus 2016; 2: 113–21. doi: 10.1016/j.euf.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 9.Johnson W, Taylor MB, Carrington BM, Bonington SC, Swindell R. The value of hyoscine butylbromide in pelvic MRI. Clin Radiol 2007; 62: 1087–93. doi: 10.1016/j.crad.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 10.Hayashi N, Tochigi H, Shiraishi T, Takeda K, Kawamura J. A new staging criterion for bladder carcinoma using gadolinium-enhanced magnetic resonance imaging with an endorectal surface coil: a comparison with ultrasonography. BJU Int 2000; 85: 32–6. doi: 10.1046/j.1464-410x.2000.00358.x [DOI] [PubMed] [Google Scholar]
- 11.Narumi Y, Kadota T, Inoue E, Kuriyama K, Horinouchi T, Kasai K, et al. Bladder wall morphology: in vitro Mr imaging-histopathologic correlation. Radiology 1993; 187: 151–5. doi: 10.1148/radiology.187.1.8451403 [DOI] [PubMed] [Google Scholar]
- 12.Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-Weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol 2007; 17: 201–4. doi: 10.1007/s00330-006-0281-7 [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009; 251: 112–21. doi: 10.1148/radiol.2511080873 [DOI] [PubMed] [Google Scholar]
- 14.Saito W, Amanuma M, Tanaka J, Heshiki A. Histopathological analysis of a bladder cancer stalk observed on MRI. Magn Reson Imaging 2000; 18: 411–5. doi: 10.1016/S0730-725X(00)00124-7 [DOI] [PubMed] [Google Scholar]
- 15.Wang H-J, Pui MH, Guan J, Li S-R, Lin J-H, Pan B, et al. Comparison of early submucosal enhancement and tumor stalk in staging bladder urothelial carcinoma. AJR Am J Roentgenol 2016; 207: 797–803. doi: 10.2214/AJR.16.16283 [DOI] [PubMed] [Google Scholar]
- 16.Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol 2005; 184: 121–7. doi: 10.2214/ajr.184.1.01840121 [DOI] [PubMed] [Google Scholar]
- 17.Kim B, Semelka RC, Ascher SM, Chalpin DB, Carroll PR, Hricak H. Bladder tumor staging: comparison of contrast-enhanced CT, T1- and T2-weighted MR imaging, dynamic gadolinium-enhanced imaging, and late gadolinium-enhanced imaging. Radiology 1994; 193: 239–45. doi: 10.1148/radiology.193.1.8090898 [DOI] [PubMed] [Google Scholar]
- 18.Johnson RJ, Carrington BM, Jenkins JP, Barnard RJ, Read G, Isherwood I, et al. Accuracy in staging carcinoma of the bladder by magnetic resonance imaging. Clin Radiol 1990; 41: 258–63. doi: 10.1016/S0009-9260(05)81661-7 [DOI] [PubMed] [Google Scholar]
- 19.Donaldson SB, Bonington SC, Kershaw LE, Cowan R, Lyons J, Elliott T, et al. Dynamic contrast-enhanced MRI in patients with muscle-invasive transitional cell carcinoma of the bladder can distinguish between residual tumour and post-chemotherapy effect. Eur J Radiol 2013; 82: 2161–8. doi: 10.1016/j.ejrad.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 20.Ueno Y, Takeuchi M, Tamada T, Sofue K, Takahashi S, Kamishima Y, et al. Diagnostic accuracy and interobserver agreement for the vesical Imaging-Reporting and data system for muscle-invasive bladder cancer: a multireader validation study. Eur Urol 2019; 76: 54–6. doi: 10.1016/j.eururo.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 21.Barchetti G, Simone G, Ceravolo I, Salvo V, Campa R, Del Giudice F, et al. Multiparametric MRI of the bladder: inter-observer agreement and accuracy with the vesical Imaging-Reporting and data system (VI-RADS) at a single reference center. Eur Radiol 2019; 29: 5498–506. doi: 10.1007/s00330-019-06117-8 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Luo C, Zhang F, Guan J, Li S, Yao H, et al. Multiparametric MRI for bladder cancer: validation of VI-RADS for the detection of detrusor muscle invasion. Radiology 2019; 291: 668–74. doi: 10.1148/radiol.2019182506 [DOI] [PubMed] [Google Scholar]
- 23.Kim SH. Validation of vesical imaging reporting and data system for assessing muscle invasion in bladder tumor. Abdom Radiol 2020; 45: 491–8. doi: 10.1007/s00261-019-02190-1 [DOI] [PubMed] [Google Scholar]
- 24.Del Giudice F, Barchetti G, De Berardinis E, Pecoraro M, Salvo V, Simone G, et al. Prospective assessment of vesical imaging reporting and data system (VI-RADS) and its clinical impact on the management of high-risk non-muscle-invasive bladder cancer patients candidate for repeated transurethral resection. Eur Urol 2020; 77: 101–9. doi: 10.1016/j.eururo.2019.09.029 [DOI] [PubMed] [Google Scholar]
- 25.Makboul M, Farghaly S, Abdelkawi IF. Multiparametric MRI in differentiation between muscle invasive and non-muscle invasive urinary bladder cancer with vesical imaging reporting and data system (VI-RADS) application. Br J Radiol 2019; 92: 20190401. doi: 10.1259/bjr.20190401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ark JT, Keegan KA, Barocas DA, Morgan TM, Resnick MJ, You C, et al. Incidence and predictors of understaging in patients with clinical T1 urothelial carcinoma undergoing radical cystectomy. BJU Int 2014; 113: 894–9. doi: 10.1111/bju.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]