Abstract
Objective:
To assess the safety and efficacy of endovascular implantation of a portal vein stent combined with iodine-125 seed-strips followed by transcatheter arterial chemoembolization with sorafenib (PVS-125I-TACE-S) for the treatment of hepatocellular carcinoma (HCC) with portal vein tumor thrombosis (PVTT).
Methods:
Between January 2015 and July 2017, 18 patients with PVTT caused by HCC that were treated with PVS-125I-TACE-S were reviewed. The technical success, complications, changes in liver function from baseline values due to subsequent endovascular implantation of a portal vein stent combined with iodine-125 seed-strips (PVS-125I), time-to-tumor progression (TTP) and overall survival (OS) were observed.
Results:
The technical success rate was 100%. Adverse events (AEs) were managed successfully, with no occurrence of procedure-related deaths. Liver function test values after PVS-125I were not significantly different than baseline values (P>0.05). The median TTP was 7.0 months (range: 4.2–9.9 months). In Vp3 PVTT, the TTP was 9.7 months (range: 8.8–10.5 months), and in Vp4 PVTT, the TTP was 4.2 months (range: 2.8–5.6 months). The median OS was 10.0 months (range: 7.0–13.1 months). In Vp3 PVTT, OS was 11.9 months (range: 9.2–14.5 months), and in Vp4 PVTT, OS was 7.2 months (range: 3.8–10.7 months).
Conclusions:
PVS-125I-TACE-S is safe for patients with HCC with PVTT and may extend the TTP and survival of patients with Vp4 PVTT.
Advances in knowledge:
PVS implantation promptly restored flow in the obstructed portal vein, which can reduce the risk of hepatic failure and upper gastrointestinal bleeding. Implantation of iodine-125 seed-strips may directly expose the portal tumor thrombus to radiation and kill cancer cells. Their combined use with TACE-S has a strong scientific rationale.
Introduction
Hepatocellular carcinoma (HCC) is among the most common malignant tumors worldwide. This disease accounts for 5.6% of all human cancers, and its incidence has gradually increased over time.1 It has a great propensity to invade the portal venous system and cause portal vein tumor thrombosis (PVTT).2 PVTT can cause partial or total portal vein occlusion and lead to gastrointestinal bleeding and ascites. Finally, the prognosis of patients is affected.3–5 Extant literature reports that the median survival for HCC patients with PVTT is 2.7–4.0 months if patients are not treated.6 In theory, surgical resection is the standard treatment for HCC with PVTT, but the surgical indications, high surgical risk, and high postoperative recurrence are important restricting factors.1,7 To improve prognosis, a variety of non-surgical therapeutic techniques have been attempted in HCC with PVTT. These include percutaneous interventions (ethanol and radiofrequency ablation), transarterial interventions (embolization, chemoperfusion or chemoembolization), and therapeutic drugs, including gene and immune therapy.1
Among these, transcatheter arterial chemoembolization with sorafenib (TACE-S) is effective for Barcelona Clinic Liver Cancer (BCLC) stage C HCC with PVTT when the main portal vein is unobstructed or when substantial collateral circulation is generated, confirmed by imagological examination.8–10 However, TACE is more efficacious only when the blood supply is principally from the hepatic artery, rather than the portal vein or other vessels around the porta hepatis, in PVTT.11 It has also been reported that sorafenib provides modest treatment efficacy towards PVTT, especially for type Vp3 or Vp4 with a response rate lower than 10%.12 Thus, combined treatment is generally reserved as a palliative therapy.
In recent years, endovascular implantation of a portal vein stent combined with iodine-125 seed-strips (PVS-125I) has been utilized for the treatment of HCC with PVTT.13 A retrospective study was performed to determine whether PVS-125I followed by transcatheter arterial chemoembolization with sorafenib (PVS-125I-TACE-S) is safe and an effective treatment for HCC with PVTT.
Methods and materials
Design and patients
This retrospective study was approved by the Institutional Ethics Committee (LDYYLL2018-116). All patients provided written informed consent.
Records from the institutional database from January 2015 to July 2017 were accessed to identify all patients with HCC and PVTT who underwent PVS-125I-TACE-S. Inclusion criteria were as follows: (1) aged 18–70 years; (2) diagnosis of HCC; (3) Vp3 or Vp4 PVTT (Liver Cancer Study Group of Japan-classification); and (4) Child-Pugh Class A or B. Exclusion criteria were as follows: (1) Vp1 or Vp2 PVTT; (2) absence of baseline imaging information; (3) coagulation disorders that could not be corrected; (4) massive ascites; and (5) patients lost to follow-up. Diagnosis of HCC was confirmed in accordance with the criteria of the American Association for the Study of Liver Diseases.14 Diagnosis of PVTT was confirmed following identification of a low attenuation intraluminal mass, partially or completely occluding the portal vein, or filling a defect in the portal vein, as observed using three-phase dynamic CT or MRI.13 PVTT was classified according to Liver Cancer Study Group of Japan classification as follows: Vp0 = no PVTT, Vp1 = segmental portal vein invasion, Vp2 = right anterior/-posterior portal vein thrombosis, Vp3 = right/left portal vein thrombosis, and Vp4 = thrombus in the main trunk.15
After diagnosis of HCC with PVTT, patients were informed in detail and presented with the range of different treatment choices, including sorafenib, TACE-S, PVS-125I-TACE-S, etc. The advantages and disadvantages of all treatment methods were provided to the patients, the final option selected by each patient. A total of 25 patients were identified, although preoperatively enhanced CT/MRI was not available for five patients and two that had been lost to follow-up were excluded. Finally, the study population comprised 18 patients. Median interval from PVS-125I to the start of TACE and the start of sorafenib was 4 weeks and 1 week, respectively.
Materials
Iodine-125 seeds (25.9 MBq with a half-life of 59.6 days, with principal photon emissions: 27.4–31.5 keV x-rays and 35.5 keV γ-rays) were obtained from Shanghai GMS Pharmaceutical Co. Ltd., China. Self-expandable stents (14 mm in diameter, 60–100 mm long) were acquired from ev3 Inc., Plymouth, MN, USA. Doxorubicin hydrochloride was purchased from Shanghai Hisun Pfizer Pharmaceutical Co. Ltd., China. Lipiodol was obtained from Beijing Wh-Medical Apparatus and Instruments Co. Ltd., China. Polyvinyl alcohol particles were supplied by Hangzhou ALICON Pharmaceutical Science and Technology Co. Ltd., China. Sorafenib was acquired from Nexavar®, Bayer HealthCare, Leverkusen, Germany.
PVS-125I
After administration of local anesthesia to patients, the second-order portal branch was punctured and cannulated using a 5-Fr NEFF set (Cook, Inc.) under ultrasound guidance. A 6-Fr sheath (Terumo, Tokyo, Japan) was then exchanged, and portography performed to confirm the location and length of the thrombosis using a 4-Fr Cobra catheter (Terumo, Tokyo, Japan). Two Amplatz Super Stiff Guidewires (Boston Scientific, Marlborough, Massachusetts) were inserted into the splenic vein. A self-expandable stent was then placed along the stiff guidewire and released into the obstructed segment. The 6-Fr sheath was fed into the obstructed section again over the second stiff guidewire. The 6-Fr sheath was then withdrawn and iodine-125 seed-strips released, becoming fixed firmly between the PVTT and stents, as shown in Figure 1. Finally, blocking of the intrahepatic puncture channel was accomplished using a 3–3 spring coil block. For construction of the iodine-125 seed-strips, iodine-125 seeds were arranged linearly and sealed into a 4-Fr sterile plastic tube (Cannula Create Medic Co., Kanagawa, Japan). The number of seeds was dependent on the length of the portal vein thrombus.
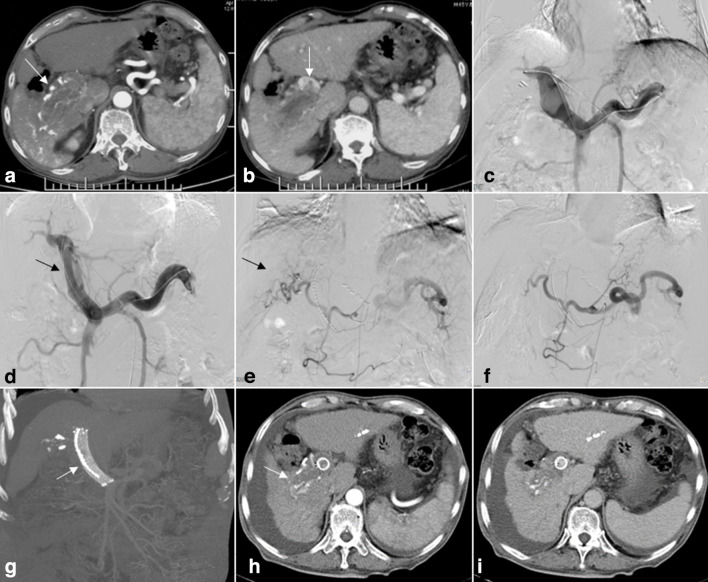
Figure 1.
A 65-year-old male who had hepatocellular carcinoma (HCC) with Vp4 portal vein tumor thrombosis (PVTT). (A) Contrast-enhanced CT cross-sectional image demonstrating HCC lesions in segment 6 (arrows). (B) PVTT from the right branch of the portal vein extending to the main portal vein (arrows). (C) The left patent intrahepatic portal vein branch was punctured, portography showing an irregular filling defect at the confluence of the left portal vein and main portal trunk. The right portal vein branch is not shown. (D) Self-expandable stents and iodine-125 seed-strips were placed in the obstructed main portal vein and the posterior segment of the left intrahepatic portal vein (arrows). (E) After advancing the catheter into the feed artery, injection of contrast medium revealed the tumor. (F) Occlusion of the tumor feeding arteries following TACE. (G-I) Results from a 6-month follow-up review. Iodine-125 seed-strips fixed firmly between the PVTT and stent. Treated lesions were observed to have decreased in size, the stent exhibiting satisfactory patency (arrows).
TACE procedure
TACE was performed under local anesthesia using 1% lidocaine. A 5-Fr RH (Terumo Corporation) catheter was introduced through the femoral artery, using a microcatheter (2.7 Fr; Progreat; Terumo) to survey tumor feeding arteries. An emulsion of 5–20 ml of lipiodol and 60–80 mg of doxorubicin hydrochloride was administered into the feeding vessels. If flow within the tumor-feeding artery remained rapid after the 20 ml upper limit was reached, polyvinyl alcohol particles were used to embolize the vessel until a sufficiently sluggish flow was observed. In patients with an arterioportal shunt, embolization with polyvinyl alcohol particles was used. TACE was performed on demand.
Sorafenib
Sorafenib was taken at a dose of 400 mg bd. However, the dose was adjusted according to the severity of toxicity. If adverse events (AEs) occurred, the dose was reduced to 400 mg once daily or an interruption in treatment was initiated. Patients underwent sorafenib tablets for as long as possible, even if progress was observed, or until death.
Follow-up
Patient follow-up was conducted 1 and 3 months after the operation and every 3 months thereafter until the patient’s death. Follow-up assessment included a combination of physical examination, laboratory testing and contrast-enhanced abdominal CT or MRI. Success of the technique was defined as release of the stent at the target location, with iodine-125 seed-strips completely irradiating the PVTT. Complications were recorded using the Common Terminology Criteria for Adverse Events v.3.0 classification. Levels of bilirubin, serum albumin, and prothrombin time were recorded before and 3 days after surgery. Time-to-tumor progression (TTP) was defined as the time from start of the PVS-125I until the date that tumor progression was radiologically confirmed. Overall survival (OS) was defined time from PVS-125I to death.
Data analysis
Statistical analyses were performed using SPSS v.24.0. Levels of bilirubin, serum albumin, and prothrombin time before and 3 days after surgery were analyzed using T tests. TTP and OS were calculated using Kaplan-Meier curves.
Results
Study population
A total of 18 patients with HCC with PVTT received PVS-125I-TACE-S. Detailed baseline patient characteristics are shown in Table 1. An average of 18.8 ± 1.4 (range: 16.0–20.0) 125I seeds were implanted, with mean radioactivity of 13.1 mCi ± 0.9 (range: 11.2–14.0 mCi). The estimated total radiation dose was approximately 40–50 Gy, as determined at specific dose reference points based upon calculations conducted by a computerized treatment planning system (FTT Technology Ltd. Co, Beijing, China).
Table 1.
Baseline characteristics of study patients (n = 18)
| Characteristic | Value |
|---|---|
| Sex | |
| Male | 17 (94.4%) |
| Female | 1 (5.6%) |
| Age (years) | 55.2 ± 7.4 |
| Child-Pugh score | |
| A | 17 (94.4%) |
| B | 1 (5.6%) |
| ECOG performance | |
| 0 | 5 (27.8%) |
| 1 | 13 (72.2%) |
| 2 | 0 |
| Etiology | |
| HBV | 12 (66.7%) |
| HCV | 1 (5.6%) |
| Alcohol and other | 5 (27.8%) |
| Classifications of PVTT | |
| Vp3 | 12 (66.7%) |
| Vp4 | 6 (33.3%) |
| α-Fetoprotein level (ng/mL) | |
| >400 | 9 (50.0%) |
| ≤400 | 9 (50.0%) |
| Maximum diameter | |
| >5 cm | 9 (50.0%) |
| ≤5 cm | 9 (50.0%) |
| Ascites | |
| Present | 4 (22.2%) |
| Absent | 14 (77.8%) |
| TACE times | 2.4 ± 0.9 |
| Distant metastasis | |
| Present | 1 (5.6%) |
| Absent | 17 (94.4%) |
Mean ± standard deviation or percentage is given. HBV: hepatitis B virus, HCV: hepatitis C virus.
Technical success and complications
The technical success rate was 100%, with no treatment-related deaths. Grades 1/2 AEs included fever (88.9%), abdominal pain (77.8%), nausea and vomiting (61.1%), fatigue (55.6%), diarrhea (38.9%), hypertension (33.3%), and hand-foot syndrome (27.8%). Grades 3/4 AEs included diarrhea (16.7%), hypertension (11.1%), and hand-foot syndrome (5.6%). No iodine-125 seed shedding or displacement occurred during follow-up. The complication rates are shown in Table 2. Liver function test values were not significantly different compared with baseline values and after PVS-125I (P>0.05), as shown in Table 3.
Table 2.
Postoperative complications (n = 18)
| Parameter | Value |
|---|---|
| Fever | |
| Grade 1–2 | 16 (88.9%) |
| Grade 3–4 | 0 |
| Abdominal pain | |
| Grade 1–2 | 14 (77.8%) |
| Grade 3–4 | 0 |
| Nausea and vomiting | |
| Grade 1–2 | 11 (61.1%) |
| Grade 3–4 | 0 |
| Fatigue | |
| Grade 1–2 | 10 (55.6%) |
| Grade 3–4 | 0 |
| Diarrhea | |
| Grade 1–2 | 7 (38.9%) |
| Grade 3–4 | 3 (16.7%) |
| Hypertension | |
| Grade 1–2 | 6 (33.3%) |
| Grade 3–4 | 2 (11.1%) |
| Hand-foot syndrome | |
| Grade 1–2 | 5 (27.8%) |
| Grade 3–4 | 1 (5.6%) |
Percentage is given.
Table 3.
Change in liver function, baseline values and 3 days after PVS-125I (n = 18)
| Characteristic | Before operation | 3 days after operation | T | P |
|---|---|---|---|---|
| Total bilirubin level(μmol/L) | 26.4 ± 8.0 | 27.2 ± 8.0 | −0.50 | 0.63 |
| Serum albumin level (g/L) | 39.1 ± 4.5 | 38.4 ± 3.4 | 1.22 | 0.24 |
| Prothrombin time (sec) | 12.4 ± 1.3 | 12.6 ± 1.0 | −0.99 | 0.34 |
Mean ± standard deviation is given.
PVS-125I,endovascular implantation of a portal vein stent combined with iodine-125 seed-strips.
There was no statistically significant difference in prothrombin time and total bilirubin and serum albumin levels before and 3 days after surgery, p > 0.05.
Survival analyses
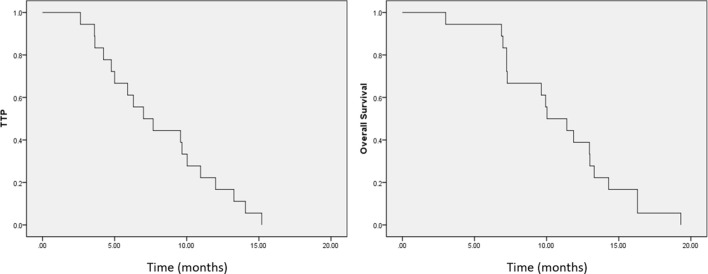
Median follow-up duration was 10.0 months (range: 3.0–19.3 months). At the time of writing, all patients had died. The median TTP was 7.0 months (range: 4.2–9.9 months). In Vp3 PVTT, the TTP was 9.7 months (range: 8.8–10.5 months), and in Vp4 PVTT, the TTP was 4.2 months (range: 2.8–5.6 months). The median OS was 10.0 months (range: 7.0–13.1 months). In Vp3 PVTT, OS was 11.9 months (range: 9.2–14.5 months), and in Vp4 PVTT, OS was 7.2 months (range: 3.8–10.7 months), as shown in Figure 2.
Figure 2.
Time-to-tumor progression (TTP) and Kaplan-Meier overall survival curves. (A) Median TTP was 7.0 months (range: 4.2–9.9 months). (B) Median survival was 10.0 months (range: 7.0–13.1 months).
Discussion
Macroscopic PVTT has been found to be present in 10–40% of patients at the time HCC is first diagnosed.2 PVTT usually worsens the prognosis of HCC, as characterized by aggressive progression of the disease, impaired liver function, and tolerance to treatment.4 Thus, such thrombosis represents a typical difficult yet common situation that must be treated in patients with HCC.16
Currently, non-surgical treatment strategies for HCC with PVTT are available, such asendovascular radiofrequency ablation (RFA), which kills tumor cells through heating, forming a cylindrical necrotic area. Because PVTT forms close to the bile duct, this approach is associated with a relatively high risk of injury to both the duct and the portal vein itself.17,18 Hepatic artery infusion of chemotherapy (HAIC) can deliver high concentrations of anticancer agents directly to the tumor through the hepatic artery, which minimizes systemic toxicity due to first-pass effects. However, it is not recommended as a standard treatment because clinical data of the technique are mostly limited to Japanese patients, without any randomized controlled trials.19 For stereotactic body radiotherapy (SBRT), the benefit is that tumors can directly receive higher radiation doses and organs are protected from substantial radiation. The size of lesion represents the limiting factor, for which the rate of control is 74% for tumors larger than 5 cm in diameter and 91% for those less than 5 cm.20 In addition, where transarterial radioembolization (TARE) has been used, a lower risk of hepatic ischemia and infarction has been observed. However, it is unavailable for a number of markets at this time, such as mainland China.2
In recent years, PVS combined with iodine-125 seeds has achieved significant results for the treatment of portal vein tumor thrombus. PVS can relieve portal vein obstruction, improve liver function, and reduce the risk of esophageal bleeding or the occurrence of stomach varices. Iodine-125 seeds release continuous low doses of x- and γ-rays (half-life of 59.6 d with a radiation diameter of 1.7 cm). Such a continuous high dose of radiation causes damage to the cancer cells, preventing PVTT progression and prolonging stent patency. The principle of the treatment is the emission of ionized water molecules by γ-rays that causes ionization and DNA damage, resulting in single- and double-stranded DNA breaks, while supplying a sharply reduced dose to the healthy tissues, leading to a reduced incidence of complications.21,22 Combined with TACE-S, providing a synergistic effect, the result is a greater probability of survival for HCC patients with PVTT.
In the present study, no treatment-related deaths occurred. AEs such as fever, abdominal pain, nausea and vomiting, fatigue, diarrhea and hypertension were managed successfully with spontaneous or conservative management. Complications such as iodine-125 seed shedding or displacement did not occur during the follow-up period because the iodine-125 seed-strips were fixed to the site stent placement and tumor thrombus. These results confirm that PVS-125I-TACE-S is a safe procedure tolerated by patients.
It has also been reported that bilirubin levels are significantly increased after PVS-125I due to damage to the bile duct close to the portal vein.23 However, in this study, liver function test values were not significantly different than baseline values after PVS-125I (P>0.05). This indicates that PVS-125I does not reduce liver function in patients with HCC combined with PVTT. This was principally because portal vein puncture was conducted under the guidance of ultrasound, a relatively effective image guidance method which avoids damage to the bile ducts or blood vessels. In addition, PVS-125I prevented obstruction of the portal vein, guaranteeing blood supply to the liver and further improving liver function.24
The present study demonstrated that TTP was 9.7 months (range: 8.8–10.5 months) in Vp3 PVTT, and 4.2 months (range: 2.8–5.6 months) in Vp4 PVTT, a promising outcome compared with the durations reported in the literature using conventional TACE-S.8 TACE combined with sorafenib is an appropriate treatment option considering their complementary effects, which may prolong the TTP of HCC with PVTT.25 Embolization of the hepatic artery by TACE reduces blood supply to the HCC with PVTT.3 Additionally, sorafenib can directly inhibit tumor cell proliferation by blocking the cell signal transduction pathway mediated by RAF/MEK/ERK and indirectly inhibit tumor cell growth by inhibiting VEGFR and platelet-derived growth factor (PDGF) receptor on HCC with PVTT.9 However, the effect of TACE-S is still limited. The principal reason is due to the inability to achieve complete embolization of the portal vein thrombus in blood vessels when using TACE, especially with an arterio-portal fistula.26 In addition, it has been reported in the literature that the availability of sorafenib is also limited in PVTT.27 Therefore, a PVTT might continue to grow or from metastases. However, PVS implantation can open the portal vein, with implantation of iodine-125 seed-strips directly exposing the portal tumor thrombus to radiation, killing the cancer cells and preventing occlusion, resulting in a promising outcome regarding TTP in the PVS-125I-TACE-S group.28
In addition, the survival of patients with Vp4 PVTT was promising, compared with conventional TACE-S, as reported in the literature,8 with OS of 7.2 months (range: 3.8–10.7 months). PVTT is a critical factor that can worsen the prognosis of patients with HCC, especially in Vp4 PVTT, due to invasion of the main portal vein causing marked obstruction to portal vein blood flow, impairment of liver function, finally resulting in liver failure or death, and potentially putting a stop to the required treatment. Additionally, increased portal venous pressure leads to intractable ascites and rupture of gastric/esophageal varices. In addition, Vp4 PVTT is more susceptible to invasion than Vp3 PVTT, leading to the spread of tumors.13,21,29,30 However, neither TACE nor sorafenib can immediately remove portal obstruction, with the restriction of PVTT somewhat limited.24 The PVS can be placed for malignant portal vein stenosis/occlusion. In particular, for Vp4 PVTT, the stent can quickly restore blood flow to the liver, improve liver function, intractable ascites, and prevent fatal gastric/esophageal variceal hemorrhage. Implantation of iodine-125 seed-strips that releases more uniform radiation energy along the PVTT not only eliminates tumor cells by maintaining them in a resting state and changing their immune-phenotype, it additionally inhibits intimal hyperplasia. This thereby decreases metastasis and prolongs stent patency.13,24,31 Therefore, promising outcome survival can be achieved in HCC patients with Vp4 PVTT.
This study has several limitations. Firstly, as the total number of patients included was relatively small, it is difficult to draw powerful statistical conclusions. In addition, this study was retrospective in nature and despite our efforts to control potential confounding factors a future multicenter randomized-controlled trial is required to validate these results. Finally, measurement using a dosemeter at the surface of the skin after iodine-125 seed implantation was not performed. Therefore, penetration by the radiation is unclear. However, prudent precautions are taken to reduce exposure to other individuals.
In conclusion, PVS-125I-TACE-S represents a safe therapeutic strategy for the treatment of HCC with PVTT and can prolong the TTP and survival in patients with Vp4 PVTT. It is a promising technique for combining recanalization of an occluded portal vein and TACE-S for HCC with PVTT. However, further studies are required to clarify the therapeutic power of the technique.
Footnotes
Conflicts of interest: No conflicts of interest, including specific financial interests, relationships or affiliations relevant to the subject matter or materials discussed in the manuscript are disclosed.
Contributor Information
Shuangxi Li, Email: daohaosiliangjia@163.com.
Lei Li, Email: jierudaoguanshier@163.com.
Baohua Li, Email: fabiaowenzhang@163.com.
Wenhui Wang, Email: 470090101@qq.com.
REFERENCES
- 1.Zhu Y, Cui P-J, Yao J, Zhang Z-Y, Yang J. Efficacy of transarterial chemoembolisation with or without antiviral therapy for patients with hepatocellular carcinoma after radical hepatectomy. Gastroenterol Res Pract 2018; 2018: 1–7. doi: 10.1155/2018/6414759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Zhang X-P, Zhong B-Y, Lau WY, Madoff DC, Davidson JC, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing East and West. Lancet Gastroenterol Hepatol 2019; 4: 721–30. doi: 10.1016/S2468-1253(19)30178-5 [DOI] [PubMed] [Google Scholar]
- 3.Wang J-C, Xia A-L, Xu Y, Lu X-J. Comprehensive treatments for hepatocellular carcinoma with portal vein tumor thrombosis. J Cell Physiol 2019; 234: 1062–70. doi: 10.1002/jcp.27324 [DOI] [PubMed] [Google Scholar]
- 4.Li T, Yu Y, Liu J, Tian X, Kong M, Wu L, et al. PIVKA-II level is correlated to development of portal vein tumor thrombus in patients with HBV-related hepatocellular carcinoma. Infect Agent Cancer 2019; 14: 13. doi: 10.1186/s13027-019-0229-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SW, Lee HL, Han NI, Kwon JH, Nam SW, Jang JW, et al. Transarterial infusion of epirubicin and cisplatin combined with systemic infusion of 5-fluorouracil versus transarterial chemoembolization using doxorubicin for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a retrospective analysis. Ther Adv Med Oncol 2017; 9: 615–26. doi: 10.1177/1758834017728018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X-P, Gao Y-Z, Chen Z-H, Chen M-S, Li L-Q, Wen T-F, et al. An eastern hepatobiliary surgery Hospital/Portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a multicenter study. Hepatology 2019; 69: 2076–90. doi: 10.1002/hep.30490 [DOI] [PubMed] [Google Scholar]
- 7.Huo YR, Chan MV, Chan C. Resection plus post-operative adjuvant transcatheter arterial chemoembolization (TACE) compared with resection alone for hepatocellular carcinoma: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2020; 43: 572–86. doi: 10.1007/s00270-019-02392-6 [DOI] [PubMed] [Google Scholar]
- 8.Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology 2014; 272: 284–93. doi: 10.1148/radiol.14131946 [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Yin X, Tang B, Ma H, Zhang L, Li L, et al. Transarterial chemoembolization (TACE) combined with sorafenib in treatment of HBV background hepatocellular carcinoma with portal vein tumor thrombus: a propensity score matching study. Biomed Res Int 2019; 2019: 1–6. doi: 10.1155/2019/2141859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan T, Li X-S, Xie Q-K, Wang J-P, Li W, Wu P-H, XS L, PH W, et al. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol 2014; 69: e553–61. doi: 10.1016/j.crad.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Shi J, Huang B, Cheng F, Guo W, Lau WY, et al. The degree of hepatic arterial blood supply of portal vein tumor thrombus in patients with hepatocellular carcinoma and its impact on overall survival after transarterial chemoembolization. Oncotarget 2017; 8: 79816–24. doi: 10.18632/oncotarget.19767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan SL, Chong CCN, Chan AWH, Poon DMC, Chok KSH. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol 2016; 22: 7289–300. doi: 10.3748/wjg.v22.i32.7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Guo J-H, Zhu H-D, Zhu G-Y, Chen L, Teng G-J. Safety and efficacy of irradiation stent placement for malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: a single-center experience. J Vasc Interv Radiol 2017; 28: 786–94. doi: 10.1016/j.jvir.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 14.Lee IJ, Lee J-H, Lee YB, Kim YJ, Yoon J-H, Yin YH, et al. Effectiveness of drug-eluting bead transarterial chemoembolization versus conventional transarterial chemoembolization for small hepatocellular carcinoma in Child-Pugh class A patients. Ther Adv Med Oncol 2019; 11: 1758835919866072. doi: 10.1177/1758835919866072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mähringer-Kunz A, Steinle V, Düber C, Weinmann A, Koch S, Schmidtmann I, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: the more, the worse? Liver Int 2019; 39: 324–31. doi: 10.1111/liv.13988 [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Guo L, Li H, Zhang B, Sun J, Zhou C, et al. Postoperative adjuvant Trans-Arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg Oncol 2018; 25: 2098–104. doi: 10.1245/s10434-018-6438-1 [DOI] [PubMed] [Google Scholar]
- 17.Chen Z-W, Lin Z-Y, Chen Y-P, Chen J, Chen J. Clinical efficacy of endovascular radiofrequency ablation in the treatment of portal vein tumor thrombus of primary hepatocellular carcinoma. J Cancer Res Ther 2018; 14: 145–9. doi: 10.4103/jcrt.JCRT_784_17 [DOI] [PubMed] [Google Scholar]
- 18.Yuan D, Gao Z, Zhao J, Zhang H, Wang J. 125I seed implantation for hepatocellular carcinoma with portal vein tumor thrombus: A systematic review and meta-analysis. Brachytherapy 2019; 18: 521–9. doi: 10.1016/j.brachy.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 19.Han K, Kim JH, Ko G-Y, Gwon DI, Sung K-B, Kichang H, Gi-Young D, Gwon K-B. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol 2016; 22: 407–16. doi: 10.3748/wjg.v22.i1.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Shin I-S, Yoon WS, Koom WS, Rim CH. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: meta-analyses and a systematic review. Radiother Oncol 2020; 145: 63–70. doi: 10.1016/j.radonc.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Si G, Jiao D-C, Han X, Zhang W, Li Y, et al. Portal Vein Stenting Combined with 125I Particle Chain Implantation Followed by As2O3 in the Treatment of Hepatocellular Carcinoma with Portal Vein Tumour Thrombus. Biomed Res Int 2020; 2020: 1–7. doi: 10.1155/2020/4109216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Jiang H, Yang W, Jiang N, Zheng Q, Huang N, et al. Predictive factors of benefit from iodine-125 brachytherapy for hepatocellular carcinoma with portal vein tumor thrombosis. Brachytherapy 2019; 18: 233–9. doi: 10.1016/j.brachy.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Yan Z, Liu Q, Qu X, Wang J. Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vasc Interv Radiol 2011; 22: 479–89. doi: 10.1016/j.jvir.2010.11.029 [DOI] [PubMed] [Google Scholar]
- 24.Tan T, Xiao Y, Zhou S, Ma C, Zhang Z. Y-configuration stent combined with iodine-125 seeds strand for the treatment of hepatocellular carcinoma with tumor thrombosis in portal vein branches: a case report. Medicine 2017; 96: e8660. doi: 10.1097/MD.0000000000008660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Wang K, Wang M, Yang G, Ye X, Wu M, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget 2017; 8: 29416–27. doi: 10.18632/oncotarget.15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang M, Lin Q, Wang H, Chen J, Bai M, Wang L, et al. Survival benefit of chemoembolization plus Iodine125 seed implantation in unresectable hepatitis B-related hepatocellular carcinoma with PVTT: a retrospective matched cohort study. Eur Radiol 2016; 26: 3428–36. doi: 10.1007/s00330-015-4198-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol 2015; 50: 445–54. doi: 10.1007/s00535-014-0978-3 [DOI] [PubMed] [Google Scholar]
- 28.Chuan-Xing L, Xu H, Bao-Shan H, Yong L, Pei-Jian S, Xian-Yi Y, Li Chuan-Xing HX, Li Yong SP-J, et al. Efficacy of therapy for hepatocellular carcinoma with portal vein tumor thrombus: chemoembolization and stent combined with iodine-125 seed. Cancer Biol Ther 2011; 12: 865–71. doi: 10.4161/cbt.12.10.17676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su F, Chen K-H, Liang Z-G, Wu C-H, Li L, Qu S, et al. Comparison of three-dimensional conformal radiotherapy and hepatic resection in hepatocellular carcinoma with portal vein tumor thrombus. Cancer Med 2018; 7: 4387–95. doi: 10.1002/cam4.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan W, Yuan G, Fan H, Li F, Wu Y, Zhao Y, et al. Apatinib combined with transarterial chemoembolization in patients with hepatocellular carcinoma and portal vein tumor thrombus: a multicenter retrospective study. Clin Ther 2019; 41: 1463–76. doi: 10.1016/j.clinthera.2019.04.036 [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa T, Kubota T, Abe H, Nagashima A, Hirose K, Togashi T, et al. Percutaneous transhepatic portal vein stent placement can improve prognosis for hepatocellular carcinoma patients with portal vein tumor thrombosis. Hepatogastroenterology 2014; 61: 413–6. [PubMed] [Google Scholar]