Abstract
Objectives:
The chest CT findings that can distinguish patients with corona virus disease 2019 (COVID-19) from those with clinically suspected COVID-19 but subsequently found to be COVID-19 negative have not previously been described in detail. The purpose of this study was to determine the distinctions among patients with COVID-19 by comparing the imaging findings of patients with suspected confirmed COVID-19 and those of patients initially suspected to have COVID-19 who were ultimately negative for the disease.
Methods:
28 isolated suspected in-patients with COVID-19 were enrolled in this retrospective study from January 22, 2020 to February 6, 2020. 12 patients were confirmed to have positive severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) RNA results, and 16 patients had negative results. The thin-section CT imaging findings and clinical and laboratory data of all the patients were evaluated.
Results:
There were no significant differences between the 12 confirmed COVID-19 (SARS-Cov-2-positive) patients and 16 SARS-CoV-2-negative patients in epidemiology and most of the clinical features or laboratory data. The CT images showed that the incidence of pure/mixed ground-glass opacities (GGOs) was not different between COVID-19 and SARS-CoV-2-negative patients [9/12 (75.0%) vs 10/16 (62.5%), p = 0.687], but pure/mixed GGOs in the peripheral were more common in patients with COVID-19 [11/12 (91.7%) vs 6/16 (37.5%), p = 0.006]. There were no significant differences in the number of lesions, bilateral lung involvement, large irregular/patchy opacities, rounded opacities, linear opacities, crazy-paving patterns, halo signs, interlobular septal thickening or air bronchograms.
Conclusions:
Although peripheral pure/mixed GGOs on CT may help distinguish patients with COVID-19 from clinically suspected but negative patients, CT cannot replace RT-PCR testing.
Advances in knowledge:
Peripheral pure/mixed GGOs on-chest CT findings can be helpful in distinguishing patients with COVID-19 from those with clinically suspected COVID-19 but subsequently found to be COVID-19 negative.
Introduction
Corona virus disease 2019 (COVID-19), caused by SARS-CoV-2, was first reported in Wuhan, China on December 31, 2019, and rapidly spread to various places.1–3 By April 06, the disease had affected 206 countries, with more than 1,293,560 confirmed patients and 70,645 deaths.
Currently, positive SARS-CoV-2 RNA findings by reverse-transcriptase polymerase chain reaction (RT-PCR) is considered the gold standard for diagnosing COVID-19, but this test is time-consuming, and a shortage of supply test kits may not meet the demand for testing a large number of patients with suspected COVID-19. Furthermore, the SARS-CoV-2 RNA findings may be falsely negative due to laboratory error or insufficient specimens.4 Therefore, it is necessary to find a rapid and accurate method for the differential diagnosis of suspected COVID-19 patients.
Radiological examination, especially thin-section chest CT, is a non-invasive, quick method and has the advantages of discovering lung pathological changes early and reflecting the severity of the disease. Some previous radiographical studies of chest CT findings in patients with COVID-19 indicate that bilateral ground-glass opacities (GGOs) or consolidation on-chest CT should remind radiologists to make a possible diagnosis of COVID-19.5 However, all these data came from confirmed patients, and the characteristics to be aware of in suspected patients are not clear.
In this study, we aimed to discover the key points for diagnosis by comparing the chest CT characteristics of 12 patients with confirmed COVID-19 and 16 clinically suspected but SARS-CoV-2-negative patients.
Methods and materials
Patients
This retrospective study was approved by the Ethic Committees of the Third Affiliated Hospital of Sun Yat-Sen University. As a retrospective study, informed consent was waived. We included all patients admitted to the Third Affiliated Hospital of Sun Yat-Sen University in Guangzhou from January 22, 2020 to February 6, 2020 (Figure 1). Our inclusion criteria (suspected patients) were patients with any epidemiological risk history and any two of the clinical characteristics as follows: (1) epidemiological history within 14 days prior to the onset of the disease: history of travel to or residence in Wuhan and its surrounding areas; close contact with COVID-19 patients or other patients from Wuhan with fever; clustered cases; and (2) clinical characteristics: fever and/or respiratory symptoms, imaging findings of pneumonia, normal or decreased total white blood cell count, or decreased lymphocyte count in the early stages of disease. Patients were confirmed by SARS-COV-2 RNA detection with respiratory or blood samples by the Guangdong Centers for Disease Control and Prevention (CDC). If the SARS-COV-2 RNA test was negative twice more than 24 h apart, the suspected patient was defined as negative. The data on demographic, epidemiological, clinical and laboratory characteristics were abstracted from medical records.6 A total of 1119 patients were screened in the fever clinic at the Third Affiliated Hospital of Sun Yat-Sen University, and 28 suspected patients were enrolled, including 12 confirmed and 16 negative patients.
Figure 1.
Flowchart of the study population. Note: #1 The interval between two times was more than 24 h; #2 The last follow-up was on February 20, 2020; CT = computed tomography; COVID-19 = corona virus disease 2019; SARS-CoV-2=severe acute respiratory syndrome corona virus 2.
Chest CT
All these patients performed thin-section CT scans. The median duration from the onset of initial symptoms to CT scan was 4 days, ranging from 1 day to 2 weeks. All CT examinations were performed using an Aquilion ONE scanner (Toshiba Medical Systems; Tokyo, Japan) or an IQon Spectral scanner (Philips Healthcare, Best, the Netherlands). All CT images were obtained with the patient in the supine position during end-inspiration and without contrast medium. The CT protocol is as follows: 120 kV; automatic tube current (180 mA–440 mA); iterative reconstruction technique; detector, 32/160 mm; rotation time, 0.5 sec; section thickness, 1 mm; collimation, 0.5 mm; pitch, 1.5; and matrix, 512*512. An image reconstruction kernel was used to smooth the lung with a thickness of 1 mm and an interval of 1 mm. The CT images were viewed with lung (window width, 1,600 HU; window level, −600 HU) and mediastinal (window width, 300 HU; window level 40 HU) window settings.
All thin-section CT images were reviewed by two experienced cardiothoracic radiologists (SidongXie and Xiuzhen Chen) using a viewing console. The images were reviewed independently and reached a decision in consensus. In case of disagreements between the two primary radiologists’ interpretations, a third cardiothoracic radiologist with 20 years of experience (Jie Qin) adjudicated a final decision.
The major CT findings were described using international standard nomenclature defined by the Fleischner Society glossary and peer-reviewed literature on viral pneumonia.7–10 In all 28 patients, the initial CT findings were evaluated for the following features: (1) type of opacity: pure/mixed GGO or consolidation; (2) lesion location: peripheral pulmonary/subpleural or central distribution/scattered; (3) morphology of lesions: large irregular/patchy opacities, rounded opacities or linear opacities; (4) the number of lung lobes affected by GGOs or consolidation and whether there was bilateral lung involvement; (5) interstitial involvement of the lung: peribronchovascular interstitial thickening, interlobular septal thickening, tree-in-bud pattern, honeycombing, subpleural curvilinear line, and pleural thickening; (6) crazy-paving pattern; (7) halo sign; (8) air bronchogram; (9) lymphadenopathy (lymph node size of ≥10 mm in short-axis diameter); and (10) underlying lung diseases such as emphysema or bronchiectasis; and (11) other abnormalities (e.g., solid pulmonary nodule, cavitation and pleural effusion).
Statistical analysis
All data were statistically analysed using SPSS v.22.0 (IBM Corp, USA). Continuous variables are expressed as the mean (SD) or median (IQR) and were compared with the t-test or Mann-Whitney U test; categorical variables are expressed as frequency (%) and were compared by χ² test or Fisher’s exact test between the confirmed and suspected but negative patients. P values < 0.05 were considered to be a statistically significant difference.
Results
Baseline characteristics, clinical features and laboratory data
All 28 clinically suspected patients admitted to The Third Affiliated Hospital of Sun Yat-Sen University were enrolled, including 12 confirmed patients with COVID-19 and 16 SARS-CoV-2-negative patients. The confirmed patients included six males and six females; the median age was 40 years; in contrast, four men and twelve females with a median age of 35 years composed the suspected but negative patients. Patients in both groups mainly manifested fever and cough. The confirmed COVID-19 patients had more myalgia (91.6% vs 31.2%, p = 0.002), and the median number of neutrophils in SARS-CoV-2-negative patients was higher than that in confirmed patients (3.2 × 109/L vs 4.6 × 109/L,p < 0.05).The baseline characteristics, epidemiological factors, clinical features and laboratory data of the 12 confirmed COVID-19 patients and 16 SARS-CoV-2-negative patients are shown in Table 1.
Table 1.
Comparison of the clinical and laboratory features between confirmed patients with COVID-19 and SARS-CoV-2-negative patients
| Characteristics | Confirmed patients (N = 12) | Negative patients (N = 16) | P values |
| Age, years, median (IQR) | 40 (30–61) | 35 (21–53) | 0.265 |
| Sex | 0.243 | ||
| Females(n, %) | 6 (50.0) | 4 (25.0) | |
| Males(n, %) | 6 (50.0) | 12 (75.0) | |
| Chronic medical illness(n, %) | 4 (33.3) | 4 (25.0) | 0.691 |
| Signs and symptoms | |||
| Fever(n, %) | 12 (100) | 13 (81.2) | 0.238 |
| Cough(n, %) | 9 (75.0) | 15 (93.7) | 0.285 |
| Shortness of breath(n, %) | 2 (16.6) | 2 (12.5) | 1 |
| Myalgia(n, %) | 11 (91.6) | 5 (31.2) | 0.002 |
| Sore throat(n, %) | 1 (8.3) | 2 (12.5) | 1 |
| Diarrhoea(n, %) | 2 (16.6) | 4 (25.0) | 0.673 |
| Nausea and vomiting(n, %) | 3 (25.0) | 3 (18.75) | 1 |
| Epidemiological survey | |||
| History of stay in Wuhan(n, %) | 8 (66.6) | 4 (25.0) | 0.053 |
| Contact with Wuhan residents(n, %) | 11 (91.6) | 10 (62.5) | 0.184 |
| Cluster outbreak(n, %) | 6 (50.0) | 4 (25.0) | 0.243 |
| Blood routine | |||
| Leucocytes (×109/L, normal range 3.5–9.5) | 4.9 (4.3–6.9) | 6.8 (5.4–9.1) | 0.095 |
| Neutrophils (×109/L, normal range 1.8–6.3) | 3.2 (2.7–3.8) | 4.6 (3.4–6.1) | 0.029 |
| Lymphocytes (×109/L, normal range 1.1–3.2) | 1.2 (1.0–1.7) | 1.0 (0.7–1.8) | 0.246 |
| Platelets (×109/L, normal range 125.0–350.0) | 189.5 (144.7–223.7) | 197.5 (154.7–253.2) | 0.546 |
| Haemoglobin (g/L, normal range 130.0–175.0) | 143.0 (133.5–149.7) | 138.5 (123.2–148) | 0.416 |
| Blood biochemistry | |||
| Alanine aminotransferase (U/L, normal range 3.0–35.0) | 14.5 (12.2–23.7) | 23.0 (14.5–50.7) | 0.21 |
| Total bilirubin (μmol/L, normal range 4.0–23.9) | 7.3 (4.9–11.6) | 8.1 (6.1–15.2) | 0.39 |
| Albumin (g/L, normal range 36.0–51.0) | 47.5 (41.5–49.0) | 44.5 (34.6–49.8) | 0.21 |
| Erythrocyte sedimentation rate (mm/h, normal range 0–15) | 12.5 (7.0–30.5) | 13.0 (6.0–35.0) | 0.807 |
| C-reaction protein (mg/L, normal range 0–6) | 8.1 (6.7–28.2) | 13.5 (2.2–106.8) | 0.889 |
| Procalcitonin (ng/mL, normal range 0–0.05) | 0.03 (0.02–0.06) | 0.08 (0.03–0.19) | 0.064 |
Data are n (%) and median(IQR). IQR=interquartile range. N is the total number of patients withavailable data. SARS-CoV-2=severe acuterespiratory syndrome coronavirus 2. P values for the comparisons between two groups were derived using Fisher’s exact test for categorized variables and the Mann-Whitney U test for continuous variables.
CT imaging findings
The initial thin-section chest CT findings of 28 clinically suspected patients with COVID-19 are presented in Table 2. The main chest CT findings of clinically suspected patients included bilateral multi-focal lesions in the lungs, halo signs and pure/mixed GGOs, which were more frequently located in the peripheral pulmonary/subpleural areas in the confirmed COVID-19 patients than in the suspected but negative patients (91.7% vs 37.5%, p = 0.006).
Table 2.
Comparison of the frequencies of CT features between confirmed patients with COVID-19 and SARS-CoV-2-negative patients
| Feature | Confirmed patients (N = 12) | Negative patients (N = 16) | P values |
|---|---|---|---|
| Type of exudative lesions | 0.687 | ||
| Pure/mixed GGO | 9 (75.0) | 10 (62.5) | |
| Consolidation | 3 (25.0) | 6 (37.5) | |
| Lesion location | 0.006 | ||
| Peripheral/subpleural | 11 (91.7) | 6 (37.5) | |
| Central distribution/scattered | 1 (8.3) | 10 (62.5) | |
| Number of lesions | 0.429 | ||
| Multifocal | 11 (91.7) | 16 (100.0) | |
| Unifocal | 1 (8.3) | 0 (0.0) | |
| Bilateral lung involvement | 10 (83.3) | 10 (62.5) | 0.401 |
| Morphology of lesions | |||
| Large irregular/patchy opacities | 12 (100.0) | 16 (100.0) | 1.000 |
| Rounded opacities | 4 (33.3) | 10 (62.5) | 0.127 |
| Linear opacities | 7 (58.3) | 7 (43.8) | 0.445 |
| Crazy-paving pattern | 5 (41.7) | 4 (25.0) | 0.432 |
| Halo sign | 9 (75.0) | 8 (50.0) | 0.253 |
| Air bronchogram | 5 (41.7) | 7 (43.8) | 0.912 |
| Peribronchovascular interstitial thickening | 6 (50.0) | 6 (37.5) | 0.508 |
| Interlobular septal thickening | 8 (66.7) | 6 (37.5) | 0.127 |
| Tree-in-bud pattern | 4 (33.3) | 6 (37.5) | 1.000 |
| Subpleural curvilinear line | 2 (16.7) | 0 (0.0) | 0.175 |
| Honeycombing | 0 (0.0) | 0 (0.0) | 1.000 |
| Pleural thickening | 2 (16.7) | 1 (6.2) | 0.560 |
| Solid pulmonary nodule | 4 (33.3) | 1 (6.2) | 0.133 |
| Pleural effusion | 0 (0.0) | 3 (18.8) | 0.238 |
| Cavitation | 0 (0.0) | 0 (0.0) | 1.000 |
| Lymphadenopathy | 0 (0.0) | 1 (6.2) | 1.000 |
| Emphysema | 0 (0.0) | 0 (0.0) | 1.000 |
| Bronchiectasis | 2 (16.7) | 3 (18.8) | 1.000 |
Data are n (%), N is the total number of patients with available data. Percentages may not add up to 100% because of rounding. P values for the comparisons between two groups were derived using Fisher’s exacttest for categorized variables. GGOs = ground-glass opacities; COVID-19 =coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
The number of lesions, bilateral lung involvement, large irregular/patchy opacities, rounded opacities and linear opacities, crazy-paving patterns, interlobular septal thickening, air bronchograms, peribronchovascular interstitial thickening, tree-in-bud patterns, subpleural curvilinear lines and pleural thickening were apparent in confirmed and suspected but negative patients, and there were no significant differences. (Figures 2–4)
Figure 2.
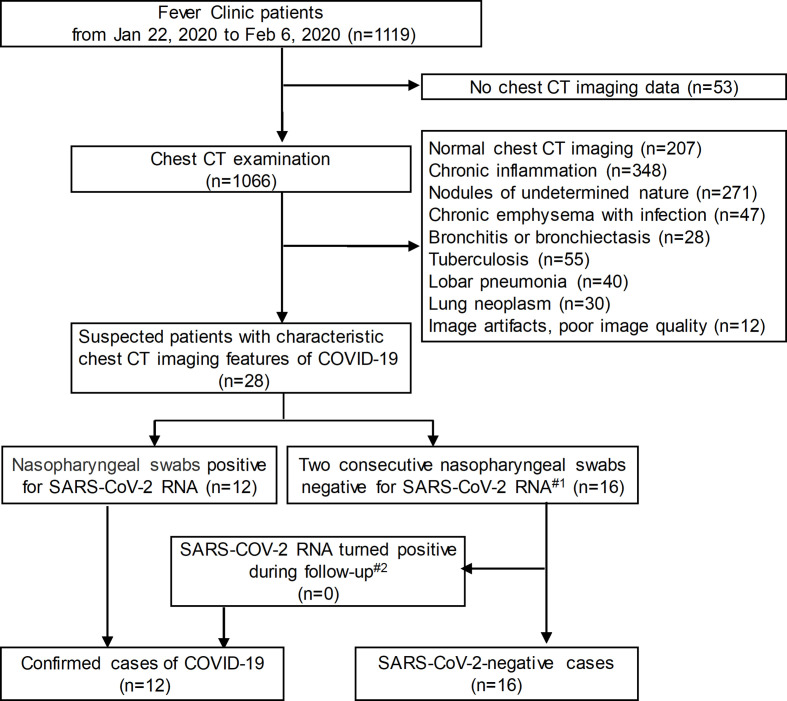
(a-b) An axial CT image obtained without intravenous contrast in a 43-year-old male showed peripheral/subpleural patchy consolidation in the anterior of the left upper lobe, with interlobular septal thickening, air bronchogram and linear opacities. He had a history of living in Xiaogan, Hubei Province and presented with fever for 1 week. Nasopharyngeal swabs for SARS-CoV-2 RNA were positive.
Figure 3.
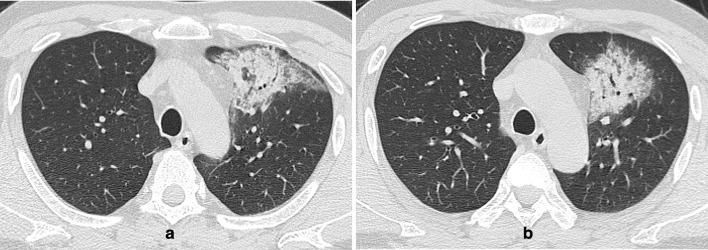
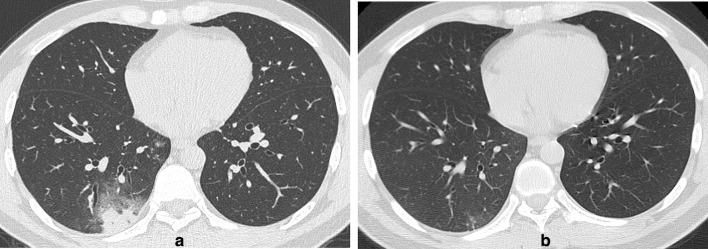
(a-b) A31-year-old male with a history of travelling on a cruise presented with fever, diarrhoea, sore throat and fatigue. (a) Axial thin-section non-contrast CT image on February 1, 2020, showed peripheral/subpleural, patchy, rounded, mixed GGOs in the right lower lobe, with interlobular septal thickening and crazy-paving patterns. (b) Follow-up CT image on February 9, 2020, showed that the lesions were obviously resolved after anti-infection therapy. Nasopharyngeal swabs for SARS-CoV-2 RNA were negative.
Figure 4.
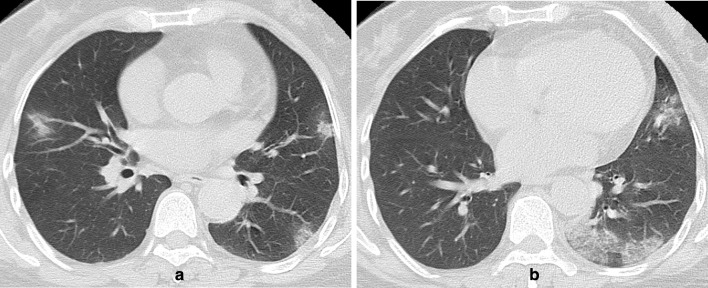
(a-b) A 56-year-old female with a history of travelling to Guangzhou from Wuhan presented with fever, cough and diarrhoea for 2 days. (a) (b) Axial thin-section non-contrast CT image showed bilateral peripheral/subpleural, patchy, pure/mixed GGOs with interlobular septal thickening and crazy-paving patterns. Two consecutive nasopharyngeal swabs for SARS-CoV-2 RNA(the interval between the two swabs more than 24 h) were negative.
Discussion
To screen for patients with COVID-19 and control the spread of the epidemic, clinically suspected patients need to be isolated. Chest imaging findings are important criteria for suspected cases. In our study, clinically suspected patients had bilateral multi-focal lung lesions, halo signs and pure/mixed GGO opacities, which were more peripheral/subpleural in the confirmed COVID-19 patients. Because COVID-19 mainly involves the respiratory system, chest CT is more sensitive than chest X-ray (CXR) in differential diagnosis initial evaluation, response evaluation and follow-up of COVID-19. CXR is usually no abnormal findings in the early stage of COVID-19, whereas CT findings may be present even before symptom onset.11,12 Furthermore, CT imaging have been proven to have diagnostic value in several studies with initially false-negative RT-PCR screening results.4,13,14 Therefore, to identify COVID-19 patients early and control the source of the infection, CT is often a first-line investigation for COVID-19 in mainland China. Many studies have concluded that the CT findings of COVID-19 include bilateral pulmonary parenchymal pure/mixed GGOs in the lung periphery, accompanying crazy-paving patterns, consolidation, intralobular interstitial thickening and interlobular septal thickening3-5, 15, 16. The common CXR findings are similar to those described for CT: bilateral, peripheral, consolidation and/or GGOs.12,15 when the disease developed from the intermediate to rapid progression stages, CXR may show bilateral lung diffuse consolidative opacities—the features of acute respiratory distress syndrome (ARDS).12 However, unlike in confirmed COVID-19 patients, whether these imaging findings exist in the suspected but negative patients has not been reported. It is clinically important to identify negative patients from suspected COVID-19 patients.
In our study, pure/mixed GGOs were also common in negative patients. Many pulmonary infectious diseases, such as influenza virus pneumonia, and non-infectious conditions, including interstitial pulmonary oedema, pulmonary haemorrhage, respiratory bronchiolitis, hypersensitivity pneumonitis, organising pneumonia and alveolar proteinosis, can show similar imaging manifestations.8,9 In addition, the type of exudative lesions is related to the different stages of COVID-19 pneumonia. Pan et al16 reported that typical mild COVID-19 pneumonia initially presented as small subpleural, unilateral or bilateral GGOs in the lower lobes, which then changed into crazy-paving patterns and subsequent consolidation in a few days.
However, pure/mixed GGOs in the peripheries was a special imaging feature for differential diagnosis to distinguish patients with COVID-19 from clinically suspected patients in our study. The lesions of COVID-19 patients were predominately distributed in the peripheral/subpleural pulmonary regions.3,16,17 We speculate that this is related to the pathophysiological mechanism of the disease. COVID-19 is caused by SARS-CoV-2, which is approximately 50–200 nm in diameter and is prone to staying in the terminal bronchioles and causing lung damage. In addition, SARS-CoV-2 mainly invades cells containing angiotensin-converting enzyme 2 (ACE2) receptors, which are found in regions rich in bronchial epithelial cells and type 2 alveolar epithelial cells, especially the latter.18–20As a result, the peripheral lung is attacked first and suffers the most damage. Nevertheless, the GGOs of both H1N1 pneumonia and SARS are also distributed more peripherally.21,22 Therefore, the single factor of peripheral/subpleural lung distribution in COVID-19 is not very unique.
In addition, other CT features, including interlobular septal thickening, crazy-paving patterns, halo signs and consolidation, were not specific for a diagnosis of COVID-19 and could be seen in the negative patients. The crazy-paving pattern has been shown to occur in many other diseases, such as usual interstitial pneumonia, infection, pulmonary oedema, haemorrhage, ARDS, alveolar proteinosis, bronchiolitis obliterans organising pneumonia (BOOP), andradiation pneumonitis.10,23
In our study, more confirmed COVID-19 patients had myalgia, but there were no differences between the two groups patients in other clinical symptoms. The neutrophil count in SARS-CoV-2-negative patients was higher than that in confirmed patients. This suggests the possibility of bacterial infection in suspected but negative patients, which may be an identifying point.
Because the clinical features and imaging findings are not unique to confirmed patients with COVID-19, aetiologic evidence, including SARS-CoV-2 RNA and serum IgM antibodies, remains the diagnostic standard for COVID-19.
There are several limitations to our study. First, the sample size of confirmed and suspected but negative patients was small. Second, the pathogens of most patients who were SARS-CoV-2 negative were not clear. Third, there were no pathological results of lung tissue available to investigate the correlation between radiological and histopathological findings.
In conclusion, although peripheral pure/mixed GGOs on CT may help distinguish patients with COVID-19 from clinically suspected but negative patients, CT cannot replace RT-PCR testing.
Footnotes
Acknowledgment: The authors would like to express their appreciation for all of the emergency services, nurses, doctors and other hospital staff for their efforts to combat the COVID-19 outbreak. We also appreciate all patients involved in the study.
Conflict of Interest: The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding: This study has received funding from the National Natural Science Foundation of China (grant number 81101096); the Medical Scientific Research Foundation of Guangdong Province (grant number B2011102); Science and Technology Planning Project of Guangdong Province (grant number 2015A020212017); and the Natural Science Foundation of Guangdong Province (grant numbers 2016A030313323 and 2017A030313841); Medical Scientific Research Foundation of Guangdong Province(grant number A2015109).
Sidong Xie and Ziying Lei have contributed equally to this study and should be considered as co-first authors.
Contributor Information
Sidong Xie, Email: xiesidong2001@163.com.
Ziying Lei, Email: leiziy@mail.sysu.edu.cn.
Xiuzhen Chen, Email: chenxiuzhen2013@sina.com.
Weimin Liu, Email: liuwm8@mail.sysu.edu.cn.
Xiaohong Wang, Email: wxhcrz@163.com.
Yunxu Dong, Email: 526842162@qq.com.
Yuefei Guo, Email: yuefei.guo@vip.163.com.
Yani Duan, Email: dyn9539@163.com.
Huijuan Cao, Email: caohjuan@mail.sysu.edu.cn.
Jie Qin, Email: jason020@163.com.
Bingliang Lin, Email: linbingl@mail.sysu.edu.cn.
REFERENCES
- 1.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. . Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63: 457–60. doi: 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. . Novel coronavirus (2019-nCoV) pneumonia. RADIOLOGY 2019; 295: 210–72020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 2020; 200343: 200343. doi: 10.1148/radiol.2020200343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology 2020; 295: 16–17. doi: 10.1148/radiol.2020200241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.General office of national health Committee. office of state administration of traditional Chinese medicine. Notice on the issuance of a programme for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia 2020;[Google Scholar]. In. [Google Scholar]
- 7.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 8.Franquet T. Imaging of pulmonary viral pneumonia. Radiology 2011; 260: 18–39. doi: 10.1148/radiol.11092149 [DOI] [PubMed] [Google Scholar]
- 9.Koo HJ, Lim S, Choe J, Choi S-H, Sung H, Do K-H. Radiographic and CT features of viral pneumonia. Radiographics 2018; 38: 719–39. doi: 10.1148/rg.2018170048 [DOI] [PubMed] [Google Scholar]
- 10.Wong KT, Antonio GE, Hui DSC, Lee N, Yuen EHY, Wu A, et al. . Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology 2003; 228: 395–400. doi: 10.1148/radiol.2283030541 [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. . Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;13 Feb 2020. doi: 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A, Disease C. COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 2019; 2020: 1–7. [DOI] [PubMed] [Google Scholar]
- 13.Huang P, Liu T, Huang L, Liu H, Lei M, Xu W, et al. . Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology 2020; 295: 22–3. doi: 10.1148/radiol.2020200330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. . Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 200642: 200642. doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong HYF, Lam HYS, Fong AH-T, Leung ST, Chin TW-Y, Lo CSY, HYS L, Lo CSY LMM, et al. . Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology 2019; 201160: 201160. doi: 10.1148/radiol.2020201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. . Ct imaging features of 2019 novel coronavirus (2019-nCoV. Radiology 2020; 295: 202–7. doi: 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. . Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. RADIOLOGY 2020; 200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. . Angiotensin-Converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426: 450–4. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020; 94: e00127-20. doi: 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–3. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan Y, Tao X-F, Shi Y-X, Liu S-Y, Chen J-Q. Initial HRCT findings of novel influenza A (H1N1) infection. Influenza Other Respir Viruses 2012; 6: e114–9. doi: 10.1111/j.1750-2659.2012.00368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong K-tak, Antonio GE, Hui DSC, Ho C, Chan P-nin, Ng W-hung, et al. . Severe acute respiratory syndrome: thin-section computed tomography features, temporal changes, and clinicoradiologic correlation during the convalescent period. J Comput Assist Tomogr 2004; 28: 790–5. doi: 10.1097/00004728-200411000-00010 [DOI] [PubMed] [Google Scholar]
- 23.Johkoh T, Itoh H, Müller NL, Ichikado K, Nakamura H, Ikezoe J, et al. . Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology 1999; 211: 155–60. doi: 10.1148/radiology.211.1.r99ap10155 [DOI] [PubMed] [Google Scholar]