Abstract
Objective:
To compare the diagnostic performance of fecal biomarkers and 18F-fludeoxyglucose (18F-FDG) positron emmision tomography-MR (PET-MR) in the assessment of disease activity in patients with ulcerative colitis.
Methods:
This study was conducted under the framework of a single-center clinical trial (clinicaltrials.gov [NCT03781284]). N = 50 participants were enrolled. Fecal samples were collected before bowel preparation. All patients underwent whole-body 18F-FDG PET-MR followed by ileocolonoscopy within 24 h. Diagnostic performance of five fecal biomarkers (calprotectin, lactoferrin, polymorphonuclear leukocyte elastase, S100A12 and eosinophil-derived neurotoxin), MR morphological parameters (MRmorph), diffusion-weighted imaging and PET in detecting active disease determined by Rachmilewitz endoscopic activity index (EAI) were evaluated and compared with each other. Correlations between fecal biomarkers, PET and endoscopy were calculated.
Results:
According to EAI, n = 38 patients presented with endoscopically active disease (16 mild, 19 moderate and 3 severe). All five biomarkers, PET and MRmorph could differentiate endoscopically active disease from endoscopic remission without significant difference regarding their operating characteristics (accuracies between 0.673 for calprotectin and 0.898 for lactoferrin). In predicting endoscopically moderate to severe disease, PET showed the highest diagnostic performance (accuracy = 0.857) compared to calprotectin and lactoferrin (accuracy = 0.633 and 0.735). PET had also the strongest correlation with endoscopy (ρ = 0.685, p < 0.001), while within fecal biomarkers the levels of lactoferrin and eosinophil-derived neurotoxin correlated significantly with EAI (ρ = 0.423 and 0.528, both p < 0.05).
Conclusion:
Both fecal biomarkers and PET-MR were excellent non-invasive diagnostic tools in the assessment of disease activity in ulcerative colitis.
Advances in knowledge:
Both fecal biomarkers and PET-MR parameters are able to predict endoscopically active disease with comparable diagnostic performance. PET had the highest correlation with endoscopy and outperformed fecal biomarkers in differentiating moderate to severe from mild disease.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by relapsing and remitting courses. Assessment of disease extent and severity is crucial for therapeutic decision-making. In an evidence-based expert consensus, a composite of clinical and endoscopic remission was proposed as treatment target.1 However, the invasive nature, high cost and low patient’s acceptance are known drawbacks of endoscopy, which impede its frequent use in clinical practice.
Over the last two decades, accumulating data have demonstrated the diagnostic value of fecal biomarker (FB) in distinguishing functional disorder from organic disease, indicating presence of mucosal healing and predicting the risk of future relapse in IBD.2–5 The clinical utility of FB facilitates non-invasive estimation of disease activity and helps to avoid non-necessary invasive diagnostic procedures.6 To date, fecal calprotectin and lactoferrin are two most investigated surrogate markers in UC, but thresholds that indicate endoscopically mucosal healing differ significantly within studies and remain debated.7,8 Comparison of different FB regarding their diagnostic performance and validation of thresholds in a randomized prospective fashion are therefore necessary.
Several preliminary studies have revealed the promising results of 18F-Fludeoxyglucose (18F-FDG) positron emission tomography-MR (PET-MR) in the assessment of Crohn’s disease (CD). Hybrid biomarkers that combined PET and MR parameters outperformed each submodality alone, not only in differentiating fibrostenotic from inflammatory strictures but also in predicting endoscopically active inflammation.9–11 Recently, PET-CT and PET-MR have been proven as reliable imaging tools for evaluating disease activity of UC.12,13 Growing evidences suggested that the change of metabolic activity of the bowel in UC was mainly associated with the grade of neutrophil infiltrate in the mucosa, which might explain to some extent the intense correlation between PET and histologic findings.14–16 Since fecal stream is in direct contact with the inflamed sites, disease activity could also be reflected by the levels of neutrophil-derived FB. It is of clinical interest and relevance to find out which diagnostic tool is better suited to predict inflammatory condition in UC and which FB correlate significantly with PET.
Therefore, the aim of our study was to compare the diagnostic performance of PET-MR and FB in detecting and grading endoscopic inflammation in UC. Furthermore, correlations between FB, PET and endoscopy were calculated.
Methods and materials
Study design and patient population
This study was conducted from November 2015 to April 2017 under the framework of a single-center randomized controlled trial (clinicaltrials.gov NCT03781284), in which disease extent and severity of 50 patients with UC were assessed with both 18F-FDG PET-MR and ileocolonoscopy. This study was approved by ethics committee and performed in accordance with the declaration of Helsinki. Written informed consents were obtained from all patients.
Stool samples were obtained before bowel preparation to evaluate FB. After an initial enrollment of 10 patients for PET-MR protocol optimization, the following 40 participants were randomized into 2 study arms, in which bowel preparation was performed either before (n = 19) or after (n = 21) 18F-FDG PET-MR followed by ileocolonoscopy.
The eligible criteria included confirmed diagnosis of UC (based on clinical, endoscopic and histological findings) and clinical symptoms of suspect flare-ups (e.g. abdominal pain, diarrhea/altered bowel habit or rectal bleeding) requiring endoscopy as initial assessment or follow-up after medical treatment. The exclusion criteria were age <18, pregnancy, contraindications of MR (e.g. cardiac pacemaker or non-removable metal implants) and severe renal failure (glomerular filtration rate <30 ml/min).
Measurement of fecal biomarkers
Stool specimens were collected prior to bowel preparation by the patients within 72 h. After collection all samples were frozen immediately at −30°C and transferred to laboratory for further analysis (Labor L + S AG, Bad Bocklet-Großenbrach, Germany). Calprotectin, lactoferrin, polymorphonuclear leukocyte elastase (PMN-e), S100A12 and eosinophil-derived neurotoxin (EDN) were analyzed in every specimen using quantitative enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. The results of the test samples were expressed as µg/g for calprotectin, lactoferrin, EDN and S100A12 or ng/ml for PMN-e.
18F-FDG PET-MR imaging protocol and analysis
All participants underwent PET-MR examination on a whole-body 3.0 T PET-MR scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany). Body weight-adapted 18F-FDG (mean activity 178 ± 46 MBq) was administered intravenously 1h prior to scan. Applied PET-MR imaging protocol and MR sequences were summarized in supplementary materials (Table 1). Two radiologists (both with more than 4 years of experiences in PET-MR) analyzed independently the PET-MR images utilizing a dedicated post-processing software (Syngo.via, VB30B, Siemens Healthcare, Erlangen, Germany). They were blinded to the clinical and endoscopic findings of the patients.
Table 1.
Sociodemographic and clinical characteristics (mean ± standard deviation (range))
| N = 49 | |
|---|---|
| Age years | 43 ± 12 (23 - 67) |
| Sex n (%) | |
| Female | 32 (65.3%) |
| Male | 17 (34.7%) |
| Patterns of disease in the history n (%) | |
| Proctitis | 11 (22.4%) |
| Left-sided colitis | 19 (38.8%) |
| Pancolitis | 19 (38.8%) |
| Time since diagnosis years | 12 ± 8.94 (1 - 42) |
| Smokers n (%) | 3 (6.1%) |
| EAI | |
| Remission n (%) | 11 (22.4%) |
| Mild n (%) | 16 (32.7%) |
| Moderate n (%) | 19 (38.8%) |
| Severe n (%) | 3 (6.1%) |
| SUVmean | 1.41 ± 0.72 (0.6–4.36) |
| Blood values | |
| CRP | 1.01 ± 1.2 (0.0–4.8) |
| BSR | 17.59 ± 16.65 (2 - 70) |
| Leucocytes | 8235.37 ± 3258.81 (874–18330) |
| Thrombocytes | 343.28 ± 119.66 (135 - 673) |
| Medication n (%) | |
| Steroids | 23 (46.9%) |
| ThiopurineMTX | 5 (10.2%) |
| Biologics | 8 (16.3%) |
| Mesalamine | 38 (77.6%) |
| Other | 22 (44.9%) |
BSR, blood sedimentation rate; CRP, C-reactive protein; EAI, Rachmilewitz Endoscopic Activity Index; SUVmean, mean value of maximum of standardized uptake value ratio gut/liver.
From rectum to terminal ileum, seven ileocolonic segments were divided (rectum, sigmoid colon, descending colon, transverse colon, ascending colon, cecum and terminal ileum). The maximum of standard uptake value (SUVmax) of 18F-FDG was measured by placing a spherical volume of interest (mean size 9 ± 3.6 cm3) in the most FDG-avid region of each segment. The SUVmax of liver was measured by drawing a larger spherical volume (mean size 50.2 ± 20.4 cm3) on the right liver lobe. As the inflammatory process of UC classically involves the rectum and spreads proximally, the mean metabolic activity of the potentially involved bowel segments in each patient was calculated as the mean value of SUVmax (SUVmean) from rectum to terminal ileum relative to liver.
Three MR morphological criteria (MRmorph) were evaluated: (1) hyperintense mucosal layer in the contrast-agent supported T1 weighted sequence; (2) positive comb-sign indicating hyperemia and engorged vasa recta; (3) wall thickening compared to ileal segment. If all three morphological criteria were fulfilled, the corresponding bowel segment was considered actively inflamed. In addition, hyperintensity of the bowel in the b1000 images of diffusion-weighted imaging (DWI) was evaluated. Both MRmorph and DWI were assessed only in rectum, since the inflammation characteristically commences in the rectum.17
Endoscopic disease activity index
Endoscopic procedures were performed after PET-MR scan within 24 h to avoid potentially increased mucosal FDG-uptake in the biopsied areas. All examinations were performed by a board-certificated gastroenterologist and documented on videos, which were independently reviewed by a second gastroenterologist with more than 10 years of experience in endoscopy. Both gastroenterologists graded the endoscopic findings according to Rachmilewitz endoscopic activity index (EAI)18 and discrepancies were resolved by a consensus reading.
Based on four endoscopic variables, EAI ranges from 0 to 12 point. Endoscopic remission was defined as EAI ≤ 3, mild activity 4–6, moderate activity 7–9, and high activity 10–12 points.3,19
Granulation scattering reflected light: [No, 0; Yes, 2 points].
Vascular pattern: [normal, 0; faded/disturbed, 1; completely absent, 2 points].
Vulnerability of mucosa: (none, 0; slightly increased [contact bleeding], 2; greatly increased [spontaneous bleeding], 4 points).
Mucosal damage: [mucus, fibrin, exudates, erosions, ulcer] [none, 0; slight, 2; moderate, 3; pronounced, 4].
Statistics
Endoscopically active disease (mild, moderate and severe activity) was defined as EAI >3 and remission as EAI ≤3. Mann–Whitney U tests were used to test whether metric variables (FB and SUVmean) differed significantly between patients’ groups with endoscopic remission and with active diseaseas well as between patients’ groups with endoscopically mild activity (EAI 4–6) and moderate to severe activity (EAI 7–12). Receiver operating characteristics (ROC) with calculated area under the curves (AUC) were performed for metric variables in predicting both active disease and moderate to severe disease on a patient basis. Optimal cut-off-values were determined by the maximum of Youden’s index. DeLong’s tests were used to compare ROCs. Accuracies of metric variables in predicting active disease and moderate to severe disease were compared with each other with two-sides exact mcNemar test. The frequencies of the nominal parameters (MRmorph and DWI) were compared with the endoscopic findings (endoscopic remission or active disease) using contingency tables and the differences were tested separately utilizing Fisher’s exact test. Correlations between FB, SUVmean and EAI were analyzed using Spearman’s rank correlation-test.
p-Values were adjusted for multiple testing using Holm-Bonferroni method.20 A p-value < 0.05 was considered significant. All statistical tests were performed with R-Software environment for statistical computing (v. 3.4, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
Due to sampling error of 1 patient, stool samples of 49 participants (n = 32 females) could be analyzed and included in the study. The median time since diagnosis of UC was 10 ± 8.94 years (range from 1 to 42 years). Based on the endoscopic findings, 11 patients had endoscopic remission while the rest (n = 38) had active disease (n = 16 with mild, n = 19 with moderate and n = 3 with severe activity). The sociodemographic data of the patients are listed in Table 1.
Diagnostic performance of fecal biomarkers and PET-MR parameters in predicting endoscopically active disease
According to Mann–Whitney U tests, the levels of all five FB and SUVmean were significantly different between patient’s groups with endoscopic remission and with active disease (Table 2). Within patients with active disease, significant differences between patients with only mild inflammatory activity and patients with moderate to severe inflammatory activity could be found only for SUVmean and EDN.
Table 2.
Comparison of levels of FB and SUVmean in patients’ groups with endoscopic remission and with active disease
| SUVmean | Calprotectin (µg/g) | Lactoferrin (µg/g) | PMN-e (ng/ml) | EDN (µg/g) | S100A12 (µg/g) | |
|---|---|---|---|---|---|---|
| Endoscopic remssion | 0.85 ± 0.24 | 64.64 ± 169.07 | 2.21 ± 13.81 | 0.08 ± 0.14 | 1.03 ± 0.68 | 15.02 ± 54.08 |
| Endoscopically active disease | 1.47 ± 0.76 | 344.66 ± 614.72 | 36.63 ± 139.61 | 0.23 ± 0.19 | 2.56 ± 1.8 | 88.19 ± 53.39 |
| p-values* | 0.003 | 0.017 | <0.001 | 0.007 | 0.002 | 0.009 |
| Endoscopically mild activity | 1 ± 0.36 | 529.93 ± 748.01 | 42.6 ± 156.37 | 0.1 ± 0.149 | 1.55 ± 1.6 | 60.98 ± 64.87 |
| Endoscopically moderate to severe activity | 1.63 ± 0.79 | 309.86 ± 493.19 | 36.64 ± 127.54 | 0.29 ± 0.21 | 3.31 ± 1.77 | 90.24 ± 42.53 |
| p- Values* | <0.001 | 0.672 | 1.000 | 0.069 | 0.036 | 0.145 |
EDN, eosinophil-derived neurotoxin; PMN-e, polymorphonuclear leukocyte elastase; SUVmean, mean value of maximum of standardized uptake value ratio gut/liver.
Note: * Mann–Whitney U test.
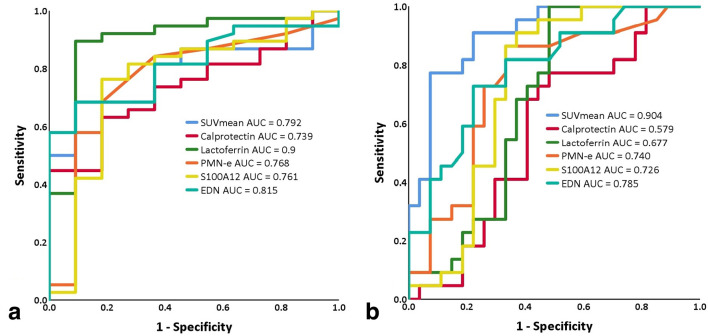
In predicting endoscopically active disease, lactoferrin showed the largest AUC of 0.9 in the ROC analysis and the highest diagnostic performance (sensitivity of 0.895 [95% CI 0.797–0.992], specificity of 0.909 [95% CI 0.739–1] and accuracy of 0.898 [95% CI 0.894–0.902])compared to other biomarkers and SUVmean (Figure 1 and Table 3). However, no significant difference regarding their ROC was present according to DeLong’s test (Supplementary Material 1) and the accuracy of lactoferrin was only significantly different from that of calprotectin (Supplementary Material 1).
Figure 1.
Receiver operating characteristics of FBs and SUVmean in predictingendoscopically active disease and moderate to severe disease. All five FB and SUVmean of PET performed similarly well in predicting patients with endoscopically active disease (a). In detecting patients with endoscopically moderate to severe disease activity, SUVmean outperformed all five biomarkers with a significant difference to calprotectin and lactoferrin (b). AUC, area under thecurve; EDN, eosinophil-derived neurotoxin; FB, fecal biomarker; PMN-e, polymorphonuclear leukocyte elastase; SUVmean, mean valueof maximum of standardized uptake value ratio gut/liver.
Table 3.
Diagnostic performance of fecal biomarkers and SUVmean in predicting endoscopically active disease
| Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | Optimal Cutoff | AUC of ROC | p values* | |
|---|---|---|---|---|---|---|
| SUVmean | 0.684 (0.536–0.832) | 0.909 (0.739–1) | 0.735 (0.727 0.742) | 1.16 (µg/g) | 0.792 | 0.012 |
| Calprotectin | 0.632 (0.478–0.785) | 0.818 (0.59–1) | 0.673 (0.665–0.682) | 139.02 (µg/g) | 0.739 | 0.021 |
| Lactoferrin | 0.895 (0.797–0.992) | 0.909 (0.739–1) | 0.898 (0.894–0.902) | 9.90 (µg/g) | 0.9 | <0.001 |
| PMN-e | 0.684 (0.536–0.832) | 0.818 (0.59–1) | 0.714 (0.706–0.722) | 0.105 (ng/ml) | 0.768 | 0.021 |
| EDN | 0.684 (0.536–0.832) | 0.909 (0.739–1) | 0.734 (0.727–0.742) | 1.68 (µg/g) | 0.815 | 0.01 |
| S100A12 | 0.763 (0.628–0.898) | 0.818 (0.59–1) | 0.776 (0.769–0.782) | 58.12 (µg/g) | 0.761 | 0.021 |
AUC, area under the curve; CI, Confidence interval; EDN, eosinophil-derived neurotoxin; PMN-e, polymorphonuclear leukocyte elastase ; ROC, receiver operating characteristics; SUVmean, mean value of maximum of standardized uptake value ratio gut/liver.
p values was determined by receiver operating characteristics
As nominal MR parameters, MRmorph led to a sensitivity of 0.974 (37/38), specificity of only 0.364 (4/11) and accuracy of 0.836 (p = 0.007, fisher’s exact test), while DWI resulted in a sensitivity of 0.947 (36/38), specificity of 0.273 (3/11) and accuracy of 0.796 (p = 0.068).
Diagnostic performance of fecal biomarkers and PET-MR parameters in predicting endoscopically moderate to severe disease activity
Based on endoscopic findings, 22 out of 49 patients had endoscopically moderate to severe activities. SUVmean with an AUC of 0.904 outperformed all the FB (AUC between 0.579 and 0.785) (Figure 1 and Table 4) with a significant difference to calprotectin and lactoferrin (Supplementary Material 1). With an AUC of 0.579 for calprotectin and 0.677 for lactoferrin, both FB failed to predict patients with endoscopically moderate to severe disease activity.
Table 4.
Diagnostic performance of fecal biomarkers and SUVmean in predicting endoscopically moderate to severe disease
| Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | Optimal Cutoff | AUC of ROC | p values* | |
|---|---|---|---|---|---|---|
| SUVmean | 0.773 (0.598–0.948) | 0.926 (0.827–1) | 0.857 (0.852–0.862) | 1.49(µg/g) | 0.904 | <0.001 |
| Calprotectin | 0.773 (0.598–0.948) | 0.519 (0.330–0.707) | 0.633 (0.623–0.642) | 105.6(µg/g) | 0.579 | 0.345 |
| Lactoferrin | 1.00 (1.000–1.000) | 0.518 (0.330–0.707) | 0.735 (0.727–0.742) | 9.09(µg/g) | 0.677 | 0.07 |
| PMN-e | 0.864 (0.720–1) | 0.667 (0.489–0.844) | 0.756 (0.748–0.762) | 0.105(ng/ml) | 0.740 | 0.016 |
| EDN | 0.727 (0.541–0.913) | 0.778 (0.621–0.935) | 0.755 (0.748–0.762) | 2.23(µg/g) | 0.785 | 0.005 |
| S100A12 | 0.864 (0.789–1) | 0.667 (0.447–0.812) | 0.755 (0.748–0.762) | 64.385(µg/g) | 0.726 | 0.021 |
AUC, area under the curve; CI, Confidence interval; EDN, eosinophil-derived neurotoxin; PMN-e, polymorphonuclear leukocyte elastase; ROC, receiver operating characteristics; SUVmean, mean value of maximum of standardized uptake value ratio gut/liver.
p values was determined by receiver operating characteristics.
Within the 38 patients with active disease, SUVmean and EDN were the only 2 surrogate markers that could differentiate moderate to severe from mild disease (AUC = 0.869 for SUVmean, p < 0.001; AUC = 0.702 for EDN, p = 0.036). Despite the highest diagnostic specificity of SUVmean (0.926 [95% CI 0.827–1]), the overall accuracies of SUVmean and EDN in predicting moderate to severe disease were not significantly different (0.857 [95% CI 0.852–0.862] vs 0.755 [95% CI 0.748–0.762], Supplementary Material 1).
Correlations between SUVmean, fecal biomarkers and endoscopic activity index
Compared to FB, SUVmean showed the strongest correlation with EAI (Spearmann’s rho = 0.685; p < 0.001). Within all the FB, only lactoferrin and EDN correlated significantly with EAI (ρ = 0.423 and 0.528; p < 0.05). Compared to other FB, EDN showed the highest correlation with SUVmean (ρ = 0.603; p < 0.001).
Discussion
The main objective of our study was to compare the diagnostic performance of FB and PET-MR as non-invasive diagnostic tools in the assessment of disease activity in UC. All five FB, SUVmean of PET and MRmorph could differentiate patients with endoscopically active disease from patients with endoscopic remission with similar diagnostic performance. PET had the highest diagnostic performance in predicting endoscopically moderate to severe disease. PET showed also the strongest correlation with endoscopic activity index (ρ = 0.685, p < 0.001), while among FB only the levels of lactoferrin and EDN correlated significantly with endoscopy (ρ = 0.423 and 0.528, both p < 0.05).
Discrepancies between clinical symptoms and endoscopic findings in UC have been well-reported in clinical trials.21,22 Non-invasive and objective assessments of mucosal inflammatory conditions are desirable, at least to select patients warranting further endoscopic evaluation in resource-limited settings. In line with previous reports,23–25 our study results confirmed the significantly higher fecal levels of neutrophil-derived biomarkers (calprotectin, lactoferrin, PMN-e and S100A12) in patients with endoscopically active inflammation. Indeed, immunohistochemical analysis of biopsy samples revealed dense neutrophil infiltrate in the colonic mucosa of UC patients with active disease, whereas only a few neutrophils were present in patients under quiescent disease.26
Lactoferrin presented the best diagnostic performance of all analyzed FB in predicting active disease with an overall accuracy of 0.898 (AUC = 0.9, p < 0.001) in our study. Hence, lactoferrin was considered as the most suitable neutrophil-derived biomarker for clinical application, not only because of its superior diagnostic accuracy but also due to its efficient extracellular release and higher stability in feces.23,27 SUVmean that reflected the average metabolic condition of ileocolon showed the same specificity of 0.909 but inferior sensitivity of 0.684 ( vs 0.895 for lactoferrin), though no significant difference regarding their ROC performance was noted (AUC = 0.792 vs.0.9, p > 0.05). Nevertheless, SUVmean correlated stronger with endoscopy than lactoferrinand enabled differentiation of endoscopically moderate to severe disease from mild ones.
In comparison to other FB and PET, calprotectin in our study showed the lowest accuracy of 0.67 (threshold = 139 µg/g, AUC = 0.739, p = 0.021) regardless of the strongest correlation with lactoferrin (rho = 0.593, p < 0.001) in predicting endoscopically active disease. Similar results for calprotectin in predicting endoscopically mucosal healing (threshold = 121 µg/g, sensitivity = 0.79, specificity = 0.57) were reported in 63 IBD patients receiving anti-tumor necrosis factor α therapy.28 In a recently published data investigating fecal calprotectin in 39 UC patients,5 a cut-off of 187 µg/g was chosen for active disease but with considerably higher diagnostic performance (AUC = 0.915 vs 0.739 in our study). Two possible explanations are the different definition of active disease (Ulcerative Colitis Endoscopic Index of Severity ≥ 4 vs Rachmilewitz EAI ≥ 4) and the variability of endoscopic experience from different endoscopists.
Apart from neutrophil-derived biomarkers, the fecal level of eosinophil-derived surrogate markers like EDN was also markedly increased under inflamed condition in our study. The same finding was strengthened by previous reports investigating stool samples of 42 UC patients.29 It is of notice that among all five FB EDN was the only one, the concentrations of which were significantly different between patients with endoscopically mild disease and those with moderate to severe disease (median value 1.55 ± 1.6 vs 3.31±1.77 µg/g, p = 0.036). Intriguingly, compared to neutrophil-derived biomarkers, EDN correlated stronger with endoscopy (rho = 0.528, p < 0.01) as well as with PET (rho = 0.603, p < 0.001). It is well-studied that both eosinophils and neutrophils participate in different inflammatory stages of UC.30 Lampinen et al found that activation of neutrophils was restricted at the sides of active inflammation, whereas beside high percentage of eosinophil infiltrate in the rectum in patients with distal colitis high degree of eosinophil activity was found in the terminal ileum as well.26 Based on this finding, the authors assumed that eosinophils might play a role in the propagation and genesis of early lesions. This immunohistopathological finding might explain the higher correlation between EDN and PET, since SUVmean of PET reflected the average metabolic activity from rectum to terminal ileum instead of only inflamed sides.
Combing three morphological criteria of inflammation, MRmorph also showed high diagnostic accuracy of 0.836 in predicting endoscopically active disease. Similar diagnostic accuracies of MR morphological parameters were demonstrated in a previous study, which investigated DWI-MR without bowel preparation in 35 UC patients.31 It could be shown in that study among all categorical MR parameters the best diagnostic performance (sensitivity = 0.882, specificity = 0.833, p = 0.0001) was achieved by the presence of hyperintense mucosal layer, which indeed reflected florid inflammatory cell infiltrate in the mucosa (Figure 2). However, given the recent controversial discussions about gadolinium deposition in the brain,32 intravenous administration of contrast agent required for this MR sequence raises concerns about its frequent use in IBD patients. The persistence of histologic inflammation in patients with endoscopic remission might partially explain the extremely high sensitivity and low specificity of MRmorph against endoscopic findings in our study.33 On the other hand, DWI as functional parameter without the need of contrast agent failed in predicting active disease in our study, probably because of the susceptibility artifacts to the often times-present rectal air.
Figure 2.
18F-FDG PET-MR imaging in a female patient with pancolitis 18F-FDG PET-MR imaging revealed active pancolitis in a female patient with endoscopically moderate disease activity. The fecal levels of calprotecin, lactoferrin, PMN-e, S100A12 and EDN were 37.43, 46.92, 0.06, 90.14, 3.27, while the SUVmean was 1.93. According to the optimal cut-off values (Table 4), only EDN and SUVmean correctly predicted the endoscopically disease activity. (A) Hyperintense mucosal layer and wall thickness in the T2 weighted imaging demonstrating mucosal edema in the ascending and descending colon (black arrows). (B) Hyperintense appearance of mucosal layer (white arrow) and adjacent comb sign indicating hyperemia in the contrast-supported T1 weighted imaging with fat-saturation. (C) Fused imaging of PET and MR. (D) Hyperintensity of bowel wall shown in the b1000 of diffusion-weighted imaging. (E) PET. (F) Maximum intensity projection of PET in the coronal plane demonstrating enhanced 18F-FDG-uptake of the whole colorectumcompared to liver. EDN, eosinophil-derivedneurotoxin; 18F-FDG, 18F-fludeoxyglucose; PET, positronemission tomography; PMN-e, polymorphonuclear leukocyte elastase; SUVmean, mean value of maximum of standardized uptakevalue ratio gut/liver.
As cross-sectional imaging modality, PET-MR has several advantages over FB in the assessment and management of UC. Firstly, it enables direct visualization of the entire gastrointestinal tract and localization of the inflamed sides, offering a more precise assessment of disease activity and extent. Secondly, extraintestinal manifestation of IBD could be simultaneously detected by PET-MR. MR signs of primary sclerosing cholangitis were detected in one female patient and this finding was of paramount clinical importance because of increased risk of developing cholangiocarcinoma.34 Thirdly, compared to FB such as calprotectin, the interpretation of PET results is associated with higher diagnostic confidence due to the limited variability of published cut-off value and excellent interobserver concordant reading.16,35 Lastly, the results of PET-MR are available in most instances on the same day and help to guide an immediate decision-making, whereas the laboratory results of FB take often days. PET-MR has also advantages over PET-CT because of its superior tissue contrast provided by simultaneously acquired MR and lower radiation exposure due to lack of CT component. However, the limited availability, high cost, patient´s discomfort, the associated radiation exposition of PET and potential gadolinium deposition in the brain of MR are known disadvantages for PET-MR, making its clinical utility only in selected patients or clinical trials.
The main strengths of our study are its prospective design and short time interval between collection of FB, PET-MR examination and endoscopy. Since ileocolonoscopy was performed after PET-MR examination within 24 h, irritation of bowel mucosa due to biopsies and the potentially associated false-positive interpretation of PET could be avoided. Another strength of our study is the enrollment of both neutrophil- and eosinophil-derived FB for multiple testing and our results revealed stronger correlation of EDN with both PET and endoscopy.
The major limitation of our study is the different timing of bowel preparation conducted under pre-defined randomization of clinical trial. A total of 20 patients underwent bowel purgation before PET-MR, which might lead to false-positive interpretation of PET36 because of potentially increased metabolic activity of bowel in histologically quiescent colonic segments.16 Therefore, the expected diagnostic performance of PET without bowel preparation should be higher. Another limitation is the restriction of inflammatory evaluation with MRmorph and DWI only in rectum. Although UC is known as chronic inflammatory bowel disease that classically involves the rectum, atypical distribution of mucosal inflammation or rectal-sparing type were also reported.37 This limitation might mislead to false-negative results for MR and DWI.
Conclusion
Both FBs and PET-MR parameters are able to predict endoscopically active disease with comparable diagnostic performance. Due to their wide availability, lower cost and absent radiation exposure, FBs particularly lactoferrin should be preferred in selecting UC patients with suspected active disease. PET might be used to grade inflammatory activity and to identify those with severe disease because of its intense correlation with endoscopy and superb diagnostic performance to FBs.
Footnotes
Funding: This study was performed as an independent sub-study of a registered clinical trial (clinicaltrials.gov [NCT03781284]), which was funded by the Broad Medical Research Program at Crohn’s & Colitis Foundation of America (Proposal No. 360668) and the German Research Foundation (Grant Number 239242301). Granters had no influence on collection, analysis or interpretation of data.
Contributor Information
Yan Li, Email: yan.li@uk-essen.de.
Michael Khamou, Email: michael.khamou@uk-essen.de.
Benedikt Michael Schaarschmidt, Email: benedikt.schaarschmidt@uk-essen.de.
Lale Umutlu, Email: lale.umutlu@uk-essen.de.
Michael Forsting, Email: michael.forsting@uk-essen.de.
Aydin Demircioglu, Email: aydin.demircioglu@uk-essen.de.
Johannes Haubold, Email: johannes.haubold@uk-essen.de.
Anna Katharina Koch, Email: a.koch@kem-med.com.
Nils-Martin Bruckmann, Email: nils-martin.bruckmann@med.uni-duesseldorf.de.
Lino Morris Sawicki, Email: linomorris.sawicki@med.uni-duesseldorf.de.
Ken Herrmann, Email: ken.herrmann@uk-essen.de.
James Hunter Boone, Email: jhboone@techlab.com.
Jost Langhorst, Email: j.langhorst@kem-med.com.
REFERENCES
- 1.Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for Treat-to-Target. Am J Gastroenterol 2015; 110: 1324–38. doi: 10.1038/ajg.2015.233 [DOI] [PubMed] [Google Scholar]
- 2.Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflamm Bowel Dis 2008; 14: 359–66. doi: 10.1002/ibd.20336 [DOI] [PubMed] [Google Scholar]
- 3.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis 2009; 15: 1851–8. doi: 10.1002/ibd.20986 [DOI] [PubMed] [Google Scholar]
- 4.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 2000; 119: 15–22. doi: 10.1053/gast.2000.8523 [DOI] [PubMed] [Google Scholar]
- 5.Walsh A, Kormilitzin A, Hinds C, Sexton V, Brain O, Keshav S, et al. Defining faecal calprotectin thresholds as a surrogate for endoscopic and histological disease activity in ulcerative Colitis-a prospective analysis. J Crohns Colitis 2019; 13: 424–30. doi: 10.1093/ecco-jcc/jjy184 [DOI] [PubMed] [Google Scholar]
- 6.Lopez RN, Leach ST, Lemberg DA, Duvoisin G, Gearry RB, Day AS. Fecal biomarkers in inflammatory bowel disease. J Gastroenterol Hepatol 2017; 32: 577–82. doi: 10.1111/jgh.13611 [DOI] [PubMed] [Google Scholar]
- 7.Lin J-F, Chen J-M, Zuo J-H, Yu A, Xiao Z-J, Deng F-H, et al. Meta-Analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis 2014; 20: 1407–15. doi: 10.1097/MIB.0000000000000057 [DOI] [PubMed] [Google Scholar]
- 8.Dai C, Jiang M, Sun MJ, Cao Q. Fecal lactoferrin for assessment of inflammatory bowel disease activity: a systematic review and meta-analysis. J Clin Gastroenterol 2019;. [DOI] [PubMed] [Google Scholar]
- 9.Catalano OA, Gee MS, Nicolai E, Selvaggi F, Pellino G, Cuocolo A, et al. Evaluation of quantitative PET/MR Enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology 2016; 278: 792–800. doi: 10.1148/radiol.2015150566 [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Langhorst J, Koch AK, Demircioglu A, Nensa F, Kirchner J, et al. Assessment of ileocolonic inflammation in Crohn's disease - Which surrogate marker is better? MaRIA, Clermont or PET-MR index? Initial results of a feasibility trial. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2018;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Beiderwellen K, Nensa F, Grüneisen J, Dobos G, Herrmann K, et al. 18F]FDG PET/MR enterography for the assessment of inflammatory activity in Crohn's disease: comparison of different MRI and PET parameters. Eur J Nucl Med Mol Imaging 2018; 45: 1382–93. doi: 10.1007/s00259-018-3962-y [DOI] [PubMed] [Google Scholar]
- 12.Berry N, Sinha SK, Bhattacharya A, Prasad KK, Vaishnavi C, Vaiphei K, et al. Role of positron emission tomography in assessing disease activity in ulcerative colitis: comparison with biomarkers. 2018; 63: 1541–50. [DOI] [PubMed] [Google Scholar]
- 13.Shih I-L, Wei S-C, Yen R-F, Chang C-C, Ko C-L, Lin B-R, et al. Pet/Mri for evaluating subclinical inflammation of ulcerative colitis. J Magn Reson Imaging 2018; 47: 737–45. doi: 10.1002/jmri.25795 [DOI] [PubMed] [Google Scholar]
- 14.Bettenworth D, Reuter S, Hermann S, Weckesser M, Kerstiens L, Stratis A, et al. Translational 18F-FDG PET/CT imaging to monitor lesion activity in intestinal inflammation. J Nucl Med 2013; 54: 748–55. doi: 10.2967/jnumed.112.112623 [DOI] [PubMed] [Google Scholar]
- 15.Hindryckx P, Staelens S, Devisscher L, Deleye S, De Vos F, Delrue L, et al. Longitudinal quantification of inflammation in the murine dextran sodium sulfate-induced colitis model using μPET/CT. Inflamm Bowel Dis 2011; 17: 2058–64. doi: 10.1002/ibd.21578 [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Schaarschmidt B, Umutlu L, Forsting M, Demircioglu A, Koch AK, et al. 18)F-FDG PET-MR enterography in predicting histological active disease using the Nancy index in ulcerative colitis: a randomized controlled trial. Eur J Nucl Med Mol Imaging 2019;. [DOI] [PubMed] [Google Scholar]
- 17.Stange EF, Travis SPL, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Warren BF, Hommes DW, Mantzaris GJ, Herfarth H, Fléjou JF, et al. European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis 2008; 2: 1–23. doi: 10.1016/j.crohns.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 18.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 1989; 298: 82–6. doi: 10.1136/bmj.298.6666.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis 2013; 7: 982–1018. doi: 10.1016/j.crohns.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 20.Holm S. A simple sequentially Rejective multiple test procedure. Scandinavian Journal of Statistics 1979; 6: 65–70. [Google Scholar]
- 21.Jharap B, Sandborn WJ, Reinisch W, D'Haens G, Robinson AM, Wang W, et al. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. 2015; 42: 1082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narula N, Alshahrani A-A, Yuan Y, Reinisch W, Colombel J-F. Patient-Reported outcomes and endoscopic appearance of ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019; 17: 411–8. doi: 10.1016/j.cgh.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 23.Langhorst J, Boone J, Lauche R, Rueffer A, Dobos G, Lactoferrin F. Faecal lactoferrin, calprotectin, PMN-elastase, CRP, and white blood cell count as indicators for mucosal healing and clinical course of disease in patients with mild to moderate ulcerative colitis: post hoc analysis of a prospective clinical trial. J Crohns Colitis 2016; 10: 786–94. doi: 10.1093/ecco-jcc/jjw044 [DOI] [PubMed] [Google Scholar]
- 24.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008; 103: 162–9. doi: 10.1111/j.1572-0241.2007.01556.x [DOI] [PubMed] [Google Scholar]
- 25.Däbritz J, Langhorst J, Lügering A, Heidemann J, Mohr M, Wittkowski H, et al. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm Bowel Dis 2013; 19: 1130–8. doi: 10.1097/MIB.0b013e318280b1cd [DOI] [PubMed] [Google Scholar]
- 26.Lampinen M, Rönnblom A, Amin K, Kristjansson G, Rorsman F, Sangfelt P, et al. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut 2005; 54: 1714–20. doi: 10.1136/gut.2005.066423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugi K, Saitoh O, Hirata I, Katsu K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am J Gastroenterol 1996; 91: 927–34. [PubMed] [Google Scholar]
- 28.Guidi L, Marzo M, Andrisani G, Felice C, Pugliese D, Mocci G, et al. Faecal calprotectin assay after induction with anti-tumour necrosis factor α agents in inflammatory bowel disease: prediction of clinical response and mucosal healing at one year. Dig Liver Dis 2014; 46: 974–9. doi: 10.1016/j.dld.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 29.Saitoh O, Kojima K, Sugi K, Matsuse R, Uchida K, Tabata K, et al. Fecal eosinophil granule-derived proteins reflect disease activity in inflammatory bowel disease. Am J Gastroenterol 1999; 94: 3513–20. doi: 10.1111/j.1572-0241.1999.01640.x [DOI] [PubMed] [Google Scholar]
- 30.Al-Haddad S, Riddell RH. The role of eosinophils in inflammatory bowel disease. 2005; 54: 1674–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard M-A, Régent D, et al. Diffusion-Weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 2010; 59: 1056–65. doi: 10.1136/gut.2009.197665 [DOI] [PubMed] [Google Scholar]
- 32.Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 2015; 275: 783–91. doi: 10.1148/radiol.2015150337 [DOI] [PubMed] [Google Scholar]
- 33.Bryant RV, Winer S, Travis SPL, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is 'complete' remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014; 8: 1582–97. doi: 10.1016/j.crohns.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 34.Wee A, Ludwig J, Coffey RJ, LaRusso NF, Wiesner RH. Hepatobiliary carcinoma associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hum Pathol 1985; 16: 719–26. doi: 10.1016/S0046-8177(85)80158-1 [DOI] [PubMed] [Google Scholar]
- 35.Das CJ, Makharia GK, Kumar R, Kumar R, Tiwari RP, Sharma R, et al. Pet/Ct colonography: a novel non-invasive technique for assessment of extent and activity of ulcerative colitis. Eur J Nucl Med Mol Imaging 2010; 37: 714–21. doi: 10.1007/s00259-009-1335-2 [DOI] [PubMed] [Google Scholar]
- 36.Tu D-G, Chen C-R, Wang Y-W, Tu C-W, Huang YC. Bowel-Cleansing methods affecting PET-CT image interpretation. Nucl Med Commun 2011; 32: 570–4. doi: 10.1097/MNM.0b013e328345327b [DOI] [PubMed] [Google Scholar]
- 37.Robert ME, Skacel M, Ullman T, Bernstein CN, Easley K, Goldblum JR. Patterns of colonic involvement at initial presentation in ulcerative colitis: a retrospective study of 46 newly diagnosed cases. Am J Clin Pathol 2004; 122: 94–9. doi: 10.1309/XLXK-J84C-3JCW-3RCH [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.