Abstract
Narcolepsy type 1 is a debilitating chronic neurological disorder, whose main symptoms of excessive daytime sleepiness and cataplexy may partially improve with time, but typically do not fully resolve. The irreversible loss of orexin neurons is considered to be the pivotal mechanistic link underlying the development of cataplectic attacks in narcolepsy type 1. Here we describe a case of untreated narcolepsy type 1with low cerebrospinal orexin levels (< 50 pg/mL), where cataplexy fully resolved in the first 5–6 years after disease onset, whereas excessive daytime sleepiness persisted.
Citation:
Wasserman D, Bassetti CLA, Rosenzweig I. Narcolepsy with resolution of cataplexy and persisting orexin deficiency. J Clin Sleep Med. 2020;16(8):1383–1386.
INTRODUCTION
The underlying mechanisms of human narcolepsy are a source of continued debate.1 Notwithstanding this, there has been significant progress in our understanding of the etiology, pathophysiology, and the clinical manifestations of narcolepsy since the beginning of the 21st century.1 Narcolepsy has been argued to result from the interplay of distinct genetic, epigenetic, and environmental factors.1
To date, several biomarkers of narcolepsy have been established, including cataplexy, sleep-onset rapid eye movement (REM) sleep periods, positivity for major histocompatibility complex, class II, DQ beta 1 gene (HLA-DQB1)*06:02, and orexin/hypocretin deficiency in cerebrospinal fluid (CSF).1 Of these, cataplexy is considered to be one of the cardinal symptoms of narcolepsy,2 and the presence of cataplexy, alongside with orexin deficiency, categorizes narcolepsy as type 1 (NT1).1 Cataplexy typically follows orexin deficiency and development of excessive daytime somnolence (EDS), although the onset of cataplexy prior to overt reduction in orexin levels below < 110 pg/mL has also been described.3 Severity of EDS and cataplexy has been correlated with the extent of loss of orexin neurons, and with the decrease in CSF levels of orexin.1 Additionally, low levels of orexin in CSF in NT2 patients have been shown to predict the subsequent development of cataplexy.1 However, the specific mechanisms through which loss of orexin signaling may result in cataplexy remain uncertain (for detailed review see1).
The projected psychosocial impact of chronic neurologic disorders such as narcolepsy is significant, and it has been described as “an integral part of the disease or of the human reactions to it.”4 In the background of this, the current perception of NT1 as a chronic neurologic disorder with symptoms that might fluctuate and partially improve with time, but that will never disappear completely,5 presents a potentially devastating and psychologically damaging starting point for many patients who receive this diagnosis. Anecdotal clinical experience and scarce reports in the literature,6 however, dispute this perception. In further support of this notion, we here present a rare case of patient with NT1 whose symptoms of cataplexy completely remitted in the course of the illness.
REPORT OF CASE
A 41-year-old woman presented with a diagnosis of NT1 from our center, complaining of increased sleepiness associated to significant pressures at work. The patient described losing contact with the clinical team for more than 2 decades due to “hectic family and work life” (sic). The inspection of her old medical notes revealed that she first presented to our center when she was 21 years old, with complaint of EDS. A several-year-long history of sudden loss of muscle tone evoked by positive emotions (eg, laughter), with varied frequency and with preserved full consciousness, was described. Although unsure when they originally started or how frequent they were initially, the patient recalled the increased frequency during school days when she would be incessantly teased by her schoolmates in her late teens (16 or 17 years old) who would try to provoke her attacks by making her laugh at their jokes. Most often this would lead to her developing “funny faces” (sic) and inability to speak appropriately or to lose coordination of her upper or lower limbs, predominantly bilaterally. Following school parties when she would not manage to sleep or when teasing was especially intense, she would also experience a progressive full body collapse with falls. Cataplexy attacks fully resolved a few months prior to her initial presentation to our center, presumably some 5 to 6 years into her illness, while EDS persisted. A history of excessive dreaming, sleep fragmentation, and sleep talking was also reported. Her past investigations included neuroimaging (computed tomography) that was reported normal, and the polysomnographic recording that demonstrated abrupt sleep and REM activity within 30 seconds after background alpha rhythm drop-out. An initiation of modafinil was recorded, but no further follow up was noted in the medical notes.
At the time of her second presentation she was on no regular medication, with an Epworth Sleepiness Score of 21/24. Despite self-reported scoring high on Epworth Sleepiness Score, the patient described a productive and functional everyday life as a hands-on mother of 3 children of school age and working full time in a demanding workplace. She resented, however, a lack of social life, reported falling asleep during long meetings at work, and having to retire prematurely every day. She denied ever experiencing hypnagogic hallucinations or sleep paralysis. She remembered being offered pharmacologic intervention during her first encounter with our center but confessed to taking it for several weeks only due to concerns about possible side-effects and the dislike of stimulants. Physical examination was normal, except for increased body mass index (41), and her past medical history was unremarkable except for asthma, headache disorder, and the historic diagnosis of NT1.
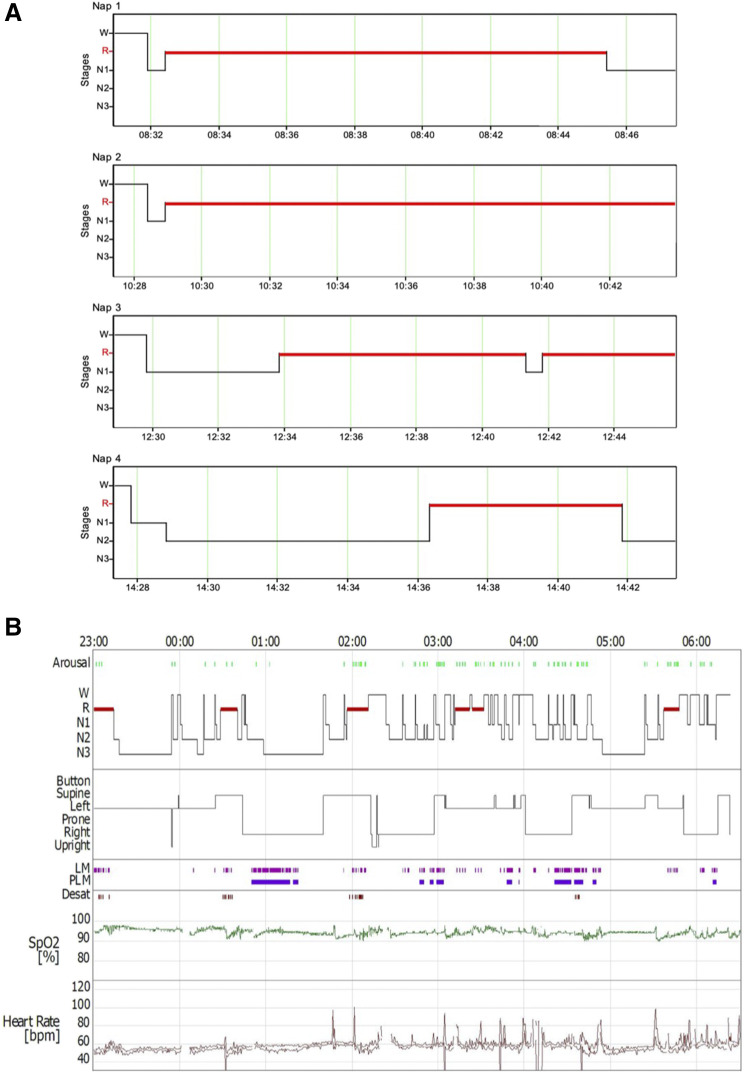
Investigations were repeated and magnetic resonance imaging of the brain was reported normal. HLA typing confirmed HLA-DQB1*0602 haplotype. Nocturnal polysomnography, performed as per current recommendations,7 revealed REM sleep latency of 1 minute, arising from N1, an increased periodic limb movement index (23.1 events/h). Also, reduced sleep efficiency of 81.2% with overall normal arousal rate (14.6 events/h) and no REM sleep behavior events were recorded. Several respiratory events were observed in supine REM sleep, leading to an overall normal apnea-hypopnoea index (4.4 events/h). Actigraphy performed for 8 days prior to the nocturnal polysomnography indicated an average sleep of 7 hours and 46 mins, with an average sleep time of 5 hours and 50 mins, and with average sleep efficiency of 75.4%. The multiple sleep latency test revealed an multiple sleep latency of 0.9 minutes and 4 of 4 sleep-onset REM sleep periods (Figure 1). Three sleep-onset REM sleep periods were recorded arising from stage N1 sleep, and the last was recorded as arising from stage N2 sleep. A lumbar puncture revealed low CSF orexin < 50 pg/mL (Neuroimmunology Laboratory, University of Oxford). All other laboratory investigations were reported normal.
Figure 1. Test results.
The schematic graph depicting results of multiple sleep latency test findings (A) and the recorded sleep architecture (B) during nocturnal polysomnography investigation. LM = limb movements, PLM = periodic limb movement, R = rapid eye movement, SpO2 = functional arterial oxygen saturation as estimated with a pulse oximeter, W = awake.
Subsequent to investigations, several nonpharmacologic treatment strategies were discussed and initiated. Regular physical activity, adequate use of caffeine, and a balanced diet avoiding large amounts of carbohydrates were recommended. Behavioral therapy was also initiated, and it included planning of scheduled daytime napping as well as regular nocturnal sleep scheduling.8 The patient initially refused pharmacological treatment but later agreed to a try an antidepressant. Venlafaxine, sustained release 75 mg in the morning, was initiated with no major side-effects reported and to good effect.
DISCUSSION
We describe a patient with NT1 who presented with complete resolution of initially severe symptoms of cataplexy in the presence of a persisting orexin deficiency. The full remission occurred some 5 to 6 years in the course of illness, with no relapse reported over an additional 2 decades.
Improvement (and disappearance) of cataplexy over time is well known by clinicians and usually thought to reflect behavioral modifications of emotional processing, with a development of aberrant coping strategies in the course of illness (eg, avoiding heightened emotional situations).9 The current report suggests the possibility that other factors may underlie spontaneous remission. Recent animal studies suggested that orexin-producing cells could be induced by long-term administration of morphine.10 In addition, morphine administration was shown to decrease cataplexy in mice made narcoleptic by the depletion of orexin neurons.10 Although our patient had no record of an overt opiate addiction, the notion that similar molecular pathways (eg, via a modulation of the endogenous opioid system) might have played role in the remission of the cataplexy is intriguing. To date we know very little about the endogenous opioid system and its modulation; however, social feedback, emotional well-being, and emotional regulation, diet, exercise and a variety of age, genetic, and epigenetic factors appear to play the role.11–14 Alternatively, adaptive modifications of other circuits and neurotransmitter (eg, monoamines) signaling systems could be postulated as well.
Arguably, this case is one of very few NT1 cases reported in the literature that have implied a complete remission of cataplexy, and crucially, in all but 1 of those reported cases, measurement of CSF orexin levels was lacking.2,6,15 Nonetheless, experienced clinicians have long argued that the frequency, severity, and the nature of adult cataplexy, and possibly that of other NT symptoms, may alter and ebb over the course of patients’ lives.16,17 Pizza and colleagues2 suggested a natural evolution in the phenotype of untreated childhood cataplexy, implying an ongoing neuroplasticity. A gradual transformation of cataplexy symptoms over the course of 3 years has been described, from a complex movement disorder with persistent hypotonia and prominent facial involvement into the classically reported adult phenotype, characterized by paroxysmal episodes of loss of antigravity muscle tone evoked by emotions.2 Notably, a natural evolution of the adult symptoms in the absence of pharmacologic intervention is correspondingly described in this case. In most patients with NT1, however, severity of clinical presentation necessitates an early initiation of pharmacologic treatment, which is then commonly long-lasting. Chronic iatrogenic interventions have been known to alter the naturalistic course of numerous neurologic disorders, and by extension of this paradigm, they may also affect symptoms’ evolution in genetically susceptible NT1 individuals.
In the past, the clinical evolution of NT1, especially that of childhood onset,2 has been compared with the course of autoimmune disorder, which might show a partial remission after an abrupt acute onset phase.2 Our case offers support for this notion, and further argues a possibility of complete resolution of cataplexy. Clinicians and sleep researchers should be aware of this possibility, and future studies of the (naturalistic) narcolepsy course are warranted to better inform patients and families and help manage their expectations. New approaches including multimodal neuroimaging might offer a better insight into the association between functional and anatomical changes of distinct brain circuitries and clinical phenotypes of narcolepsy and their evolution over time.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
I.R. planned the study. D.W, C.B. and I.R. were involved in reviewing and drafting of the manuscript.
ABBREVIATIONS
- CSF
cerebrospinal fluid,
- EDS
excessive daytime somnolence
- EEG
electroencephalogram
- MSLT
multiple sleep latency test,
- HLA-DQB1
major histocompatibility complex, class II, DQ beta 1 gene
- NREM
non-rapid eye movement
- NT1
narcolepsy type 1,
- REM
rapid eye movement
REFERENCES
- 1.Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. 10.1038/s41582-019-0226-9 [DOI] [PubMed] [Google Scholar]
- 2.Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain. 2013;136(12):3787–3795. 10.1093/brain/awt277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drakatos P, Leschziner G. Cataplexy with normal sleep studies and normal CSF hypocretin: an explanation?. J Clin Sleep Med. 2016;12(03):449–450. 10.5664/jcsm.5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broughton R, Ghanem Q, Hishikawa Y, Sugita Y, Nevsimalova S, Roth B. Life effects of narcolepsy: relationships to geographic origin (North American, Asian or European) and to other patient and illness variables. Can J Neurol Sci. 1983;10(2):100–104. 10.1017/S0317167100044723 [DOI] [PubMed] [Google Scholar]
- 5.NIH. Narcolepsy Fact Sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Narcolepsy-Fact-Sheet. [Google Scholar]
- 6.Almeneessier AS, Alballa NS, Alsalman BH, Aleissi S, Olaish AH, BaHammam AS. A 10-year longitudinal observational study of cataplexy in a cohort of narcolepsy type 1 patients. Nat Sci Sleep. 2019;11231–239. 10.2147/NSS.S229105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry RB, Brooks R, Gamaldo CE, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0.1. Darien, IL: American Academy of Sleep Medicine; 2013.
- 8. doi: 10.3390/medsci7120106. Morse AM. Narcolepsy in children and adults. A guide to improved recognition, diagnosis and management. Med Sci (Basel).7(12):106, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiappa C, Scarpelli S, D’Atri A, Gorgoni M, De Gennaro L. Narcolepsy and emotional experience: a review of the literature. Behav Brain Funct. 2018;14(1):19. 10.1186/s12993-018-0151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thannickal TC, John J, Shan L, et al. Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci Transl Med. 2018;10(447):eaao4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winters BL, Gregoriou GC, Kissiwaa SA, et al. Endogenous opioids regulate moment-to-moment neuronal communication and excitability. Nat Commun. 2017;8(1):14611. 10.1038/ncomms14611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pucci M, Micioni Di Bonaventura MV, Vezzoli V, et al. Preclinical and clinical evidence for a distinct regulation of mu opioid and type 1 cannabinoid receptor genes expression in obesity. Front Genet. 2019;10523. 10.3389/fgene.2019.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psych. 2010;167(8):925–933. 10.1176/appi.ajp.2010.09091348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu DT, Sanford BJ, Meyers KK, et al. Social feedback activates the endogenous opioid system. Mol Psych. 2013;18(11):1147. 10.1038/mp.2013.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez R, Barateau L, Evangelista E, et al. Temporal changes in the cerebrospinal fluid level of hypocretin-1 and histamine in narcolepsy. Sleep. 2017;40(1):zsw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattarozzi K, Bellucci C, Campi C, et al. Clinical, behavioural and polysomnographic correlates of cataplexy in patients with narcolepsy/cataplexy. Sleep Med. 2008;9(4):425–433. 10.1016/j.sleep.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 17.Furuta H, Thorpy MJ, Temple HM. Comparison in symptoms between aged and younger patients with narcolepsy. Psych. Clin Neurosci. 2001;55(3):241–242. 10.1046/j.1440-1819.2001.00841.x [DOI] [PubMed] [Google Scholar]