Abstract
Study Objectives:
Sleep-disordered breathing and nocturnal hypoxia are prevalent among patients with precapillary pulmonary hypertension (PAH). The rationale for these associations remains unclear and these relationships have not been well studied in other forms of pulmonary hypertension (PH). We hypothesized that severity of sleep-disordered breathing and nocturnal hypoxia are associated with worsening pulmonary hemodynamics, regardless of hemodynamic profile.
Methods:
Four hundred ninety-three patients were divided into 4 groups: 1) no PH, 2) postcapillary pulmonary hypertension, 3) PAH, and 4) mixed PAH/postcapillary pulmonary hypertension. The relationship between right heart catheterization measurements and apnea-hypopnea index or the percentage of sleep time spent with oxygen saturation < 90% (T90) was calculated using multiple linear regression. Analysis of variance was used for between-group comparisons. Statistical models were adjusted for known confounders.
Results:
Apnea-hypopnea index did not differ between hemodynamic subgroups (P = .27) and was not associated with right atrial pressure (.11 ± .19, P = .55), cardiac index (.25 ± 1.64, P = .88), mean pulmonary artery pressure (−.004 ± .09, P = .97), or pulmonary artery occlusion pressure (.16 ± .14, P = .26). While patients with PH had a higher T90 than those without (mean 24.2% vs 11.7%, P < .001), there was no difference in T90 between individual PH subgroups (P = .70). T90 was associated with mean pulmonary artery pressure (.55 ± .10, P < .0001), PVR (1.61 ± .49, P = .001), and right atrial pressure (.50 ± .20, P = .01), but not cardiac index (−.76 ± 1.73, P = .66), or pulmonary artery occlusion pressure (.23 ± .15, P = .13).
Conclusions:
Increased PH severity was associated with longer duration of nocturnal hypoxia regardless of hemodynamic subgroup.
Citation:
Samhouri B, Venkatasaburamini M, Mar HPY, Li M, Mehra R, Chaisson NF. Pulmonary artery hemodynamics are associated with duration of nocturnal desaturation but not apnea-hypopnea index. J Clin Sleep Med. 2020;16(8):1231–1239.
Keywords: pulmonary hypertension, hemodynamics, sleep apnea syndromes
BRIEF SUMMARY
Current Knowledge/Study Rationale: Nocturnal hypoxia and sleep-disordered breathing (SDB) are known complications of precapillary pulmonary hypertension (PH). Less is known about the pathophysiologic connections between these conditions and pulmonary hemodynamics in other types of PH. We studied a cohort of patients with varying hemodynamic profiles to assess the relationship between nocturnal hypoxia or SDB and pulmonary hemodynamics.
Study Impact: We found that regardless of hemodynamic profile, increasing PH severity (mean pulmonary artery pressure, right atrial pressure, pulmonary vascular resistance) was associated with longer duration of nocturnal hypoxia. There was no association between pulmonary hemodynamics and severity of SDB (measured by apnea-hypopnea index). Our study is the largest to evaluate these associations. The findings may assist in improving nocturnal hypoxia screening for patients with PH.
INTRODUCTION
Pulmonary hypertension (PH) occurs when blood pressure in the pulmonary circulation is elevated. There are 5 subgroups of PH, distinguished by clinical and histopathologic features. Regardless of subgroup, PH results in increased right ventricular (RV) afterload, RV failure, and eventual death.1 Sleep-disordered breathing (SDB) constitutes a group of conditions that includes both obstructive and central breathing cessation, each with its own distinct phenotype, pathophysiology, and relationship to adverse health outcomes.
In patients with pulmonary arterial hypertension (PAH), the prevalence of nocturnal hypoxia due to SDB or other causes can be as high as 89%.2 In addition, an association between nocturnal hypoxia and increased mortality has been demonstrated in this population.3,4 For these reasons, SDB and associated nocturnal hypoxia have been implicated as candidate targetable risk factors in the development of PAH and potential foci for interventions to mitigate PH-related morbidity and mortality.
A general association between nocturnal hypoxia or SDB and PAH has been established. But the unique pathophysiologic contributions of SDB and nocturnal hypoxia toward PAH still represent an understudied area. Some studies suggest that the severity of SDB and nocturnal hypoxia are directly proportional to PAH severity, whereas others do not.5–8 In addition, little is known about the association of SDB or nocturnal hypoxia with other forms of PH, namely postcapillary and mixed PH.7
We therefore chose to leverage a large clinic-based cohort to examine the association of SDB and nocturnal hypoxia with hemodynamic indices obtained from right heart catheterization (RHC). We hypothesized that both RV function and severity of PH are associated with SDB and nocturnal hypoxia severity across all forms of PH (not just PAH). Rather than define subgroups based upon the standard World Symposium for Pulmonary Hypertension etiologic definitions,9 we elected to define PH subgroups based upon the physiologic locus of PH: precapillary PH (PAH), postcapillary pulmonary hypertension (PVH), or overlap of both. This separation allowed us to better elucidate the physiologic relationships between SDB and PH.
METHODS
Patient identification and study design
Adult patients > 18 years of age were included if they completed in-laboratory polysomnography (PSG) between January 2004 and February 2017 and had an order in the electronic medical record for RHC. Patients were excluded if the PSG was performed using positive airway pressure or other treatment, if they did not demonstrate SDB on PSG, or if the RHC was not completed within 24 months of the PSG. This study was approved by the institutional review board at the Cleveland Clinic.
Patients with lung disease included those with interstitial lung disease (ILD) or chronic obstructive pulmonary disease (COPD). ILD presence was based on ILD diagnosis in the electronic medical record and either pulmonary function test findings suggestive of restrictive lung disease (forced vital capacity < 70% predicted or total lung capacity < 80% predicted) or chest computed tomography scan findings consistent with ILD. COPD presence was based on COPD diagnosis in the EMR and either forced expiratory volume in 1 second/forced vital capacity ratio < 70% or documented use of COPD medications at the time of RHC. Connective tissue disease diagnoses included scleroderma, mixed connective tissue disease, systemic lupus erythematosus, sarcoidosis, rheumatoid arthritis, Sjogren’s disease, polymyalgia rheumatica, inflammatory bowel disease, granulomatosis with polyangiitis, polyarteritis nodosa, antiphospholipid antibody syndrome, and dermatomyositis. These were identified by a diagnosis added by a physician into the “medical history” tab in the electronic medical record.
Hemodynamic evaluation
RHC was performed using Swan-Ganz catheter by a board certified cardiologist or pulmonologist. The following parameters were collected: cardiac index (CI), right atrial pressure (RAP), pulmonary vascular resistance (PVR), mean pulmonary artery pressure (mPAP), and pulmonary artery occlusion pressure (PAOP). Patients were divided into 4 groups: 1) no PH, 2) PVH, 3) PAH, and 4) mixed PAH/PVH. We chose to define PH as mPAP ≥ 25 mmHg by RHC to increase the specificity of the diagnosis. PAH was defined as mPAP ≥ 25 mmHg, pulmonary vascular resistance (PVR) ≥ 3 wood units (WU) and PAOP ≤ 15 mmHg. PVH was defined as mPAP ≥ 25 mmHg, PVR < 3 WU, and PAOP > 15 mmHg. Mixed PH was defined as mPAP ≥ 25 mmHg, PVR ≥ 3 WU, PAOP > 15 mmHg, and either transpulmonary gradient ≥ 12 mmHg or diastolic pulmonary gradient ≥ 7 mmHg.10,11 Sixty-six patients had PH but did not meet the definition of any of the 4 groups described above. These patients were excluded from the study.
Polysomnography
Attended overnight PSG studies were performed using the Polysmith (Nihon Kohden Corporation, Tokyo, Japan) system following standard clinical guidelines. The recording montage included (F3-M2, C3-M2, O1-M2, F4-M1, C4-M1, O2-M1) bilateral electrooculography, submental and bilateral anterior tibial electromyography, thoracic and abdominal respiratory inductance plethysmography, and finger pulse oximetry. Nasal airflow and nasal pressure were measured using an oronasal thermistor and nasal cannula, respectively. Hypopnea was defined as airflow ≥ 50% in the nasal pressure channel for ≥ 10 seconds resulting in an arousal or ≥ 3% oxygen desaturation.12 Apnea was defined as a decrease in amplitude of oronasal thermistor signal (or alternative) by 90% for ≥ 10 seconds based on American Academy of Sleep Medicine event definition criteria.12 AHI was defined as the number of hypopneas and apneas per hour of sleep. SDB was defined as an apnea-hypopnea index (AHI) ≥ 5 events/h. Severity of nocturnal hypoxia was assessed by recording the percentage of sleep time spent with oxygen saturation < 90% (T90). Based on prior literature, nocturnal hypoxia was defined as an oxygen saturation < 90% for greater than 1% total sleep time.5
Statistical analysis
Data were summarized as mean and standard deviation for normally distributed continuous variables. Median and interquartile ranges (P25, P75) were used for nonnormally distributed continuous variables. Counts and percentages were used for categorical variables.
Analyses of variance and Kruskal–Wallis tests were performed for normally distributed and nonnormally distributed groups, respectively, to compare AHI or T90 between the different hemodynamic groups.
Linear regression models were developed to examine the association of SDB and nocturnal hypoxemia (AHI and T90, respectively) with hemodynamic measures (RAP, CI, mPAP, PVR, and PCWP). Multivariable linear regression models included known confounders that were identified a priori: age, sex, body mass index (BMI), presence of chronic kidney disease, and presence of underlying lung disease. Pearson correlation coefficient was utilized to assess the relationship between AHI and T90 (ie, the degree to which the frequency of apnea and hypopnea episodes are related to increased nocturnal hypoxia.)
To determine the impact that the use of PH medications or supplemental oxygen during the sleep study might have on these associations, we performed selected sensitivity analyses to evaluate different scenarios. To assess the influence oxygen therapy might have on AHI or T90, we repeated our analysis excluding patients who used supplemental oxygen during PSG. We also performed the analysis excluding patients who were on PH therapy at the time of their sleep study to assess the impact PH therapy might have on T90 or AHI. Because we could not confirm if patients were on continuous positive airway pressure prior to RHC, we performed a third sensitivity analysis excluding patients who received PSG prior to RHC. Finally, a sensitivity analysis was performed using only patients who underwent RHC within 6 months of PSG.7 All analyses were performed by using the SAS 9.4 for Linux (SAS, Cary, NC). The level of statistical significance was set at P < .05 (2-tailed).
RESULTS
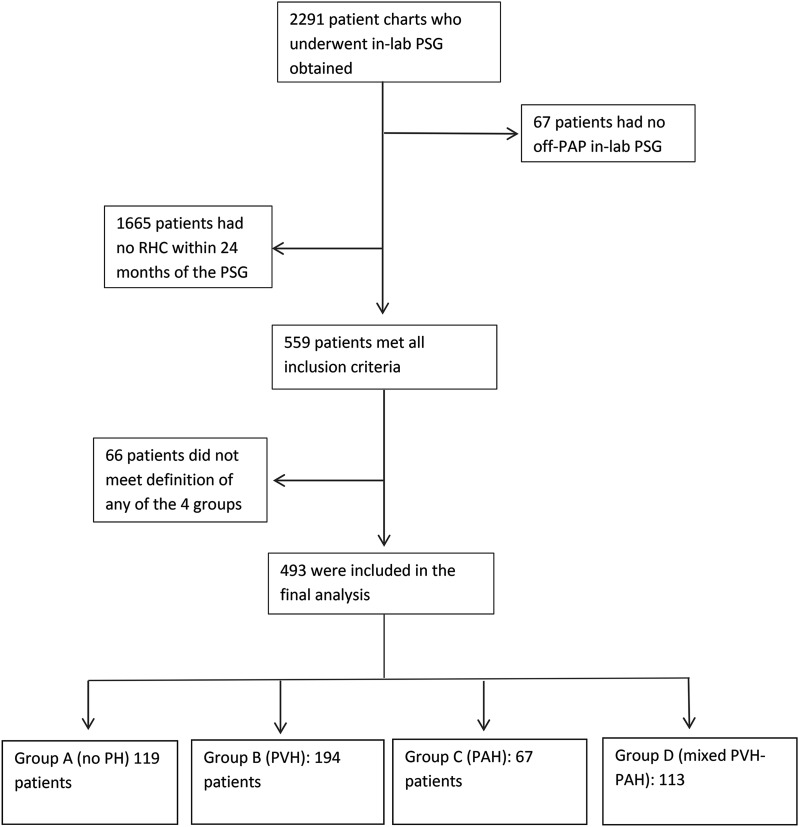
Of 2,291 patients who underwent in-laboratory PSG, 493 patients were included in the final analytic sample (Figure 1). Median duration between PSG and RHC was 157 days (range: 49–370). The mean age was 60.7 ± 12.2 years and 47% of patients were women. Mean BMI was 34.7 ± 8.8 kg/m2. Hemodynamic parameters and prevalence of comorbid conditions are listed in Table 1.
Figure 1. Flow diagram demonstrating the number of patients excluded for each of the exclusion criteria.
PAH = precapillary pulmonary hypertension, PAP = positive airway pressure; PH = pulmonary hypertension, PSG = polysomnography, PVH = postcapillary pulmonary hypertension, RHC = right heart catheterization, SDB = sleep-disordered breathing.
Table 1.
Patient demographics.
| Entire Cohort | Group A (no PH) | Group B (PVH) | Group C (PAH) | Group D (mixed PAH/PVH) | |
|---|---|---|---|---|---|
| Number of patients | 493 | 119 | 194 | 67 | 113 |
| Patient demographics | |||||
| Men, % | 53.5 | 46.2 | 62.9 | 44.8 | 50.4 |
| Age, y | 60.7 ± 12.2 | 58.9 ± 10.8 | 60.2 ± 12.9 | 60.1 ± 13.0 | 63.8 ± 11.6 |
| BMI, kg/m2 | 34.7 ± 8.8 | 33.0 ± 6.5 | 36.6 ± 10.2 | 32.7 ± 7.4 | 34.3 ± 8.7 |
| Race | |||||
| White, % | 65.1 | 68.1 | 64.9 | 64.2 | 62.8 |
| Black, % | 28.4 | 24.4 | 27.3 | 32.8 | 31.9 |
| Other, % | 6.5 | 7.5 | 7.9 | 3 | 5.3 |
| Comorbid conditions | |||||
| LVEF% | 50.1 ± 15.8 | 59.8 ± 5.5 | 45.2 ± 17.1 | 57.2 ± 9.9 | 47.7 ± 16.9 |
| Chronic kidney disease, % | 30.2 | 21 | 32.5 | 23.9 | 39.8 |
| COPD, % | 23.4 | 18.1 | 20.1 | 29.8 | 31.9 |
| ILD, % | 9.3 | 10.4 | 16.4 | 4.6 | 11.5 |
| Connective tissue disease, % | 11.4 | 14.3 | 9.3 | 17.9 | 8 |
| RHC measurements | |||||
| mPAP, mm Hg | 35.0 ± 12.7 | 19.4 ± 3.4 | 34.7 ± 6.9 | 43.9 ± 11.0 | 46.8 ± 9.9 |
| RAP, mm Hg | 12.0 ± 6.3 | 6.6 ± 3.4 | 14.0 ± 5.7 | 10.0 ± 4.9 | 15.2 ± 6.6 |
| CI, mm Hg | 2.6 ± .74 | 2.9 ± .75 | 2.6 ± .78 | 2.4 ± .68 | 2.2 ± .55 |
| PAOP, mm Hg | 19.5 ± 8.6 | 10.9 ± 4.3 | 24.8 ± 6.6 | 11.3 ± 3.2 | 23.8 ± 6.5 |
| PVR, wood units | 3.25 ± 2.85 | 1.58 ± .81 | 1.8 ± .76 | 7.3 ± 3.5 | 5.31 ± 2.58 |
| Polysomnographic findings | |||||
| AHI, events/h | 32.7 ± 27.1 | 28.3 ± 23.1 | 37.5 ± 30.1 | 23.3 ± 19.0 | 34.6 ± 27.8 |
| T90, % sleep time | 21.2 ± 27.1 | 11.7 ± 19.7 | 23.1 ± 28.4 | 26.2 ± 28.2 | 25.0 ± 28.8 |
| Proportion with nocturnal hypoxia, % | 75.1 | 59.8 | 77.2 | 81.8 | 83.8 |
| Proportion on oxygen during PSG, % | 11.6 | 8.4 | 7.2 | 29.9 | 11.5 |
| PH directed therapy at PSG, % | 3.1 | .0 | .0 | 7.5 | 9.7 |
Data are presented as mean ± standard deviation. AHI = apnea-hypopnea index, BMI = body mass index, CI = cardiac index, COPD = chronic obstructive pulmonary disease, ILD = interstitial lung disease, LVEF = left ventricular ejection fraction, mPAP = mean pulmonary artery pressure, PAH = pulmonary arterial hypertension, PAOP = pulmonary artery occlusion pressure, PSG = polysomnogram, PVH = pulmonary venous hypertension, PVR = pulmonary vascular resistance, RAP = right atrial pressure, RHC = right heart catheterization, T90 = percentage of sleep time spent with oxygen saturation < 90%.
The prevalence of nocturnal hypoxia in the entire cohort was 74%. Mean T90 was 21.2% ± 27.1. T90 was higher among patients with PH compared with those without (mean 24.2% vs 11.7%, P < .001) (Figure 2A). There was no difference in T90 between PH subgroups (P = .7) (Figure 2B). T90 was associated with PH severity (mPAP: .55 ± .10% increase in T90 per 1 mm Hg increase in mPAP, P < .0001 [Figure 2C]; PVR: 1.61 ± .49% increase in T90 per 1 WU increase in PVR, P = .001; and RAP: .50 ± .20% increase in T90 per 1 mm Hg increase in RAP, P = .01), but not with CI (−.76 ± 1.73% change in T90 per 1 L/min/m2 increase in CI, P = .66) or PAOP (.23 ± .15% increase in T90 per 1 mmHg increase in PAOP, P = .13).
Figure 2. T90, pulmonary hypertension, and pulmonary artery pressure.
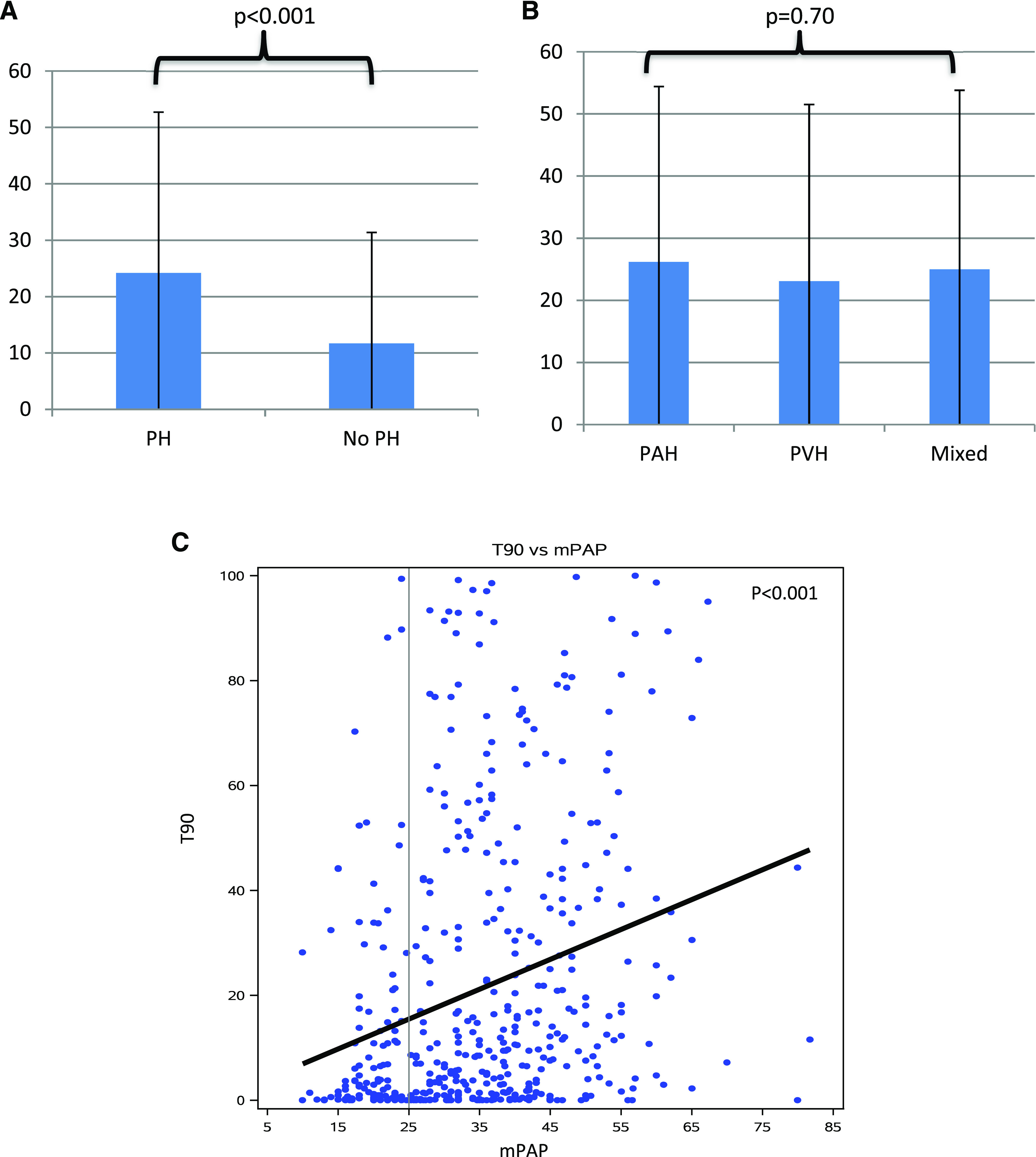
(A) Mean T90 among patients with and without pulmonary hypertension. (B) T90 among pulmonary hypertension subgroups. (C) Scatterplot demonstrating the relationship between T90 and mPAP. mPAP = mean pulmonary artery pressure, PAH = precapillary pulmonary hypertension, PH = pulmonary hypertension, PVH = postcapillary pulmonary hypertension, T90 = percentage of sleep time spent with oxygen saturation < 90%.
After performing sensitivity analyses, the associations between T90 and pulmonary hemodynamics persisted after excluding the 57 patients who used oxygen during PSG. When we excluded 39 patients who were on PH therapy at the time of PSG, the slope of regression between T90 and PAOP changed minimally. However, this relationship became statistically significant under these conditions (.33 ± .15% increase in T90 per 1 mm Hg increase in PAOP, P = .03). When 194 patients who had PSG prior to RHC were excluded, the association between RAP and T90 was unchanged in magnitude, but was not statistically significant (.49 ± .26% increase in T90 per 1 mm Hg increase in RAP, P = .06). No significant differences were noted when the analysis was limited to patients who had their PSG and RHC within 6 months of each other. These analyses are presented in Table S1 in the supplemental material.
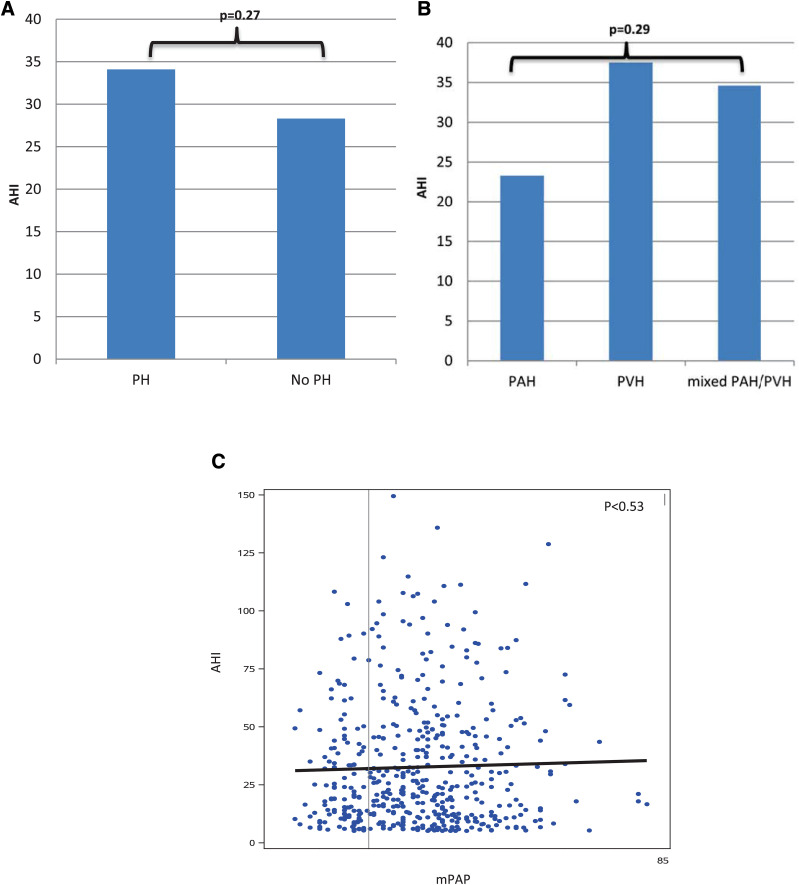
The mean AHI for the entire cohort was 32.7 ± 27.1 events/h. There was no statistically significant difference in AHI between hemodynamic groups (Figure 3). Across the entire cohort, AHI was not associated with indices of cardiac function (CI [.25 ± 1.64, P = .88], PH severity (mPAP [−.004 ± .09, P = .97], RAP [.11 ± .19, P = .55]) or left atrial pressure (PAOP [.16 ± .14, P = .26]). Within each hemodynamic subgroup, similar findings were observed (Table S2). Sensitivity analyses, as described above, were performed for AHI as well. These analyses did not demonstrate any change from the initial analysis. (Table S1).
Figure 3. AHI, pulmonary hypertension, and pulmonary artery pressure.
(A) Mean AHI among patients with and without pulmonary hypertension. (B) AHI among pulmonary hypertension subgroups. (C) Scatterplot demonstrating the relationship between AHI and mPAP. AHI = apnea-hypopnea index, mPAP = mean pulmonary artery pressure, PAH = precapillary pulmonary hypertension, PH = pulmonary hypertension, PVH = postcapillary pulmonary hypertension.
We assessed the correlation between AHI and T90 to determine if increased T90 might simply be a function of more severe SDB. The correlation was weak (Pearson correlation coefficient [r] =.27, P = .0001), suggesting that these indices may be independent of each other and may reflect distinct biological processes.
DISCUSSION
Our study is the largest we are aware of that focuses on the association of cardiopulmonary hemodynamics with SDB and nocturnal hypoxia. It is also unique because it offers a comparison of a broad array of patients with varying hemodynamic profiles, including patients with PVH. As has been previously shown,5,6,13 patients with PAH had a high prevalence of nocturnal hypoxia. We showed that similar findings exist in other forms of PH. In addition, we demonstrated that hemodynamic markers of PH severity are strongly associated with T90 severity, but not AHI, regardless of hemodynamic subgroup. Parameters relating to RV function were not associated with AHI or T90.
The poor correlation between AHI and T90 suggests that a mechanism other than SDB may be responsible for nocturnal hypoxia in PH patients. Several mechanisms are plausible and provide biologic rationale for the occurrence of nocturnal hypoxia in PH. We believe that the most likely explanation entails heterogeneity in ventilation/perfusion (V/Q) relationships throughout the different regions of the lung, with low V/Q ratio lung units existing in some areas 14,15 and high V/Q relationships in others.16 Under these circumstances, positional changes in blood flow distribution during sleep may lead to increased flow through low V/Q lung regions and subsequent hypoxia while supine. We found that T90 was associated with markers of PH severity (mPAP and PVR). When we excluded patients currently on PH therapy, we also found that T90 was associated with PAOP. These findings support the possibility that V/Q mismatch may be a result of either pulmonary vascular remodeling (high V/Q relationship) or pulmonary edema (low V/Q relationship).
Another possible explanation includes abnormal diffusing capacity of the lung for carbon monoxide (DLCO). In heart failure, DLCO is commonly diminished due to pulmonary vascular remodeling, especially in cases of chronic disease.17,18 In PAH, DLCO impairment is also common and occurs on account of vascular remodeling as well as diminished pulmonary capillary blood volume.19,20 Although severe decrements in DLCO can lead to hypoxia during exertional activity, it is less likely that this is the principal reason for hypoxia during sleep in PH patients. Prior work from our institution focused upon evaluating of differences in DLCO among patients with PAH. In this study, there was no difference in DLCO between patients who experienced nocturnal desaturation and those who did not.21 Therefore, we do not feel that this was the cause of nocturnal hypoxia in our population. However, our ability to assess the effect of DLCO on nocturnal hypoxia was limited as many of our patients did not have recorded DLCO.
Finally, respiratory muscle weakness, which can occur in PAH,22 may lead to shallower than normal breathing during sleep. Although the nuances of respiratory mechanics during sleep remain poorly described in PH, it is plausible that respiratory muscle weakness could contribute to shallow respiration followed by nocturnal atelectasis and shunting (and thus hypoxia) in this population.
One might question whether there is clinical significance to identifying nocturnal hypoxia in patients with PH. The association between nocturnal hypoxia and clinical outcomes has been demonstrated across multiple diseases. In PAH, a recent study suggested that nocturnal hypoxia is associated with increased mortality.4 In patients with COPD, nocturnal hypoxia causes increased pulmonary artery pressures and may contribute to RV dysfunction.23–25 In the same population, studies suggest that providing nocturnal oxygen therapy to patients with COPD and nocturnal hypoxia decreased further progression of cor pulmonale.26,27 In patients with end-stage renal disease, nocturnal hypoxia was associated with a 5-fold higher risk of experiencing cardiovascular events than those without.28 In patients admitted to a hospital for decompensated heart failure, nocturnal hypoxia documented prior to discharge was an independent predictor of readmission and mortality.29 The 6th World Symposium for Pulmonary Hypertension guidelines on pulmonary hypertension do not offer guidance on screening or therapy for nocturnal hypoxia in patients with PH. The broad clinical implications of nocturnal hypoxia in disease has triggered some authors to recommend all patients with PAH be screened for nocturnal hypoxia, even in the absence of daytime hypoxia.21 Our data suggest that these implications may extend beyond PAH alone and apply to other types of PH. Although further study of the clinical effect of nocturnal hypoxia in PH patients is warranted, our data support the notion that patients with severe PH (of any type) should be considered for screening for nocturnal hypoxia irrespective of daytime oxygen saturation or symptoms. Given the low cost, ease of use, wide availability, easy interpretation, high interrator reliability,30 and acceptability by the Centers of Medicare and Medicaid Services as a diagnostic tool for nocturnal hypoxia, we suggest using nocturnal oximetry as the screening modality.
Our data correspond to a few other studies that suggest no association between AHI and hemodynamic markers of PH severity or RV function (RA pressure, mPAP, PVR, and cardiac index).2,4,7,8 However, our findings differ from those of 2 smaller studies; one suggesting that PH severity (mPAP) correlates with the severity of SDB5 and the other suggesting that CI correlates with SDB.6 We believe that a primary reason our data may differ from these 2 studies is that the studies had small sample sizes (13–83 patients), which could have limited the ability to correct for well-known factors that influence SDB severity, including sex, BMI, the presence of chronic kidney disease, or underlying lung disease. Similar to other studies,31,32 our study demonstrated an association between AHI and both male sex and BMI. This suggests that correction for these risk factors is necessary, even in a population with PH. One of these studies6 also allowed a significant proportion of patients to use supplemental oxygen during a home sleep study. This fact may have altered actual AHI values used in their analysis. To avoid similar issues in our study, we performed a sensitivity analysis, excluding the 57 patients who used oxygen during PSG. No relevant change in the outcomes was identified during this analysis.
The present study has several strengths that are worth mentioning. First, we utilized gold standard diagnostic tests to obtain our data (RHC for hemodynamic parameters and in-lab PSG for SDB and nocturnal hypoxia). We also used rigorous measures to avoid confounding, including adjustment for previously known confounding factors, and performed multiple sensitivity analyses to assess the impact of additional potential influences.
There are limitations, however. First, the retrospective nature of this study prevented us from identifying the reason for PSG or RHC in most cases and prevented collection of variables that may have allowed further description of the study population. The retrospective nature also is subject to confounders that we were unable to or did not consider in our analysis. We took steps where possible to correct for known confounders and performed sensitivity analyses where we could not correct for potential confounding.
In our analysis, we allowed a maximum 24-month window between RHC and PSG. Although this time gap has the potential to limit associations between RHC and SDB, we also reported the relationships using only patients who had these tests within a 6-month window (a time established as acceptable in other studies).7 With the exception of the relationship between RAP and T90, the data from this analysis were similar to our initial analysis. Whether the absence of an association between T90 and RAP in PH patients argues against the theory of rostral fluid shift32 is unclear but deserves further evaluation.
Finally, we chose 2 parameters, AHI and T90, as representative measures of an otherwise complex phenomenon. Although there are alternate parameters that could measure SDB and nocturnal hypoxia, the parameters we selected are similar to those evaluated in the majority of studies on this topic. We obtained our data by merging an existing sleep database with a cohort of patients who had undergone RHC. Because of this, we were unable to identify some alternative measures of SDB and nocturnal hypoxia, either because the data were not available to us or it contained sufficient missing data that imputing values would have been difficult.
We conclude that PH severity is associated with an increased duration of nocturnal hypoxia, but not AHI, regardless of type of PH. This important finding may aid clinicians in screening and treatment for nocturnal hypoxia among PH patients in the future. Future investigations should focus on providing further insight into the mechanisms behind nocturnal hypoxia in PH and its effect on morbidity and mortality in PH.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Cleveland Clinic Foundation. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
cardiac index
- COPD
chronic obstructive pulmonary disease
- DLCO
diffusing capacity of the lung for carbon monoxide
- ILD
interstitial lung disease
- mPAP
mean pulmonary artery pressure
- PAH
precapillary pulmonary arterial hypertension
- PAOP
pulmonary artery occlusion pressure
- PH
pulmonary hypertension
- PSG
polysomnography
- PVH
pulmonary venous hypertension
- PVR
pulmonary vascular resistance
- RAP
right atrial pressure
- RHC
right heart catheterization
- RV
right ventricle
- SDB
sleep-disordered breathing
- T90
Percentage sleep time spent with oxygen saturation <90%
- V/Q
ventilation/perfusion
- WU
wood units
REFERENCES
- 1.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36(9):957–967. 10.1016/j.healun.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 2.Jilwan FN, Escourrou P, Garcia G, Jaïs X, Humbert M, Roisman G. High occurrence of hypoxemic sleep respiratory disorders in precapillary pulmonary hypertension and mechanisms. Chest. 2013;143(1):47–55. 10.1378/chest.11-3124 [DOI] [PubMed] [Google Scholar]
- 3.Oldenburg O, Wellmann B, Buchholz A, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37(21):1695–1703. 10.1093/eurheartj/ehv624 [DOI] [PubMed] [Google Scholar]
- 4.Nagaoka M, Goda A, Takeuchi K, et al. Nocturnal hypoxemia, but not sleep apnea, is associated with a poor prognosis in patients with pulmonary arterial hypertension. Circ J. 2018;82(12):3076–3081. 10.1253/circj.CJ-18-0636 [DOI] [PubMed] [Google Scholar]
- 5.Prisco DL, Sica AL, Talwar A, et al. Correlation of pulmonary hypertension severity with metrics of comorbid sleep-disordered breathing. Sleep Breath. 2011;15(4):633–639. 10.1007/s11325-010-0411-y [DOI] [PubMed] [Google Scholar]
- 6.Orr JE, Auger WR, DeYoung PN, Kim NH, Malhotra A, Owens RL. Usefulness of low cardiac index to predict sleep-disordered breathing in chronic thromboembolic pulmonary hypertension. Am J Cardiol. 2016;117(6):1001–1005. 10.1016/j.amjcard.2015.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104(9):1300–1306. 10.1016/j.amjcard.2009.06.048 [DOI] [PubMed] [Google Scholar]
- 8.Minic M, Granton JT, Ryan CM. Sleep disordered breathing in group 1 pulmonary arterial hypertension. J Clin Sleep Med. 2014;10(3):277–283. 10.5664/jcsm.3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J. 2019;53(1):1802148. 10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee NA, Lewis GD. Characterization of pulmonary hypertension in heart failure using the diastolic pressure gradient. JACC Heart Fail. 2015;3(1):17–21. 10.1016/j.jchf.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 11.Galiè N, Simonneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol. 2013;62(25):D1–D3. 10.1016/j.jacc.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 13.Rafanan AL, Golish JA, Dinner DS, Hague LK, Arroliga AC. Nocturnal hypoxemia is common in primary pulmonary hypertension. Chest. 2001;120(3):894–899. 10.1378/chest.120.3.894 [DOI] [PubMed] [Google Scholar]
- 14.West J, Luks AM. Ventilation-perfusion relationships: how matching of gas and blood determines gas exchange. In: Respiratory Physiology, The Essentials. 10th ed. Philadelphia, PA: Wolters Kluwer; 2016:63-86. [Google Scholar]
- 15.Liu W-H, Luo Q, Liu Z-H, et al. Pulmonary function differences in patients with chronic right heart failure secondary to pulmonary arterial hypertension and chronic left heart failure. Med Sci Monit. 2014;20960–966. 10.12659/MSM.890409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsang JYC, Hogg JC. Gas exchange and pulmonary hypertension following acute pulmonary thromboembolism: has the emperor got some new clothes yet?. Pulm Circ. 2014;4(2):220–236. 10.1086/675985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guazzi M, Galiè N. Pulmonary hypertension in left heart disease. Eur Respir Rev. 2012;21(126):338–346. 10.1183/09059180.00004612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich S, Rabinovitch M. Diagnosis and treatment of secondary (non–category 1) pulmonary hypertension. Circulation. 2008;118(21):2190–2199. 10.1161/CIRCULATIONAHA.107.723007 [DOI] [PubMed] [Google Scholar]
- 19.Farha S, Laskowski D, George D, et al. Loss of alveolar membrane diffusing capacity and pulmonary capillary blood volume in pulmonary arterial hypertension. Respir Res. 2013;14(1):6. 10.1186/1465-9921-14-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing Z-C, Xu X-Q, Badesch DB, et al. Pulmonary function testing in patients with pulmonary arterial hypertension. Respir Med. 2009;103(8):1136–1142. 10.1016/j.rmed.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 21.Minai OA, Pandya CM, Golish JA, et al. Predictors of nocturnal oxygen desaturation in pulmonary arterial hypertension. Chest. 2007;131(1):109–117. 10.1378/chest.06-1378 [DOI] [PubMed] [Google Scholar]
- 22.Meyer FJ, Lossnitzer D, Kristen AV, et al. Respiratory muscle dysfunction in idiopathic pulmonary arterial hypertension. Eur Respir J. 2005;25(1):125–130. 10.1183/09031936.04.00095804 [DOI] [PubMed] [Google Scholar]
- 23.Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med. 1985;102(1):29–36. 10.7326/0003-4819-102-1-29 [DOI] [PubMed] [Google Scholar]
- 24.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531–1536. 10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 25.Boysen PG, Block AJ, Wynne JW, Hunt LA, Flick MR. Nocturnal pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chest. 1979;76(5):536–542. 10.1378/chest.76.5.536 [DOI] [PubMed] [Google Scholar]
- 26.Fletcher EC, Levin DC. Cardiopulmonary hemodynamics during sleep in subjects with chronic obstructive pulmonary disease. The effect of short- and long-term oxygen. Chest. 1984;85(1):6–14. 10.1378/chest.85.1.6 [DOI] [PubMed] [Google Scholar]
- 27.Medical Research Council Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681–686. https://www.ncbi.nlm.nih.gov/pubmed/6110912 [PubMed] [Google Scholar]
- 28.Zoccali C, Mallamaci F, Tripepi G. Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol. 2002;13(3):729–733. https://www.ncbi.nlm.nih.gov/pubmed/11856778 [DOI] [PubMed] [Google Scholar]
- 29.Ohmura T, Iwama Y, Kasai T, et al. Impact of predischarge nocturnal pulse oximetry (sleep-disordered breathing) on postdischarge clinical outcomes in hospitalized patients with left ventricular systolic dysfunction after acute decompensated heart failure. Am J Cardiol. 2014;113(4):697–700. 10.1016/j.amjcard.2013.10.048 [DOI] [PubMed] [Google Scholar]
- 30.Ayache M, Strohl KP. High interrater reliability of overnight pulse oximetry interpretation among inexperienced physicians using a structured template. J Clin Sleep Med. 2018;14(4):541–548. 10.5664/jcsm.7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 32.Soler X, Liao S-Y, Marin JM, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): The challenge to predict OSA in advanced COPD. PLoS One. 2017;12(5):e0177289.. 10.1371/journal.pone.0177289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.