Abstract
Study Objectives:
Sleep disturbance is significantly associated with suicidal ideation. However, the majority of past research has examined the relationship between insomnia and suicidality. The current exploratory study examined the relationship of circadian rhythm dysregulation (eveningness, seasonality, and rhythmicity) with suicidality.
Methods:
We examined the association of insomnia, eveningness, seasonality, and rhythmicity with suicidal ideation in 103 participants with depression, insomnia, and suicidality within a larger 8-week double-blinded randomized control trial primarily examining whether cautious use of zolpidem extended-release or placebo reduced suicidal ideation. All participants additionally received an open-label selective serotonin reuptake inhibitor. Methodological strengths of the current analyses included consideration of multiple sleep-wake constructs, adjustment for relevant covariates, investigation of relationships over the course of treatment, and use of both self-report measures and objective measurement with actigraphy.
Results:
Over the course of treatment, self-reported eveningness and greater insomnia severity were independently correlated with greater suicidal ideation, whereas actigraphic delayed sleep timing was related to suicidal ideation at a trend level. At the end of treatment, those with greater suicidal ideation demonstrated lower actigraphic activity levels. There were no significant relationships between self-reported seasonality and actigraphic measures of sleep disturbance and suicidality.
Conclusions:
Self-reported delays in sleep timing, objectively lower activity levels, and self-reported insomnia severity correlated independently with greater suicidal ideation in those with depression, insomnia, and suicidality. These exploratory findings highlight the need to consider sleep-wake constructs more broadly in those with suicidality in future research studies in order to improve more definitively both assessment and intervention efforts.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Reducing Suicidal Ideation through Insomnia Treatment; URL: https://clinicaltrials.gov/ct2/show/NCT01689909; Identifier: NCT01689909
Citation:
Rumble ME, McCall WV, Dickson DA, Krystal AD, Rosenquist PB, Benca RM. An exploratory analysis of the association of circadian rhythm dysregulation and insomnia with suicidal ideation over the course of treatment in individuals with depression, insomnia, and suicidal ideation. J Clin Sleep Med. 2020;16(8):1311–1319.
Keywords: eveningness, rhythmicity, circadian rhythm, insomnia, suicidal ideation, depression
BRIEF SUMMARY
Current Knowledge/Study Rationale: Insomnia is a well-established correlate of suicidality. Less is known about the relationship between circadian rhythm dysregulation and suicidality. The current exploratory study examined the relationship of insomnia, eveningness, seasonality, and rhythmicity to suicidal ideation in individuals with depression, insomnia, and suicidal ideation within an 8-week randomized controlled trial primarily examining whether cautious use of zolpidem ER or placebo and an open-label selective serotonin reuptake inhibitor reduced suicidal ideation.
Study Impact: Lower levels of objective activity, self-reported eveningness, and greater insomnia severity were related to increased suicidal ideation over the course of treatment. The current exploratory study underscores the need to think more broadly in terms of potential sleep-wake assessment and treatment targets. That is, circadian rhythm dysregulation and insomnia are both likely important considerations in those with suicidal ideation.
INTRODUCTION
Suicidal ideation is defined as wishes to be dead or thoughts of killing oneself that can be accompanied by intentions and plans to kill oneself.1 As a measurement of the continuum of suicidality, data from the National Survey on Drug Use and Health demonstrated that about 4% of those 18 years of age or older within the United States (an estimated 9.5 million individuals) reported suicidal ideation within the past 12 months.2 Moreover, even suicidal ideation considered more passive, such as wishes to be dead, has been shown to be associated with increased risk of suicide death, which has steadily increased over the last two decades.3,4 Thus, suicidal ideation is an urgent public health concern with a need for both better assessment and treatment.
Sleep disturbance is a well-established correlate of increased suicidal ideation, attempts, and death and has been considered an important modifiable risk factor for suicidal ideation and behavior.5–8 However, the bulk of the research in this area has examined the relationship between insomnia and suicidality, with few studies examining important circadian and seasonal rhythm constructs that are commonly linked with mood disorders, including eveningness (eg, a preference for a later bedtime and wake-up time), greater seasonality (eg, seasonal changes in behavior, including sleep, social activity, mood, weight, appetite, and energy), and decreased rhythmicity (eg, less daily routine, including less consistent bedtimes, wake-up times, times for meals, etc.).
Several studies found relationships between higher levels of suicidality and eveningness, greater seasonality, and lower rhythmicity.9–12 However, a number of key issues hinder a better understanding the relationship of sleep disturbance and circadian rhythm dysregulation to suicidality.13 First, many studies have focused on only one aspect of sleep disturbance or circadian rhythm dysregulation in relationship to suicidality. Given the overlap among insomnia, eveningness, seasonality, and rhythmicity, it is important to examine all of these variables within the same sample. Second, many studies have not adjusted for such broader symptomatology as depressive symptoms, so examining these relationships and insuring they are independent of depression per se is key. Finally, these understudied constructs have been mostly examined from self-reported measures that may or may not correlate with objective measurement of these constructs.13
In the current exploratory study, we investigated whether questionnaire and/or actigraphic indices of insomnia, eveningness, seasonality, and rhythmicity related to suicidal ideation. Our inquiry occurred within a larger study examining primarily whether cautious use of sleep medication reduced suicidal ideation in those with depression, insomnia, and suicidality. These relationships were examined over the course of treatment while adjusting for depression severity and treatment assignment. We hypothesized that greater suicidality would be correlated with greater insomnia severity, eveningness, greater seasonality, and less rhythmicity.
METHODS
Overview
The parent REST-IT (Reducing Suicidal Ideation through Insomnia Treatment) study was a multisite randomized controlled trial (Medical College of Georgia, Duke University, University of Wisconsin, and Wake Forest University) that examined whether cautious use of sleep medication reduced suicidal ideation in those with depression, insomnia, and suicidality (ClinicalTrials.gov, Identifier: NCT01689909). Given the high risk of the study sample, all participants received open-label selective serotonin reuptake inhibitors, as randomization to placebo alone could not be ethically justified. The start of the selective serotonin reuptake inhibitors and the double-blinded randomization (zolpidem extended-release [ER] or placebo) occurred at the same time. Participants were scheduled at 1, 2, 4, 6, and 8 weeks for follow-up assessment postrandomization. All participants and study staff interacting with participants, including study physicians and assessors, were blinded, so all study measures reported here were completed without knowledge of treatment condition. A data safety monitoring board provided oversight and interim analyses and stopping rules were in effect. Please see the REST-IT study method paper for further details regarding study safety procedures.14
The primary outcome measures for the parent REST-IT study were suicidal ideation as measured by the Scale for Suicide Ideation (SSI) and Columbia Suicide Severity Rating Scale (C-SSRS), and a secondary outcome measure was insomnia severity as measured by the Insomnia Severity Index (ISI). Results from the primary aim of the parent REST-IT study have been published and demonstrated that the group allocated to receive zolpidem ER had greater improvement in suicidality as measured by the C-SSRS and greater improvement in insomnia as measured by the ISI.15 Actigraphy measurement was also stipulated as an exploratory aim for REST-IT. The current report provides a post hoc analysis of the actigraphy data and corresponding questionnaire measures of eveningness and seasonality. Although this exploratory aim was identified in ClinicalTrials.gov, the specific variables that are the main focus of this paper were not listed.
Participants
Participants were adults (18–65 years old) with Major Depressive Disorder, confirmed by the Structured Clinical Interview for DSM-IV,16 and Insomnia as determined by Research Diagnostic Criteria for Insomnia.17 Additional inclusion criteria included a 24-item Hamilton Rating Scale for Depression (HRSD-24) ≥ 2018 and a ISI score > 7.19 Participants had to report suicidal ideation as defined by a Scale for Suicide Ideation score ≥ 3.20 However, participants were required to be free of suicidal plans or intentions as measured by a C-SSRS ideation intensity score ≤ 3.1 At baseline assessment, participants were free of all psychotropic medications for 1 week or greater (4 weeks or greater for fluoxetine) and had a Mini-Mental Status Exam score ≥ 24.21 Patients were excluded if they had a diagnosis of bipolar disorder, schizophrenia, or alcohol or substance abuse as confirmed by the Structured Clinical Interview for DSM-IV or a diagnosis of another sleep disorder other than insomnia. Additional exclusions were an apnea-hypopnea index > 10 on a baseline sleep study either in the laboratory (Duke University) or at home (Medical College of Georgia and University of Wisconsin),22 body mass index > 50, positive urine drug screen, positive urine pregnancy test, non-English speaking/reading/writing, and patients who posed imminent danger to others. All participants provided written, informed consent, and the protocol was approved by each site’s institutional review board.
Randomization and treatment
At the start of study recruitment (November 2012), all participants were randomized to zolpidem ER 12.5 mg or placebo. In February 2013, the Food and Drug Administration released guidance that zolpidem ER should not be initially started at a dose greater than 6.25 mg for women.23 We then immediately adjusted our protocol so that all participants were randomized to either zolpidem ER 6.25 mg or placebo. At the end of first week of treatment, the zolpidem ER/placebo dose could be increased to 12.5 mg if there was inadequate treatment effects and no side effects. The dose of zolpidem ER could be reduced to 6.25 mg if the higher dose was intolerable. All participants received open-label fluoxetine 20 mg, sertraline 50 mg, or citalopram 20 mg daily at the time of randomization with the option of increasing the dose at the end of 4 weeks if the HRSD-24 score was > 15.
Measures
Suicidal ideation and behavior
The primary outcome for the current report was suicidal ideation as measured by the SSI. The SSI was administered at each visit from the baseline assessment to week 8 to assess suicidal ideation and behavior. The SSI is a self-rated scale consisting of 19 items that evaluate active and passive suicidal desire and specific plans for suicide.20,24,25 Items are rated on a 3-point scale (0 to 2) with higher scores denoting more severe suicidal ideation. The observer-rated Columbia Suicide Severity Rating Scale1 was also administered at each visit to measure suicidal ideation and behavior. For the current analyses, the suicidal ideation “intensity” score from 0–5 with 1 representing only wishes to be dead and 5 representing suicidal ideation with plan and intent was used to further classify the sample.
Self-reported eveningness and seasonality
Given that eveningness and seasonality are more trait-like measures, these variables were assessed by questionnaire at baseline and at the end of randomized treatment. Eveningness was measured with the Reduced Morningness-Eveningness Questionnaire (rMEQ).26 The rMEQ has 5 items and lower scores indicate greater eveningness. Seasonality was measured with the Global Seasonality Scale from the Seasonal Pattern Assessment Questionnaire.27 The Global Seasonality Scale has 6 areas, mood, sleep length, energy, appetite, social activity, and weight, that are rated 0 (no change by season) to 4 (extremely marked change by season).
Actigraphy
Participants wore an actigraphy motion biosensor (Philips Respironics) on the nondominant wrist throughout the 8-week treatment period. Baseline data were not collected due to the acuity of this patient population, which prohibited a prolonged medication-free baseline. All actigraphic data were collected in 30-second epochs. Settings for the actigraphic sleep-wake activity threshold and sleep immobility onset and offset were kept standard (ie, medium and 10 minutes, respectively). Participants were required to have 5 days of data for any time-point measurement to be included. All available data were downloaded into Actiware software (Philips) for initial analyses, and sleep statistics were computed from 30-second epoch data. From these computations, the sleep statistics used for the current analyses were sleep onset latency, sleep efficiency, and total sleep time, allowing for objective measurement of sleep disturbance. Variables of sleep timing were also used with the clock times for the start and end of the sleep interval, allowing for objective measurement of delayed sleep timing. Finally, all raw activity data were exported to Excel and then imported into SAS software (SAS Institute, Cary, NC) for hour-by-hour activity analysis using 1-minute epochs, allowing for an examination of objective rest-activity rhythmicity. For this analysis, each participant’s 1-minute epoch data were average across hour bins to provide an averaged rest-activity pattern across the 24-hour day.
Depression and insomnia severity
Depression severity was assessed at each visit with the observer-rated HRSD-24.18 The HRSD-24 has three sleep items and one suicide item. Given the aims of our study, the sleep and suicide items were excluded from the computation of the total score to create the HRSD-20. Insomnia severity was measured with the ISI at each visit.19 The ISI is a 7-item questionnaire, with each item scored 0–4, for a maximum of 28 points. A higher score on the ISI represents greater insomnia severity. This measure has demonstrated excellent reliability and validity in the general population.28
Statistical analyses
SAS software was used for all study analyses, and the P value was set to 0.05, given the exploratory aim of the study. For all multilevel modeling, an autoregressive covariance structure was chosen.
First, multilevel modeling examined how self-reported eveningness and seasonality at baseline related to suicidal ideation over the course of treatment. Similarly, multilevel modeling examined how the mean (ie, the cross-sectional effect) and person-centered values (ie, the longitudinal effect) of self-reported insomnia severity and actigraphic measures of delayed sleep timing and sleep disturbance related to suicidality over the course of treatment.29 Third, multilevel modeling was used to examine the main effects of median-split SSI group and each hour within the 24-hour day and their interaction on actigraphic rest-activity levels during week 1 and then week 8. All of the above analyses adjusted for depression severity (ie, the HRSD-20 defined as the HRSD-24 score minus the sleep and suicide items) and treatment assignment. Analyses not looking directly at insomnia severity in relation to suicidality also controlled for insomnia severity (ISI). Finally, we examined whether there were any treatment effects for any of our independent variables that were significantly related to suicidality to better understand our results in this larger treatment study. Since we conducted the current analyses in addition to the analyses for the main REST-IT report,15 there is an increased possibility that these findings are chance findings or false positives.
RESULTS
Sample characteristics
Characteristics of the sample are summarized in Table 1. Participants were on average about 41 years old (standard deviation = 13.2), and the majority of participants were women (62%), with racial/ethnic minorities composing about 39% of the sample. At baseline, the intensity of suicidal ideation, as measured by the SSI, was moderate.30 To further classify the sample in terms of suicidal ideation, on the baseline C-SSRS the average intensity score was 1.64 (standard deviation = 1.02), which is between a score of 1, indicating passive wishes to be dead, and a score of 2, indicating nonspecific suicidal thoughts (eg, “I have thought of killing myself.”). Additionally, at baseline, about 26% of the sample (n = 27) endorsed active suicidal ideation with any methods (not plan) without intent to act, or a score of 3 on the C-SSRS. Average depression and insomnia symptomatology at baseline were considered severe (ie, within the range of 27–34) and moderate (ie, within the range of 15–21), respectively.19,31
Table 1.
Characteristics of the sample.
| Characteristic | Zolpidem ER | Placebo | Overall |
|---|---|---|---|
| N = 51 | N = 52 | N = 103 | |
| Age, M [SD] | 39.7 [14.5] | 41.2 [12.0] | 40.5 [13.2] |
| Female, n (%) | 32 (63) | 32 (62) | 64 (62) |
| Race/Ethnicity, n (%) | |||
| Caucasian/White | 30 (59) | 33 (63) | 63 (61) |
| African American | 12 (24) | 16 (31) | 28 (27) |
| Hispanic | 4 (8) | 1 (2) | 5 (5) |
| Other | 5 (10) | 2 (4) | 7 (7) |
| BMI, M [SD] | 28.3 [6.4] | 28.2 [5.6] | 28.2 [6.0] |
| Lifetime Suicide Attempt(s), n (%) | 15 (29) | 16 (31) | 31 (30) |
| Baseline SSI Score, M [SD] | 12.2 [5.3] | 11.8 [5.3] | 12.0 [5.3] |
| Baseline HRSD 24 Score, M [SD] | 28.7 [4.7] | 29.6 [7.0] | 29.1 [5.9] |
| Baseline ISI, M [SD] | 20.7 [4.0] | 21.0 [4.3] | 20.9 [4.1] |
| Baseline rMEQ score, M [SD] | 12.9 [4.8] | 13.5 [4.2] | 13.2 [4.5] |
| Baseline rMEQ class, n (%) | |||
| Evening type (< 12) | 22 (43%) | 14 (27%) | 36 (35%) |
| Neither type (12-17) | 19 (37%) | 29 (56%) | 48 (47%) |
| Morning type (> 17) | 9 (18%) | 8 (15%) | 17 (16%) |
| Missing | 1 (2%) | 1 (2%) | 2 (2%) |
| Baseline GSS score, M [SD] | 8.4 [5.4] | 10.3 [5.3] | 9.3 [5.4] |
| Baseline GSS class, n (%) | |||
| More seasonal (GSS ≥ 11) | 18 (35%) | 24 (46%) | 42 (41%) |
| Less seasonal (GSS < 11) | 33 (65%) | 28 (54%) | 61 (59%) |
| Week 1 actigraphic variables, M [SD]*** | |||
| Start of the sleep interval, clock time | 11:48 pm [2.0 hours] | 12:00 am [1.9 hours] | 11:54pm [1.9 hours] |
| End of the sleep interval, clock time | 7:36 am [2.2 hours] | 7:24 am [1.9 hours] | 7:30 am [2.0 hours] |
| SOL, minutes | 23.8 [19.1] | 29.2 [29.4] | 26.3 [24.4] |
| SE, % | 82.2 [7.2] | 79.0 [8.5] | 80.7 [7.9] |
| TST, minutes | 421.5 [67.1] | 389.3 [61.3] | 406.5 [65.9] |
= sample size for actigraphic variables at week 1 was n = 60, overall; n = 32, zolpidem ER; and n = 28, placebo. BMI = body mass index; GSS = Global Seasonality Score; HRSD = Hamilton Rating Scale for Depression; ISI = Insomnia Severity Index; M = mean; rMEQ = Revised Morningness-Eveningness Questionnaire; SD = standard deviation; SE = sleep efficiency; SOL = sleep onset latency; SSI = Scale for Suicide Ideation; TST = total sleep time.
The average baseline score for the sample on the eveningness questionnaire, the Reduced Morningness-Eveningness Questionnaire, was within the range of neither evening nor morning type; however, there was variation in the sample in terms of being classified as evening, neither, and morning types. The average baseline score on the seasonality measure, the Global Seasonality Scale, was below the commonly used cut-off of 11, indicating that the sample was lower on seasonality on average; however there was variation in the sample, with a little less than half of the sample being considered more seasonal and scoring above this cut-off. During the first week of treatment, the average actigraphic start and end of the sleep interval were approximately midnight and 7:30 am, respectively. Estimates of sleep and wake with actigraphy also demonstrated an average 26-minute sleep onset latency, 81% sleep efficiency, and 6.8 hours of total sleep time.
Data availability
Actigraphy was available for 45–62% of participants at each assessment point. Please see Table 2 for the specifics of actigraphy availability. Availability of questionnaires measuring eveningness and seasonality at baseline was 98% and 100%, respectively. Availability of questionnaires of suicidality, depression severity, and insomnia severity ranged from 80–100% throughout study point assessments, with no significant differences in retention between study arms as computed by Kaplan-Meier estimates of retention (86% for zolpidem ER versus 73% for placebo at week 8 of treatment; P = 0.09).
Table 2.
Actigraphy availability at all assessment points.
| Week 1 | Week 2 | Week 4 | Week 6 | Week 8 | |
|---|---|---|---|---|---|
| Available, n (%) | 60 (58) | 64 (62) | 60 (58) | 51 (49) | 47 (45) |
| < 5 days, n (%) | 3 (3) | 6 (6) | 0 (0) | 1 (1) | 2 (2) |
| < 3 days, n (%) | 5 (5) | 5 (5) | 4 (4) | 1 (1) | 2 (2) |
| Daylight Savings Time resulted in < 5 days, n (%) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 5 (5) |
| Watch malfunction, n (%) | 2 (2) | 4 (4) | 4 (4) | 6 (6) | 3 (3) |
| No actigraphy collected, n (%) | 31 (30) | 23 (22) | 35 (34) | 44 (43) | 44 (43) |
Relationship of suicidal ideation to self-reported eveningness and seasonality
Self-reported eveningness at baseline, as measured by the continuous Reduced Morningness-Eveningness Questionnaire score, was significantly and independently associated with greater suicidal ideation over the course of treatment even when adjusting for depression severity (HRSD-20), insomnia severity (ISI), and treatment assignment (F(1,96) = 4.79, P = 0.03). Self-reported seasonality, as measured by the continuous Global Seasonality Scale score, was not significantly related to suicidal ideation over the treatment period (F(1,101) = 0.28, P = 0.60).
Relationship of suicidal ideation to self-reported insomnia severity, delayed sleep timing, and sleep disturbance
A significant longitudinal effect was found between greater self-reported insomnia severity and greater suicidal ideation (F(1,448) = 9.78, P = 0.002), adjusting for depression severity (HRSD-20) and treatment assignment. There was a trending longitudinal effect between a more delayed actigraphic start of the sleep interval and greater suicidal ideation (F(1,189) = 3.01, P = 0.08), adjusting for depression severity (HRSD-20), insomnia severity (ISI), and treatment assignment. None of the actigraphic sleep disturbance variables (ie, sleep onset latency, sleep efficiency, and total sleep time) were found to have a statistically significant longitudinal relationship to suicidal ideation (sleep onset latency, F(1,193) = 0.44, P = 0.51; sleep efficiency, F(1,192) = 0.36, P = 0.55); or total sleep time, F(1,193) = 2.09, P = 0.15).
Relationship of suicidal ideation to actigraphic rest-activity rhythmicity
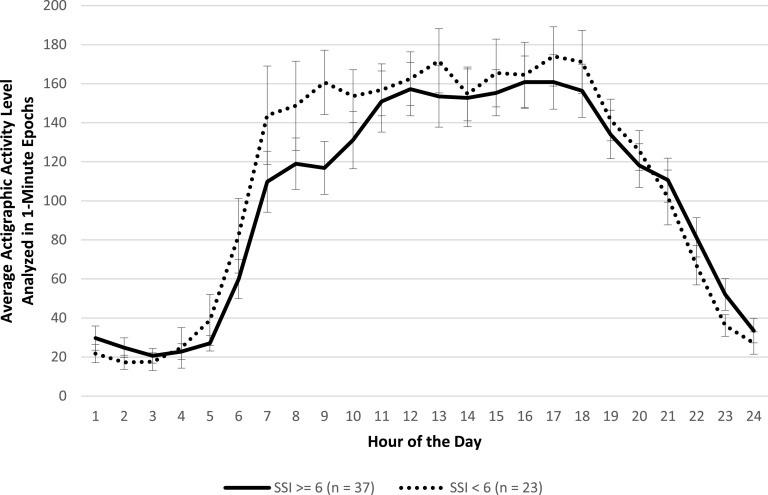
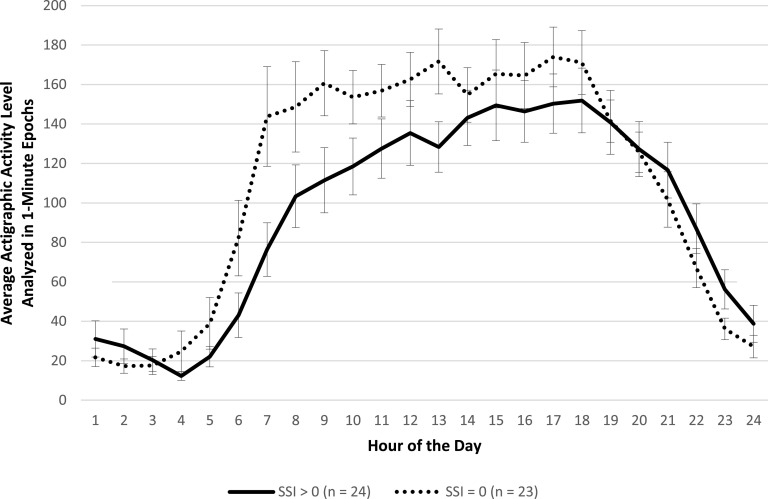
Participants with available actigraphic data were classified according to a median split on SSI scores at week 1 (SSI ≥ 6 [n = 37] or SSI < 6 [n = 23]) and week 8 (SSI > 0 [n = 24] or SSI = 0 [n = 23]). A median split was chosen to reflect the range of scoring on the SSI at week 1 and week 8. Results revealed no significant main effect for SSI score group or significant interaction between SSI score group and hour of the 24-hour day on activity level (Figure 1; F(1,55) = 1.94, P = 0.17 and F(23,1334) = 0.80, P = 0.73, respectively). That is, there were no differences in hour-by-hour activity level mappings of those with higher versus lower suicidal ideation at the start of treatment. However, at the end of treatment, results revealed significant differences between SSI score groups, in that those with higher suicidal ideation had significantly lower activity levels than those with lower suicidal ideation (Figure 2; F(1,42) = 4.68, P = 0.04), adjusting for depression severity (HRSD-20), insomnia severity (ISI), and treatment assignment. There was no significant interaction between SSI score group and hour of the 24-hour day (F(23,1035) = 1.04, P = 0.41).
Figure 1. Actigraphic data by hour during the first week of treatment (week 1) for participants with higher and lower Scale for Suicide Ideation (SSI) scores, adjusted by depression severity, insomnia severity, and treatment assignment.
Figure 2. Actigraphic data by hour during the last week of treatment (week 8) for participants with higher and lower Scale for Suicide Ideation (SSI) scores, adjusted by depression severity, insomnia severity, and treatment assignment.
Impact of zolpidem ER on eveningness, insomnia severity, delayed sleep timing, and rest-activity rhythmicity
Results revealed a significant time-by-treatment effect for insomnia severity (F(5,443) = 2.5, P = 0.03). There was no significant time-by-treatment effect for self-reported eveningness or actigraphic start of the sleep interval (F(2,83) = 0.7, P = 0.5 and F(4,192) = 0.6, P = 0.6, respectively). Similarly, there were no significant differences between those receiving zolpidem ER and placebo in terms of hour-by-hour activity mappings at the end of treatment (F(1,45) = 0.7, p 0.4). There was also no time effect for self-reported eveningness (F(5,83) = 0.6, P = 0.7). There was a time effect for actigraphic start of the sleep interval (F(4,192) = 3.6, P = 0.01); however, the time effect was only present for the start of the sleep interval becoming earlier between week 1 and week 2 (t(192) = 2.6, P = 0.01) and then returned to values not statistically different from week 1 for the remaining weeks of treatment (ie, weeks 4–8).
DISCUSSION
Greater self-reported insomnia severity was related to greater suicidality longitudinally in a well-defined sample of individuals with depression, insomnia, and suicidal ideation who were being treated for depression and insomnia with an open label selective serotonin reuptake inhibitor and zolpidem ER or placebo in a double-blind fashion. Additionally, as reported previously,15 those receiving zolpidem ER were found to have significantly improved insomnia over the course of the study in comparison with those receiving placebo. These results were expected, given the larger body of research examining the relationship between insomnia and suicidality and the aim of the REST-IT study to target insomnia.5–7,15 We also found that delayed sleep timing and less overall activity were correlated with suicidal ideation. More specifically, both self-reported delayed sleep timing and actigraphically determined lower activity levels related to greater suicidality, independent of insomnia symptoms. In support of the finding related to self-reported delayed sleep timing, actigraphically determined delayed sleep timing was found to be related to greater suicidality at a trend level. These exploratory findings are similar to previous studies that demonstrated a relationship of suicidal ideation with delayed sleep timing and decreased rhythmicity, but importantly the current findings considered a variety of objective and subjective sleep-wake constructs, included appropriate covariates, and investigated these relationships over time.9–12 Furthermore, delayed sleep timing and reduced activity level were not positively impacted by zolpidem ER in comparison to placebo. Thus, sleep-wake factors related to suicidal ideation in those with insomnia, depression, and suicidal ideation include more than just insomnia, even within this very well-defined sample; independent contributions can be made by sleep timing and rhythmicity as well.
Self-reported seasonality and actigraphic sleep disturbance variables were not associated with suicidal ideation in the current study. Regarding seasonality, the lack of association may be related to the way in which seasonality was measured. More detailed measurement of seasonality, such as longitudinal measurement of activity with actigraphy over seasons, may be more appropriate to best understand the relationship of seasonality to suicidality. Similarly, actigraphic measures of sleep disturbance in those with insomnia are known to be imprecise in terms of correlating with polysomnography,32 since actigraphy cannot reliably distinguish sleep from quiet wakefulness. However, a recent study examined the relationship between actigraphic variables and suicidal ideation using an ecological momentary assessment methodology over a 1-week period and found that objectively measured shorter sleep predicted higher levels of suicidal ideation the next day.33 Thus, further exploration of the relationships of objective sleep disturbance and seasonality to suicidality is warranted.
The current exploratory study underscores the need to think more broadly in terms of potential assessment and intervention targets in those with insomnia, depression, and suicidality. Of note, 8 weeks of treatment with a selective serotonin reuptake inhibitor and randomization to zolpidem ER did not impact self-reported eveningness, sleep timing, or rhythmicity. More specifically, this population might additionally benefit from light therapy and other behavioral strategies for delayed sleep timing,34 or from social rhythm therapy to improve behavioral activity rhythms.35 The parent REST-IT study found that randomization to cautious use of sleep medication targeting insomnia led to significantly greater improvement in the Columbia Suicide Severity Rating Scale, but not the Scale for Suicide Ideation, compared to those randomized to placebo.15 Additionally, a prior nonrandomized and uncontrolled study found that a clinically meaningful reduction in insomnia severity was associated with a significant reduction in odds of suicidal ideation in veterans receiving cognitive-behavioral therapy for insomnia.36 The findings of the current study, encouraging a broader look at insomnia and behavioral rhythms issues, are also in line with the Transdiagnostic Intervention for Sleep and Circadian Dysfunction (TranS-C).37 The TranS-C is an approach that offers flexible treatment options for the variety of sleep issues that can be comorbid with psychiatric disorders. This approach offers both core (ie, establishing regular sleep-wake times, learning wind-down and wake-up routines, improving daytime functioning, and correcting unhelpful sleep-related beliefs), and optional modules (eg, strategies to help with delayed sleep phase, additional insomnia strategies) and is currently being tested in individuals with serious mental illness.38 Thus, there are many potential and promising avenues to explore in considering best ways to support and improve insomnia and circadian functioning in those with suicidality.
This study had several strengths. First, the study sample was a well-defined group of individuals with depression, insomnia, and suicidal ideation assessed in 3 separate sites. Second, the current study offered the opportunity to look at relationships of interest over multiple assessments during an 8-week treatment period. Finally, we assessed several key areas that have not been addressed in previous studies, including examination of both insomnia and circadian dysregulation in relation to suicidality in the same group of subjects, with consideration of important covariates (eg, depression severity) and inclusion of both self-reported and objective measures.
This study also had several limitations. First, the current study examined an exploratory aim within a larger randomized clinical trial, and it is important to keep in mind that these additional analyses not clearly specified ahead of time may be chance findings or false positives. The current results need to be confirmed by future observational and clinical studies specifically designed to examine the relationships found in the current study before any clear and applicable clinical implications are formed. Second, actigraphy was only available for about half of the sample at each assessment. Despite this limitation, the presence of multiple assessments over the course of treatment helped reduce concerns about actigraphy sample sizes at any time point. Third, as already noted, there are some potential limitations with the self-reported seasonality measures and actigraphic measures of sleep disturbance. Fourth, additional potential objective markers of sleep and circadian functioning, including polysomnography and dim-light melatonin onset, were not included in the study and deserve study. Fifth, this study focused on the relationship of sleep-wake variables and suicidal ideation and included many important covariates; however, we did not fully examine other factors that are related to suicidal ideation (eg, interpersonal stressors) in the current study. Finally, the generalizability of our results is limited to adults with insomnia, depression, and suicidal ideation, so an understanding of these relationships within the context of bipolar disorder, in other psychiatric disorders, and in children and adolescents is still needed.
In summary, the current exploratory study demonstrated that both self-reported delayed sleep timing and lower objectively measured activity levels contributed independently of insomnia symptoms to heightened suicidal ideation in those with depression, insomnia, and suicidality. The current study also confirmed the relationship between increased insomnia severity and suicidality over the course of multiple assessments. This study addressed several gaps in previous research, including analyzing relationships over the course of time, examining both insomnia and circadian rhythm dysregulation in relation to suicidality, adjusting for important covariates, and using both self-reported and objective measures. Altogether, these findings highlight the need for future observational and treatment studies to consider both sleep disturbance and circadian rhythm dysregulation in those with suicidality so that we can continue to understand how to best address the pressing public health crisis of suicidal ideation and behavior.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this manuscript was performed at the authors’ affiliated institutions. This work was supported by the National Institutes of Health (grant nos. MH095776, MH095780, and MH095778). Conflicts of interest: Dr. Rumble receives grant support from Merck. Dr. McCall receives grant support from Merck, MECTA, and VistaGen, is a consultant to Sage and Jazz, is on a speakers’ bureau with CME Outfitters, and receives royalties from Wolters Kluwer. Dr. Krystal receives grant support from NIH, Janssen, Jazz, Axsome, and Reveal Biosensors, and is a consultant to Adare, Eisai, Ferring, Galderma, Harmony Biosciences, Idorsia, Janssen, Jazz, Merck, Neurocrine, and Takeda. Dr. Benca is a consultant for Eisai, Genomind, Jazz, and Merck. The other authors report no conflicts of interests.
ABBREVIATIONS
- C-SSRS
Columbia Suicide Severity Rating Scale
- HRSD-24
24-item Hamilton Rating Scale for Depression
- HRSD-20
the HRSD-24 score minus the sleep and suicide items
- ISI
Insomnia Severity Index
- REST-IT
Reducing Suicidal Ideation through Insomnia Treatment
- SSI
Scale for Suicide Ideation
- zolpidem ER
zolpidem extended-release
REFERENCES
- 1.Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: Utility and limitations of research instruments. In: First MB, ed. Review of Psychiatry, Vol. 22. Standardized Evaluation in Clinical Practice. Washington, DC: American Psychiatric Association Publishing; 2003:103–130 [Google Scholar]
- 2.Park-Lee E, Hedden SL, Lipari RN. Suicidal thoughts and behavior in 33 Metropolitan Statistical Areas update: 2013 to 2015. In: The CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration (US); 2013:1–10 [PubMed] [Google Scholar]
- 3.Brown GK, Steer RA, Henriques GR, Beck AT. The internal struggle between the wish to die and the wish to live: a risk factor for suicide. Am J Psychiatry. 2005;162(10):1977–1979. 10.1176/appi.ajp.162.10.1977 [DOI] [PubMed] [Google Scholar]
- 4.Hedegaard H, Curtin SC, Warner M. Suicide rates in the United Sates continue to increase. In: NCHS Data Brief, no 309. National Center for Health Statistics. 2018. https://www.cdc.gov/nchs/data/databriefs/db309.pdf Accessed April 27, 2020
- 5.Bernert RA, Kim JS, Iwata NG, Perlis ML. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. 2015;17(3):554. 10.1007/s11920-015-0554-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik S, Kanwar A, Sim LA, et al. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: a systematic review and meta-analysis. Syst Rev. 2014;3(1):18. 10.1186/2046-4053-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(09):e1160–e1167. 10.4088/JCP.11r07586 [DOI] [PubMed] [Google Scholar]
- 8.McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013;15(9):389. 10.1007/s11920-013-0389-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahk YC, Han E, Lee SH. Biological rhythm differences and suicidal ideation in patients with major depressive disorder. J Affect Disord. 2014;168294–297. 10.1016/j.jad.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Chan JWY, Lam SP, Li SX, et al. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 2014;37(5):911–917. 10.5665/sleep.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indic P, Murray G, Maggini C, et al. Multi-scale motility amplitude associated with suicidal thoughts in major depression. PLoS One. 2012;7(6):e38761. https://doi:10.1371/journal.pone.003876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernert RA, Hom MA, Iwata NG, Joiner TE. Objectively assessed sleep variability as an acute warning sign of suicidal ideation in a longitudinal evaluation of young adults at high suicide risk. J Clin Psychiatry. 2017;78(06):e678–e687. 10.4088/JCP.16m11193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rumble ME, Dickson D, McCall WV, et al. The relationship of person-specific eveningness chronotype, greater seasonality, and less rhythmicity to suicidal behavior: A literature review. J Affect Disord. 2018;227721–730. 10.1016/j.jad.2017.11.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCall WV, Benca RM, Rosenquist PB, et al. A multi-site randomized clinical trial to reduce suicidal ideation in suicidal adult outpatients with major depressive disorder: development of a methodology to enhance safety. Clin Trials. 2015;12(3):189–198. 10.1177/1740774515573958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCall WV, Benca RM, Rosenquist PB, et al. Reducing suicidal ideation through insomnia treatment (REST-IT): a randomized clinical trial. Am J Psychiatry. 2019;176(11):957–965. 10.1176/appi.ajp.2019.19030267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.First M, Sptizer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Asix I Disorders-Clinician Version (SCID-CV). Washington, DC: American Psychiatric Association; 1998 [Google Scholar]
- 17.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine work group. Sleep. 2004;27(8):1567–1596. 10.1093/sleep/27.8.1567 [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47(2):343–352. 10.1037/0022-006X.47.2.343 [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 22.McCall WV, Benca RM, Rumble ME, Case D, Rosenquist PB, Krystal AD. Prevalence of obstructive sleep apnea in suicidal patients with major depressive disorder. J Psychiatr Res. 2019;116147–150. 10.1016/j.jpsychires.2019.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Drug Administration . FDA requiring lower recommended dose for certain drugs containing zolpidem. US Department of Health and Human Services: Washington, DC; 2013. http://wayback.archive-it.org/7993/20170111080036/http://www.fda.gov/Drugs/DrugSafety/ucm334033.htm. Accessed June 15, 2020
- 24.Beck AT, Brown GK, Steer RA. Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behav Res Ther. 1997;35(11):1039–1046. 10.1016/S0005-7967(97)00073-9 [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Brown GK, Steer RA, Dahlsgaard KK, Grisham JR. Suicide ideation at its worst point: a predictor of eventual suicide in psychiatric outpatients. Suicide Life Threat Behav. 1999;291–9 [PubMed] [Google Scholar]
- 26.Adan A, Almirall H. Horne and Ostberg Morningness Eveningness Questionnaire–a reduced scale. Pers Individ Dif. 1991;12(3):241–253. 10.1016/0191-8869(91)90110-W [DOI] [Google Scholar]
- 27.Rosenthal NE, Bradt GH, Wehr TA. Seasonal Pattern Assessment Questionnaire. Washington, DC: National Institute of Mental Health; 1987 [Google Scholar]
- 28.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH. Fixed effects versus random effects models. In: Fitzmaurice GM, Laird NM, Ware JH, eds. Applied Longitudinal Statistics, 2nd ed. Hoboken, NJ, John Wiley & Sons; 2011: 241–264. 10.1002/9781119513469.ch9 [DOI] [Google Scholar]
- 30.McCall WV, Batson N, Webster M, et al. A psychometric cut-point to separate emergently suicidal depressed patients from stable depressed outpatients. Indian J Psychiatry. 2013;55(3):283–286. 10.4103/0019-5545.117150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 32.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14(07):1209–1230. 10.5664/jcsm.7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littlewood DL, Kyle SD, Carter LA, Peters S, Pratt D, Gooding P. Short sleep duration and poor sleep quality predict next-day suicidal ideation: an ecological momentary assessment study. Psychol Med. 2019;49(3):403–411. 10.1017/S0033291718001009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lack LC, Wright HR. Clinical management of delayed sleep phase disorder. Behav Sleep Med. 2007;5(1):57–76. 10.1207/s15402010bsm0501_4 [DOI] [PubMed] [Google Scholar]
- 35.Frank E, Swartz HA, Boland E. Interpersonal and social rhythm therapy: an intervention addressing rhythm dysregulation in bipolar disorder. Dialogues Clin Neurosci. 2007;9325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trockel M, Karlin BE, Taylor CB, Brown GK, Manber R. Effects of cognitive behavioral therapy for insomnia on suicidal ideation in veterans. Sleep. 2015;38(2):259–265. 10.5665/sleep.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey AG, Buysse DJ. Treating Sleep Problems: A Transdiagnostic Approach. New York, NY: The Guilford Press; 2018 [Google Scholar]
- 38.Harvey AG, Hein K, Dong L, et al. A transdiagnostic sleep and circadian treatment to improve severe mental illness outcomes in a community setting: study protocol for a randomized controlled trial. Trials. 2016;17(1):606. 10.1186/s13063-016-1690-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Actigraphy was available for 45–62% of participants at each assessment point. Please see Table 2 for the specifics of actigraphy availability. Availability of questionnaires measuring eveningness and seasonality at baseline was 98% and 100%, respectively. Availability of questionnaires of suicidality, depression severity, and insomnia severity ranged from 80–100% throughout study point assessments, with no significant differences in retention between study arms as computed by Kaplan-Meier estimates of retention (86% for zolpidem ER versus 73% for placebo at week 8 of treatment; P = 0.09).
Table 2.
Actigraphy availability at all assessment points.
| Week 1 | Week 2 | Week 4 | Week 6 | Week 8 | |
|---|---|---|---|---|---|
| Available, n (%) | 60 (58) | 64 (62) | 60 (58) | 51 (49) | 47 (45) |
| < 5 days, n (%) | 3 (3) | 6 (6) | 0 (0) | 1 (1) | 2 (2) |
| < 3 days, n (%) | 5 (5) | 5 (5) | 4 (4) | 1 (1) | 2 (2) |
| Daylight Savings Time resulted in < 5 days, n (%) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 5 (5) |
| Watch malfunction, n (%) | 2 (2) | 4 (4) | 4 (4) | 6 (6) | 3 (3) |
| No actigraphy collected, n (%) | 31 (30) | 23 (22) | 35 (34) | 44 (43) | 44 (43) |