Abstract
Site-selective transformation of benzylic C–H bonds into diverse functional groups is achieved via Cu-catalyzed C–H fluorination with N-fluorobenzenesulfonimide (NFSI), followed by substitution of the resulting fluoride with various nucleophiles. The benzyl fluorides generated in these reactions are reactive electrophiles in the presence of hydrogen-bond donors or Lewis acids, allowing them to be used without isolation in C–O, C–N, and C–C coupling reactions.
Graphical Abstract
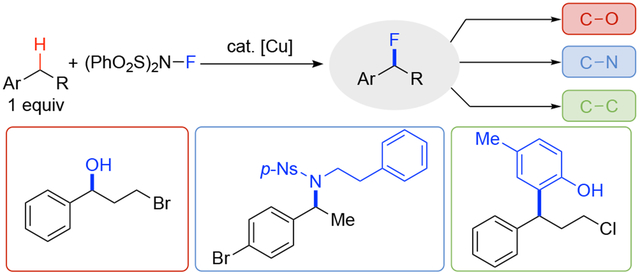
Medicinal chemistry and drug discovery efforts greatly benefit from synthetic coupling reactions that facilitate access to analogs of pharmaceutical building blocks and core structures. Functional groups that participate in efficient coupling, such as carboxylic acids, aryl halides, and boronic acids, provide the foundation for these methods.1 Expansion of latent functionalities that participate in coupling could greatly increase the scope of synthetic diversity.2 Benzylic C–H bonds are an appealing target in this context as they are prevalent in drug-like molecules and are susceptible to site-selective activation owing to their enhanced reactivity (e.g., reduced bond strength, higher acidity). Recent studies demonstrate that benzylic C–H substrates may be used as the limiting reagent in cross-coupling reactions with a number of different reaction partners, including alcohols,3 amides4, and arylboronic acids.5 Cu catalysts in combination with N-fluorobenzenesulfonimide (NFSI) are particularly effective in these reactions as they exhibit unique selectivity for benzylic C–H bonds and promote a radical relay mechanism that enables coupling with diverse reaction partners (Scheme 1).3a,4b,5a, 6 We recently discovered that a Cu/NFSI-based catalyst system switches selectivity, from C–N to C–F bond formation, when the reaction is conducted with MeB(OH)2 as a redox buffer and Li2CO3 as a Brønsted base.7 These observations provide the foundation for the present study in which we demonstrate a C–H fluorination/substitution sequence that enables benzylic C–H cross-coupling with diverse oxygen, nitrogen, and carbon nucleophiles (Scheme 1). This strategy, which takes advantage of the intrinsic lability of benzyl monofluorides,8,9 contrasts the many C–H fluorination efforts motivated by the inertness of the C–F bond.10,11 This approach allows for successful benzylic C–H cross coupling with reaction partners that are oxidatively sensitive or otherwise incompatible with direct Cu/NFSI-catalyzed methods, thereby greatly expanding the synthetic scope and versatility of benzyl C–H cross coupling.
Scheme 1.
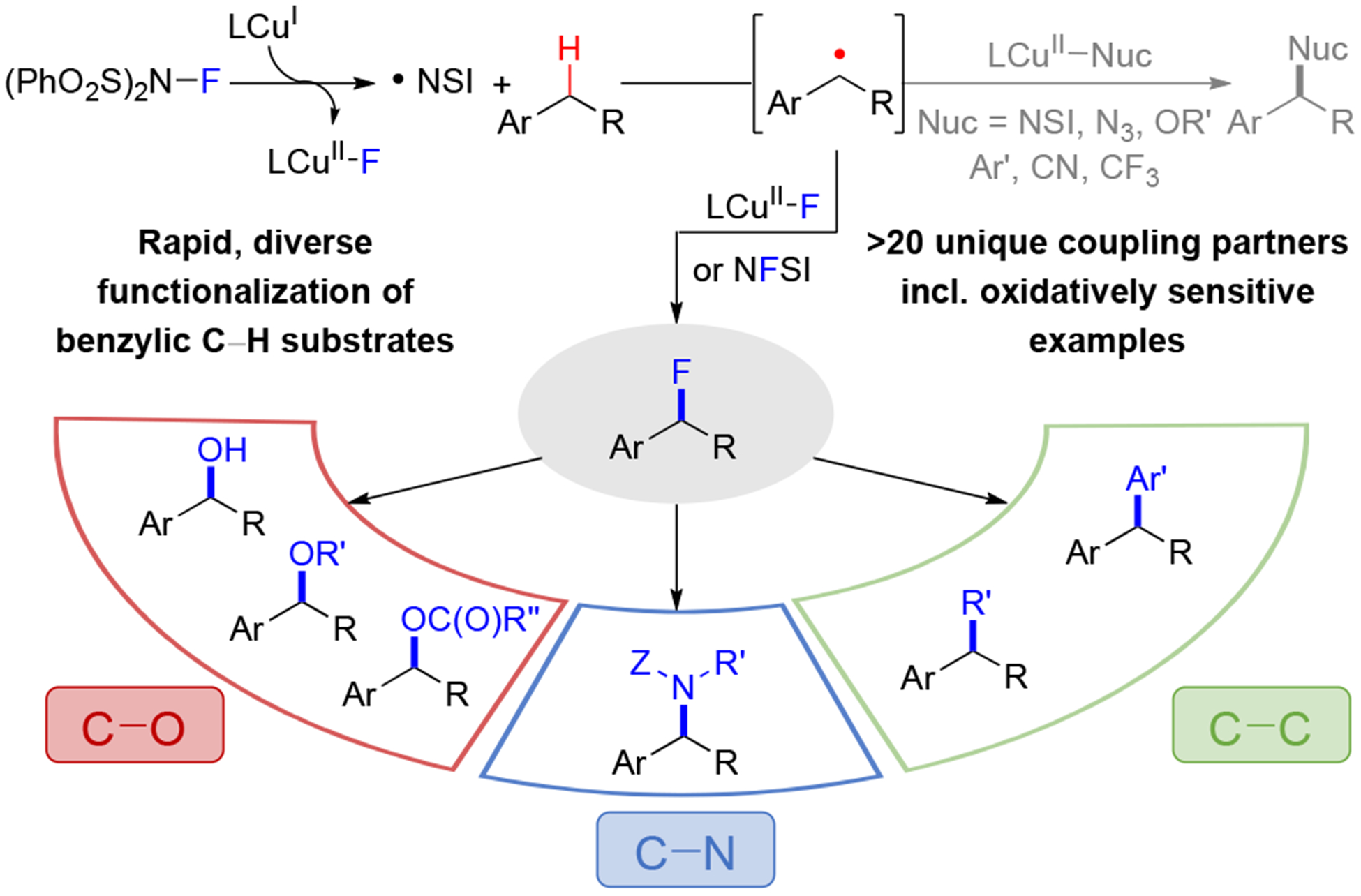
C–H Cross-Coupling via a Benzyl Fluoride
The present study began by testing the previously optimized fluorination conditions7 with a variety of benzylic C–H substrates (Scheme 2). para-Bromoethylbenzene proceeds effectively in 81% to the corresponding benzyl fluoride product 1 (Scheme 2). Use of ortho-bromoethylbenzene resulted in low conversion of the starting material (<20%), presumably reflecting the deleterious steric or σ-electron withdrawing effect of the o-halogen on hydrogen atom transfer. Empirical modification of the conditions, including use of 4 equiv of NFSI, replacement of MeB(OH)2 with B2pin2 as the reductant, and operating at 55 °C, led to a 50% yield of the desired product 2. The modified conditions, either at 55 °C or 75 °C, also proved effective with other electron- deficient substrates (2, 4, 5, 6, 9, 13, 17, 19), while the original conditions were favored for more reactive substrates (3, 7, 8, 10, 11, 12, 14). The latter group also includes celestolide, which underwent fluorination in 86% yield (18), and substrates with tertiary C–H bonds, leading to 23 and 24 in 92% and 84% yields. Overoxidation to ketone or styrene-derived side products, was observed with more activated C–H substrates, necessitating the identification of milder conditions (35 °C, 0.5 equiv MeB(OH)2). These conditions allowed several benzyl fluorides to be obtained in good yield (15, 16, 20), including a bromochroman derivative. Methylarenes appear to favor C–H sulfonimidation rather than fluorination, as observed by the formation of 21 and 22. A collection of other less successful substrates is provided in Table S9 of the Supporting Information, but, overall, these results show that the catalytic conditions may be tuned to access good fluorination reactivity for a broad range of benzylic C–H substrates.12
Scheme 2. Cu/NFSI Fluorination of Benzylic C–H Bonds.
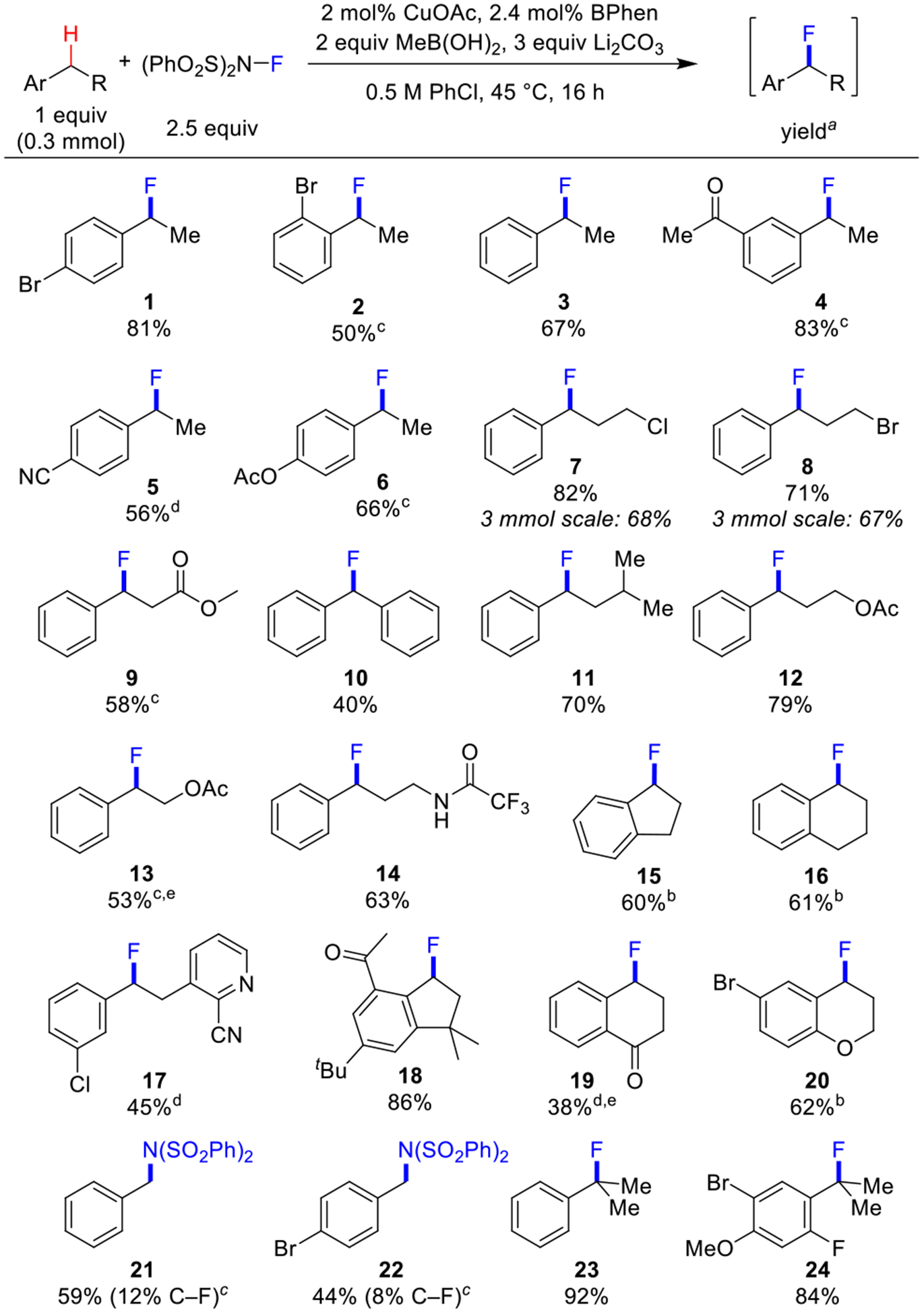
a1H or 19F NMR yields; CH2Br2 or PhCF3 as internal standard. b35 °C, 0.5 equiv MeB(OH)2. c55 °C, 1 mol% Cu/1.2 mol% BPhen, 4 equiv NFSI, 1 equiv B2pin2 instead of MeB(OH)2. d75 °C 1 mol% Cu/1.2 mol% BPhen, 4 equiv NFSI, 1 equiv B2pin2 instead of MeB(OH)2. eAcetone solvent.
Complications were encountered during product isolation. Many of the products decomposed in the presence of silica gel, and even when stored in glass vessels. These observations belie the frequent incorporation of fluorine in organic molecules to inhibit reactivity at specific sites, for example, to slow drug metabolism.10 Separately, benzylic monofluorides have been shown to undergo nucleophilic substitution in the presence of acids or hydrogen-bond donors.8,9 These insights suggest that monofluorination of benzylic C–H bonds is not a compelling end-goal for many substrates. On the other hand, they suggest that benzyl fluorides could serve as strategic intermediates in a sequential approach to benzylic C–H functionalization.
Efforts to explore sequential C–H fluorination/functionalization were initiated by testing hexafluoroisopropanol (HFIP, 10 equiv) as a hydrogen bond donor to activate the benzyl fluoride (Scheme 3).9f Initial results demonstrated conversion of benzyl fluorides to benzyl alcohols by including water as a nucleophile in the reaction mixture (25-27). Formation of 25 shows that hydrogen-bond activation supports displacement of the fluoride, even in the presence of a primary alkyl bromide. This fluorination/water-substitution sequence to access benzylic alcohols is noteworthy because C–H oxygenation strategies will typically proceed directly to ketones, reflecting the higher reactivity of alcohols relative to C–H bonds.13
Scheme 3. Benzylic C–H Cross-Coupling to C–O, C–N, and C–C Bonds via a Benzyl Fluoride.
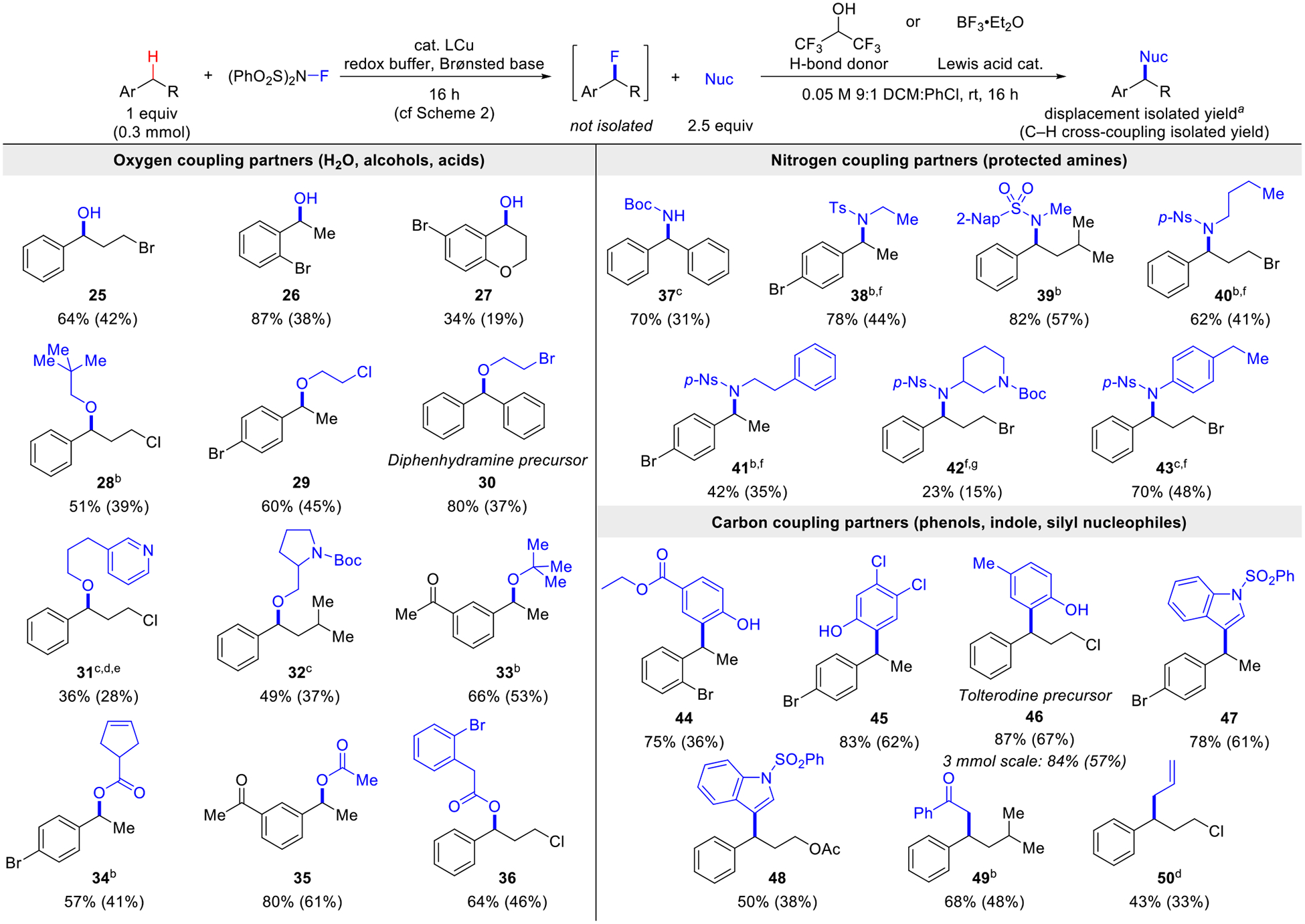
aReaction uses 10 equiv HFIP as a H-bond donor. Isolated yields calculated with respect to the 1H NMR yield of the benzyl fluoride (or the C–H substrate, in parentheses). b10 mol% BF3•Et2O used instead of HFIP. c50 mol% BF3•Et2O used instead of HFIP dBoth HFIP and BF3•Et2O used. e2.5 equiv MsOH added to the nucleophile. fUsed dichloromethane as the fluorination reaction solvent. g1.5 equiv BF3•Et2O used instead of HFIP. Isolated as the amine.
Analogous efforts were effective for the formation of benzylic ethers and esters (28–36). For less nucleophilic alcohols, like tert-butanol, more forcing conditions were needed to form the product, using BF3•OEt2 as a Lewis acid catalyst (28 and 33).9i This approach also enabled reactivity with alcohols bearing Boc-pyrrolidine or pyridine substituents (31 and 32). These results expand the scope of accessible products relative to the recently reported method for direct Cu/NFSI-catalyzed benzylic etherification,3a which shows limited compatibility with basic heterocycles, such as pyridines, and Boc-protected pyrrolidines. Carboxylic acids were also effective coupling partners (34-36). These substrates have innate acidity, but the reactions were more effective with HFIP or BF3 additives. The presence of allylic and benzylic C–H bonds in the carboxylic acids used to prepare 34 and 36 would likely complicate direct C–H carboxylation methods with these partners.
We then targeted C–N coupling reactions. Direct C–H amidation reactions typically feature primary sulfonamides or other stabilized ammonia surrogates capable of generating nitrenoid intermediates.14 Few precedents exist for oxidative coupling of C–H bonds with carbamates or secondary sulfonamides.4b,15 tert-Butyl carbamate itself proved to be an effective coupling partner when using BF3•Et2O to activate the benzyl fluoride (37). Then a range of secondary sulfonamides were shown to undergo effective displacement of the benzyl fluoride, with BF3•Et2O as an activator (38-43). The good reactivity with less nucleophilic, but more readily deprotected, nosylamides is noteworthy. Competitive Friedel-Crafts reactivity with chlorobenzene was observed in some of these reactions, but this complication was resolved by using dichloromethane as the solvent for the fluorination step (40-43).
The observation of Friedel-Crafts reactivity highlights opportunities for coupling with electron-rich arenes and other carbon nucleophiles that would not be compatible with a direct Cu/NFSI-catalyzed C–H coupling reaction. Such reactivity was demonstrated with phenols (44–46), N-sulfonyl indole (47-48), and a silyl enol ether and allyl silane (49-50).
Each of the reactions highlighted above proceeds via a straightforward two-step protocol, without isolation of the benzyl fluoride intermediate. Following the fluorination step, sodium dithionite is added to quench any unreacted NFSI. The slurry is then diluted with dichloromethane and filtered. Subsequent addition of the nucleophile and HFIP/BF3 promoter initiates the displacement reaction. As conveyed in several instances above, this two-step C–H cross-coupling sequence greatly expands the scope of useful reaction partners. Many of the electron-rich substrates and nucleophiles bearing oxidatively sensitive substituents would decompose or undergo deleterious side reactions with NFSI in a direct oxidative coupling reaction.11c,16
In summary, the results described above introduce a new strategy to achieve selective benzylic C–H cross-coupling with diverse reaction partners. The use of Cu/NFSI conditions that may be tuned to accommodate different substrate electronic properties allowed formation of benzyl fluorides that may be used without isolation as coupling partners to access products with new C(sp3)–O, –N, and –C bonds. This method joins a number of emerging strategies for C(sp3)–H cross-coupling that involve formation of strategic intermediates, such as xanthate esters, isocyanates, lactones, alkylboronates,17 that allow rapid access to diversified products.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH (R01 GM126832, R35 GM134929), including a Ruth L. Kirschstein NRSA fellowship (F32 GM129909, to JAB). Spectroscopic instrumentation was partially supported by the NIH (1S10 OD020022-1) and the NSF (CHE-1048642).
Footnotes
Supporting Information
Experimental procedures, characterization data, and NMR spectra.
The Supporting Information is available free of charge on the ACS Publications website.
The authors declare no competing financial interests.
REFERENCES
- 1.Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59, 4443–4458. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-Like Molecules. Chem. Soc. Rev 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]; (b) Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem 2018, 10, 383–394. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hu H; Chen S-J; Mandal M; Pratik SM; Buss JA; Krska SW; Cramer CJ; Stahl SS Copper-Catalyzed Benzylic C–H Coupling with Alcohols via Radical Relay Enabled by Redox Buffering. Nat. Catal 2020, 3, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee BJ; DeGlopper KS; Yoon TP Site-Selective Alkoxylation of Benzylic C–H Bonds by Photoredox Catalysis. Angew. Chem. Int. Ed 2020, 59, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Zhang Y; Dong J; Liu L; Liu L; Zhou Y; Yin S-F Manganese(III) Acetate Catalyzed Oxidative Amination of Benzylic C(sp3)–H Bonds with Nitriles. Org. Biomol. Chem 2017, 15, 2897–2901. [DOI] [PubMed] [Google Scholar]; (b) Wang A; DeOliveira CC; Emmert M Non-Directed, Copper Catalyzed Benzylic C–H Amination Avoiding Substrate Excess. ChemRxiv 2019, 10.26434/chemrxiv.8792243. [DOI] [Google Scholar]
- 5.(a) Zhang W; Chen P; Liu G Copper-Catalyzed Arylation of Benzylic C–H Bonds with Alkylarenes as the Limiting Reagents. J. Am. Chem. Soc 2017, 139, 7709–7712. [DOI] [PubMed] [Google Scholar]; (b) Yazaki R; Ohshima T Recent Strategic Advances for the Activation of Benzylic C–H Bonds for the Formation of C–C Bonds. Tet. Lett 2019, 60, 151225. [Google Scholar]
- 6.(a) Ni Z; Zhang Q; Xiong T; Zheng Y; Li Y; Zhang H; Zhang J; Liu Q Highly Regioselective Copper-Catalyzed Benzylic C–H Amination by N-Fluorobenzenesulfonimide. Angew. Chem. Int. Ed 2012, 51, 1244–1247. [DOI] [PubMed] [Google Scholar]; (b) Zhang W; Wang F; McCann SD; Wang D; Chen P; Stahl SS; Liu G Enantioselective Cyanation of Benzylic C–H Bonds via Copper-Catalyzed Radical Relay. Science 2016, 353, 1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xiao H; Liu Z; Shen H; Zhang B; Zhu L; Li C Copper-Catalyzed Late-Stage Benzylic C(sp3)–H Trifluoromethylation. Chem 2019, 5, 940–949. [DOI] [PubMed] [Google Scholar]; (d) Suh S-E Chen, S.-J. Mandal, M.; Guzei, I. A.; Cramer, C. J.; Stahl, S. S. Site-Selective Copper-Catalyzed Azidation of Benzylic C–H Bonds. J. Am. Chem. Soc 2020, 142, 11388–11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buss JA; Vasilopoulos A; Golden DL; Stahl SS Copper-Catalyzed Functionalization of Benzylic C–H Bonds with NFluorobenzenesulfonimide: Switch from C–N to C–F Bond Formation Promoted by a Redox Buffer and Brønsted Base. Org. Lett 2020, DOI: 10.1021/acs.orglett.0c02239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Amii H; Uneyama K C–F Bond Activation in Organic Synthesis. Chem. Rev 2009, 109, 2119–2183. [DOI] [PubMed] [Google Scholar]; (b) Hamel J-D; Paquin J-F Activation of C–F Bonds α to C–C Multiple Bonds. Chem. Commun 2018, 54, 10224–10239. [DOI] [PubMed] [Google Scholar]
- 9.For leading primary references, see:; (a) Bernstein J; Roth JS; Miller WT Jr. The Preparation and Properties of Some Substituted Benzyl Fluorides. J. Am. Chem. Soc 1948, 70, 2310–2314. [DOI] [PubMed] [Google Scholar]; (b) Swain CG; Spalding RET III. Mechanism of Acid Catalysis of the Hydrolysis of Benzyl Fluoride. J. Am. Chem. Soc 1960, 82, 6104–6107. [Google Scholar]; (c) Toteva MM; Richard JP Hydrogen Bonding and Catalysis of Solvolysis of 4-Methoxybenzyl Fluoride. J. Am. Chem. Soc 2002, 124, 9798–9805. [DOI] [PubMed] [Google Scholar]; (d) Blessley G; Holden P; Walker M; Brown JM; Gouverneur V Palladium-Catalyzed Substitution and Cross-Coupling of Benzylic Fluorides. Org. Lett 2012, 14, 2754–2757. [DOI] [PubMed] [Google Scholar]; (e) Champagne PA; Pomarole J; Thérien M-E; Benhassine Y; Beaulieu S; Legault CY; Paquin J-F Enabling Nucleophilic Substitution Reactions of Activated Alkyl Fluorides Through Hydrogen Bonding. Org. Lett 2013, 15, 2210–2213. [DOI] [PubMed] [Google Scholar]; (f) Champagne PA; Benhassine Y; Desroches J; Paquin J-F Friedel-Crafts Reaction of Benzyl Fluorides: Selective Activation of C–F Bonds as Enabled by Hydrogen Bonding. Angew. Chem. Int. Ed 2014, 53, 13835–13839. [DOI] [PubMed] [Google Scholar]; (g) Pryyma A; Bu YJ; Wai Y; Patrick BO; Perrin DM Synthesis and Activation of Bench-Stable 3a-Fluoropyrroloindolines as Latent Electrophiles for the Synthesis of C‑2-Thiol-Substituted Tryptophans and C‑3a-Substituted Pyrroloindolines. Org. Lett 2019, 21, 8234–8238. [DOI] [PubMed] [Google Scholar]; (h) Zhu J; Pérez M; Stephan DW C–C Coupling of Benzyl Fluorides Catalyzed by an Electrophilic Phosphonium Cation. Angew. Chem. Int. Ed 2016, 55, 8448–8451. [DOI] [PubMed] [Google Scholar]; (i) Zhou X; Ding H; Chen P; Liu L; Sun Q; Wang X; Wang P; Lv Z; Li M Radical Dehydroxymethylative Fluorination of Carbohydrates and Divergent Transformations of the Resulting Reverse Glycosyl Fluorides. Angew. Chem. Int. Ed 2020, 59, 4138–4144. [DOI] [PubMed] [Google Scholar]
- 10.Szpera R; Moseley DFJ; Smith LB; Sterling AJ; Gouverneur V The Fluorination of C–H Bonds: Developments and Perspectives. Angew. Chem. Int. Ed 2019, 58, 14824–14848. [DOI] [PubMed] [Google Scholar]
- 11.For selected primary references directed toward benzylic fluorination, see:; (a) Xia J-B; Zhu C; Chen C Visible Light-Promoted Metal-Free C–H Activation: Diarylketone-Catalyzed Selective Benzylic Mono- and Difluorination. J. Am. Chem. Soc 2013, 135, 17494–17500. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bloom S; Pitts CR; Woltornist R; Griswold A; Holl MG; Lectka T Iron(II)-Catalyzed Benzylic Fluorination. Org. Lett 2013, 15, 1722–1724. [DOI] [PubMed] [Google Scholar]; (c) Nodwell MB; Bagai A; Halperin SD; Martin RE; Knust H; Britton R Direct Photocatalytic Fluorination of Benzylic C–H Bonds with N-Fluorobenzenesulfonimide. Chem. Commun 2015, 51, 11783–11786. [DOI] [PubMed] [Google Scholar]; (d) Meanwell M; Nodwell MB; Martin RE; Britton R A Convenient Late-Stage Fluorination of Pyridylic C–H Bonds with N-Fluorobenzenesulfonimide. Angew. Chem. Int. Ed 2016, 55, 13244–13248. [DOI] [PubMed] [Google Scholar]
- 12.For previous studies of benzylic fluorination, see refs. 7, 10, 11 and references cited therein.
- 13.For important alternative strategies to convert 2° benzylic C–H bonds to alcohols, see:; (a) Li G-X; Morales-Rivera CA; Gao F; Wang Y; He G; Liu P; Chen G A Unified Photoredox-Catalysis Strategy for C(sp3)–H Hydroxylation and Amidation using Hypervalent Iodine. Chem. Sci 2017, 8, 7180–7185. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dantignana V; Milan M; Cussó O; Company A; Bietti M; Costas M Chemoselective Aliphatic C–H Bond Oxidation Enabled by Polarity Reversal. ACS Cent. Sci 2017, 3, 1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tanwar L; Börgel J; Ritter T Synthesis of Benzylic Alcohols by C–H Oxidation. J. Am. Chem. Soc 2019, 141, 17983–17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Clark JR; Feng K; Sookezian A; White MC Manganese-Catalysed Benzylic C(sp3)–H Amination for Late-Stage Functionalization. Nat. Chem 2018, 10, 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chiappini ND; Mack JBC; Du Bois J Intermolecular C(sp3)–H Amination of Complex Molecules. Angew. Chem. Int. Ed 2018, 57, 4956–4959. [DOI] [PubMed] [Google Scholar]; (c) Nasrallah A; Boquet V; Hecker A; Retailleau P; Darses B; Dauban P Catalytic Enantioselective Intermolecular Benzylic C(sp3)–H Amination. Angew. Chem. Int. Ed 2019, 58, 8192–8196. [DOI] [PubMed] [Google Scholar]; (d) Bakhoda A; Jian Q; Badiei YM; Bertke JA; Cundari TR; Warren TH Copper Catalyzed sp3 C–H Amidation: Sterically Driven Primary and Secondary C–H Site-Selectivity. Angew. Chem. Int. Ed 2019, 58, 3421–3425. [DOI] [PubMed] [Google Scholar]
- 15.Each of the following has an example of a secondary (sulfon)amide as a coupling partner:; (a) Pelletier G; Powell DA Copper-Catalyzed Amidation of Allylic and Benzylic C–H Bonds. Org. Lett 2006, 8, 6031–6034. [DOI] [PubMed] [Google Scholar]; (b) Ye Y-H; Zhang J; Wang G; Chen S-Y; Yu X-Q Cobalt-Catalyzed Benzylic C–H Amination via Dehydrogenative-Coupling Reaction. Tetrahedron 2011, 67, 4649–4654. [Google Scholar]; (c) Bosnidou AE; Muñiz K Intermolecular Radical C(sp3)–H Amination under Iodine Catalysis. Angew. Chem. Int. Ed 2019, 58, 7485–7489. [DOI] [PubMed] [Google Scholar]
- 16.(a) Jing L; Yu X; Guan M; Wu X; Wang Q; Wu X An Efficient Method for Sulfonylation of Amines, Alcohols, and Phenols with N-Fluorobenzenesulfonimide Under Mild Conditions. Chem. Res. Chin. Univ 2018, 34, 191–196. [Google Scholar]; (b) Rozatian N; Ashworth IW; Sandford G; Hodgson DR W. A Quantitative Reactivity Scale for Electrophilic Fluorinating Reagents. Chem. Sci 2018, 9, 8692–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For examples of C(sp3)–H functionalization sequences, see:; (a) Czaplyski WL; Na CG; Alexanian EJ C–H Xanthylation: A Synthetic Platform for Alkane Functionalization. J. Am. Chem. Soc 2016, 138, 13854–13857. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huang X; Zhuang T; Kates PA; Gao H; Chen X; Groves JT Alkyl Isocyanates via Manganese-Catalyzed C–H Activation for the Preparation of Substituted Ureas. J. Am. Chem. Soc 2017, 139, 15407–15413. [DOI] [PubMed] [Google Scholar]; (c) Zhuang Z; Yu J-Q Lactonization as a General Route to β-C(sp3)–H Functionalization. Nature 2020, 577, 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Oeschger R; Su B; Yu I; Ehinger C; Romero E; He S; Hartwig J Diverse Functionalization of Strong Alkyl C–H Bonds by Undirected Borylation. Science 2020, 368, 736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


